Psychological disorders are considered pressing health issues, with growing numbers of people experiencing negative consequences on health and longevity(Reference Whiteford, Degenhardt and Rehm1–3). In 2015, the global prevalence of depressive and anxiety disorders was 4·4 % (5·1 % for females, 3·6 % for males) and 3·6 % (4·6 % for females, 2·6 % for males), respectively(3). According to national statistics, depression and anxiety affect about 21 % and 20 % of the adult population in Iran(Reference Noorbala, Yazdi and Yasamy4). Therefore, low-cost strategies to address these mental health disorders would be valuable.
Many factors including genetic and lifestyle determinants (smoking, alcohol consumption and diet) affect the aetiology of psychological disorders(Reference Saveanu and Nemeroff5). Notably, the importance of food groups and dietary patterns has been recognised in the development of mental disorders(Reference Murakami and Sasaki6–Reference Perez11). For instance, dietary intake of whole grains, seeds, nuts, fruits and vegetable-rich sources of B-vitamins are associated with lower risk of psychological disorders(Reference Su, Yu and He12–Reference Anjom-Shoae, Sadeghi and Keshteli15). A biological mechanism that could mediate the association between B-vitamin intake and psychological disorders is their involvement in single-carbon transfer reactions, which is needed for the production of monoamine neurotransmitters(Reference Sánchez-Villegas, Doreste and Schlatter16,Reference Bjelland, Ueland and Vollset17) . In studies among psychiatric patients, vitamin B deficiencies have been associated with severe depressive symptoms (Reference Bell, Edman and Morrow18–Reference Carney20). Folate serum level can also affect patients’ responses to antidepressants(Reference Wesson, Levitt and Joffe21). It is important to note that such studies have been small and were conducted on highly selected patients, for whom folate deficiency might not be the cause but rather a consequence of depression (due to loss of appetite and poor dietary intake). Moreover, although several clinical trials have investigated the effects of vitamin B supplementation on psychological disorders(Reference Bryan, Calvaresi and Hughes22–Reference Deijen, Van der Beek and Orlebeke24), findings of such studies may not be generalisable to the whole population as they either used high dosage of B-vitamins or had short duration. To date, the few population-based studies that have focused on the association between dietary B-vitamin intake and depression have provided conflicting results. Although two studies (with Korean and Finnish participants) reported a positive link between vitamin B deficiencies(Reference Tolmunen, Hintikka and Ruusunen25,Reference Kim, Stewart and Kim26) and depression, it is not clear that such results are generalisable to other settings due to geographical variation in nutrient intake. Moreover, to the best of our knowledge, no previous study has considered the association between dietary B-vitamin intake with anxiety and psychological stress, especially in women. We are only aware of one study in Iran, which assessed only vitamin B6 in relation to depression and anxiety(Reference Kafeshani, Feizi and Esmaillzadeh27). Investigating potential associations between dietary B-vitamin intake and psychological disorders might provide an avenue for prevention, especially in Middle East region where there is a high prevalence of psychological disorders(Reference Noorbala, Yazdi and Yasamy4,Reference Ferrari, Somerville and Baxter28) and consumption of whole grains, meat, fruits and vegetables as dietary sources of B-vitamins is low(Reference Esteghamati, Noshad and Nazeri29,Reference Esmaillzadeh and Azadbakht30) . Therefore, in the present study, we examined the cross-sectional association between dietary vitamin B6–9–12 intake with depression, anxiety and psychological distress among Iranian women.
Materials and methods
Study population
In this population-based cross-sectional study, we used clustered random sampling to recruit 455 women who were referred to ten health centres located in southern Tehran, Iran (September 2017–September 2018). To determine the number of women sampled from each health centre, the total population covered by each centre was represented proportionally in the initial estimated sample size (n 435). Female participants were selected if they met the following inclusion criteria: (a) were age 20–50 years old; (b) had no previous diagnosis of chronic diseases, or psychological disorders by a physician; (c) were not taking any specific medications (including those that would affect weight, lipid and/or glucose metabolism, blood pressure and mental status); (d) were not following a specific dietary pattern such as a vegetarian diet; (e) were not pregnant or lactating; (f) were not women who had immigrated to Iran and (g) did not experience emotional suffering in the preceding year (e.g. reported severe financial problems, death of close friends/relatives). Participants were not included in the analysis if they showed implausibly low or high scores for total energy intake (<3347·2 kJ/d or >17572·8 kJ/d)(Reference Mozaffari, Namazi and Larijani31,Reference Mozaffari, Daneshzad and Larijani32) . The prevalence of mental disorders among Iranian women was chosen, as a main dependent variable, to estimate sample size(Reference Noorbala, Yazdi and Yasamy4), using the following formula N = [(Z1 − α/2)2 P(1 − P)]/d 2. Assuming statistical values of P = 26; α = 0·05; d = 4·12, the calculated sample size was estimated to be 435. However, because of possible missing data, we interviewed 455 female participants. After removing eight participants because of unexplained energy intake, only 447 females remained in the statistical analysis. All the participants provided informed consent prior to inclusion in the study.
Dietary intake assessment
Data on individuals’ dietary intakes were collected by completing a 168-item semi-quantitative FFQ through face-to-face interviews with a trained nutritionist. The validity and reliability of the FFQ have been shown to be adequate(Reference Mirmiran, Esfahani and Mehrabi33). Participants were asked to report on average portion sizes and frequency of consumption of foods on a daily, weekly or monthly basis. Next, the daily intake of each food was converted from household measures into grams. An adapted version of NUTRITIONIST IV modified for Iranian foods (version 7.0; N-Squared Computing) was used to compute mean energy and nutrient intakes, especially B-vitamins(Reference Azadbakht, Kimiagar and Mehrabi34).
Psychological profile assessment
To evaluate psychological disorders, the Iranian validated version of the Depression Anxiety Stress Scale-21 was applied(Reference Samani and Joukar35). This questionnaire is a 21-item self-reported structured scale that comprises three subscales: depression, anxiety and psychological distress. Each subscale contains seven items. Questions 3, 5, 10, 13, 16, 17 and 21 correspond to depression, 2, 4, 7, 9, 15, 19 and 20 correspond to anxiety and questions 1, 6, 8, 11, 12, 14 and 18 correspond to psychological distress. Answers to each item are based on a four-point Likert scale including 0 (never), 1 (little), 2 (sometimes) and 3 (always). Participants were asked to rate the extent to which they had experienced each of the states during the preceding week. To estimate the total score for each psychological disorder subscale, a bimodal scoring method (0–0 to 1–1) was used. In total, each subscale can receive a score of 0 to 21. Depression Anxiety Stress Scale-21 was developed to represent all subscales including depression, anxiety and stress; thus, it can be made equivalent to the DASS-42 by multiplying the final score of each subscale by 2. Higher scores for each subscale correspond to higher levels of depression, anxiety or psychological distress. In this study, the scores of ≥10 for depression, ≥8 for anxiety and ≥15 for psychological distress were considered indicators of psychological disorders. The validity and reliability of the Iranian version of the Depression Anxiety Stress Scale-21 have been previously evaluated by Samani and Jokar(Reference Samani and Joukar35). The test–retest validity (depression= 0·80, anxiety = 0·76, stress = 0·77) and Cronbach’s alpha coefficient (depression = 0·81, anxiety = 0·74, stress = 0·78) for each subscale of Depression Anxiety Stress Scale-21 were reported to be adequate(Reference Samani and Joukar35).
Anthropometric assessment
Anthropometric measurements (body weight and height) were collected using the WHO standard protocol and registered by a trained assistant. Participant body weight was measured using a portable digital scale (Seca725 GmbH & Co.) after participants removed their heavy outer garments. Weight was recorded with 100 g precision. Moreover, height was measured using a stadiometer or meter while participants stood barefoot against a wall with their shoulders in a comfortable position. Height was reported within a precision of 0·5 cm. BMI was calculated as body weight in kg divided by the square of height in metres (m2).
Assessment of other covariates
Socioeconomic status was assessed using a valid and reliable questionnaire developed for health research in Iran(Reference Mozaffari, Namazi and Larijani36). This questionnaire included several questions regarding level of education, participants’ employment, property (car and house ownership), number of rooms in the household, electronic appliances, number of family members and number of trips abroad or within the country during the preceding year(Reference Mozaffari, Namazi and Larijani36). To gather data on other important covariates, a demographic questionnaire contained questions on age, body shape satisfaction and the number of hours spent sleeping and spent outside the home. A trained assistant recorded the average amount of time that each participant dedicated to different physical activities during the day. Then, the time for each activity was multiplied by the corresponding metabolic equivalent task (MET-h/week) to estimate an individual’s physical activity(Reference Ainsworth, Haskell and Whitt37).
Statistical analysis
The distribution of variables was investigated using histogram curves and the Kolmogorov–Smirnov test. Energy-adjusted dietary intake of B-vitamins was obtained using the residual method(Reference Brown, Kipnis and Freedman38). Participants were categorised based on tertiles of energy-adjusted dietary B-vitamin intake: vitamin B6 (<1·21; 1·21–1·47; >1·47 mg/d), folate (>286·71; 286·71–354·80; >354·80 µg/d) and vitamin B12 (>3·66; 3·66–4·90; >4·90 µg/d). To examine continuous variables (e.g. some demographic variables and lifestyle factors) across tertiles of B-vitamin intake, one-way ANOVA was applied. The distribution of categorical variables across tertiles of B-vitamin intake was calculated using χ 2 tests. To compare participants’ dietary intakes (nutrients and food groups) within tertiles of dietary B-vitamins, ANCOVA with Bonferroni correction was used to adjust for energy intake. Before conducting binary logistic regression for each psychological disorder subscale, participants were categorised based on the reported cut-offs as follows: depression ≥10, depression, anxiety ≥8 and psychological distress ≥15. Associations between dietary intake of B-vitamins and psychiatric disorders were calculated using binary logistic regression in crude models and separate multivariable models for each disorder. We adjusted for energy intake (continuous), age (continuous), socioeconomic status (weak/moderate/strong), supplement use (yes/no), satisfaction with body shape (yes/no) and BMI (continuous) as covariates. Additionally, dietary intake of fibre (continuous), n-3 fatty acids (continuous) and Mg (continuous) were adjusted for in the final model. All the analyses were performed using SPSS software (version 19.0; SPSS Inc.), and lowest tertiles were regarded as the reference category. P values were considered statistically significant at < 0·05.
Results
The mean age of participants (n 447) was 31·68 years. General participant characteristics across tertiles of dietary B-vitamin intake are displayed in Table 1. The prevalence of depression, anxiety and psychological stress was 31, 32 and 35 %, respectively. Participants in the high tertile of dietary vitamin B6 intake were more likely to be married. However, with regard to other general characteristics, we did not observe significant differences between participants in the high tertile compared with participants in low tertile.
Table 1 General characteristics of Iranian women across tertiles of energy-adjusted dietary B-vitamin intake
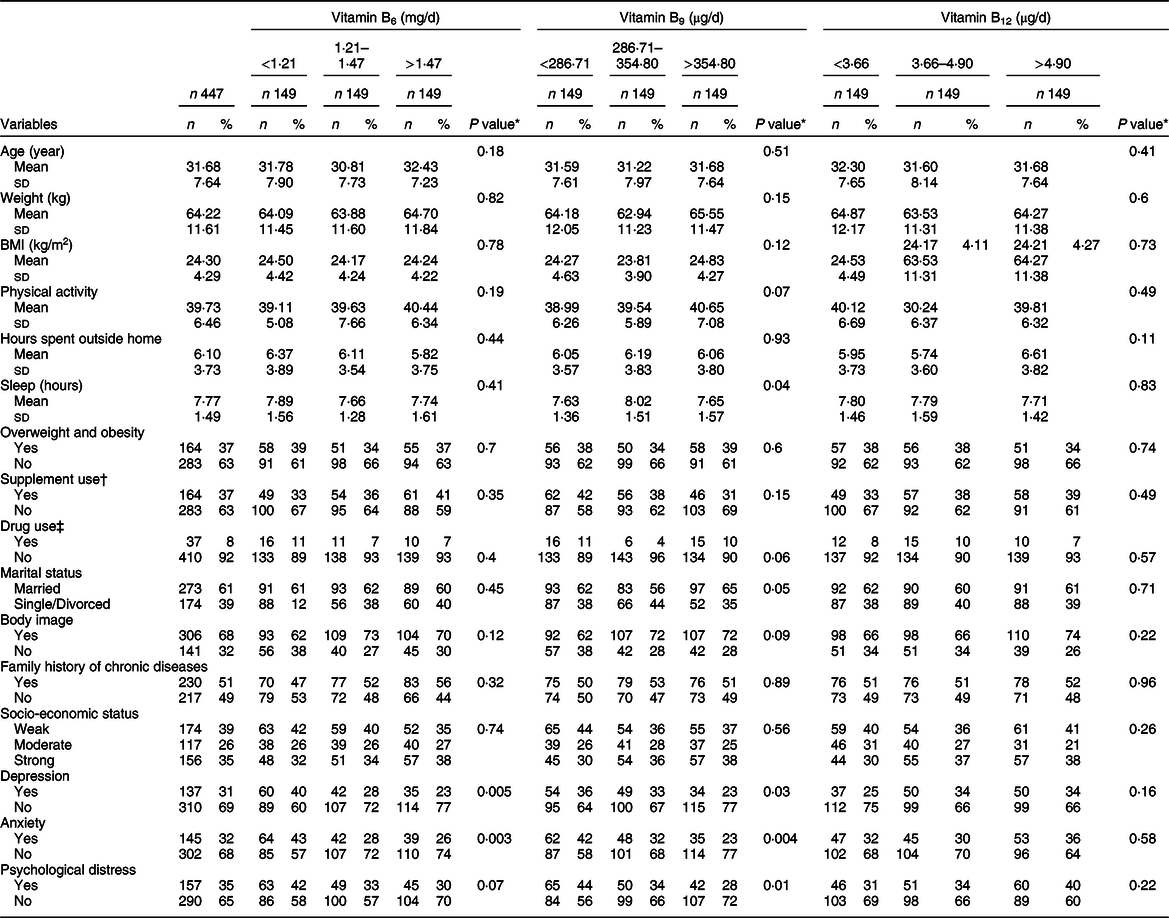
* ANOVA was calculated for continuous variables and the χ 2 test for categorical variables.
† Multivitamin and mineral supplements, n-3, vitamin D, and vitamin B supplements.
‡ Gastrointestinal medications.
Participants’ dietary B-vitamin intakes by tertitle are provided in Table 2.
-
Vitamin B 6 : Participants in the high vitamin B6 tertile had higher consumption of total protein (P value = 0·02), fibre (P value = 0·0001), Mg (P value = 0·0001), fruits (P value = 0·0001) and vegetables (P value = 0·0001) compared with those in the low tertile. However, they showed lower intake of energy (P value = 0·001) and grains (P value = 0·0001).
-
Vitamin B 9 : Participants included in the high tertile for vitamin B9 had higher intakes of proteins (P value = 0·001), carbohydrates (P value = 0·05), fibre (P value = 0·0001), Mg (P value = 0·0001), fruits (P value =0·005) and vegetables (P value = 0·0001). However, they showed lower intakes of energy (Pvalue = 0·0001), fat (Pvalue = 0·003) and grains (P value = 0·001).
-
Vitamin B 12 : Participants included in the high tertile of vitamin B12 had higher intakes of proteins (P value = 0·0001), n-3 (P value = 0·02), Mg (P value = 0·0001), meats (P value = 0·0001) and dairy products (P value =0·0001). However, they had lower intakes of energy (P value = 0·0001), carbohydrates (P value = 0·0001) and fruits (P value = 0·009).
-
OR and 95 % CI for depression, anxiety and psychological distress by tertile of dietary B-vitamin intake are provided in Table 3.
-
Vitamin B 6 : A significant inverse association was observed between vitamin B6 intake and depression (OR: 0·54; 95 %CI: 0·31, 0·95; P trend: 0·03), but not for anxiety (OR: 0·67; 95 %CI: 0·38, 1·16; P trend: 0·14) or psychological distress (OR: 0·76; 95 %CI: 0·44, 1·31; P trend: 0·32).
-
Vitamin B 9 : No association was found between dietary intake of folate with depression (OR: 0·80; 95 %CI: 0·40, 1·60; P trend: 0·56), anxiety (OR: 0·76; 95 %CI: 0·38, 1·50; P trend: 0·42) or psychological distress (OR: 0·84; 95 %CI: 0·44, 1·63; P trend: 0·57).
-
Vitamin B 12 : A positive association was found between dietary vitamin B12 intake with odds of depression (OR: 2·05; 95 % CI: 1·17, 3·60; P trend: 0·01) and psychological distress (OR: 2·00; 95 % CI: 1·17, 3·41; P trend: 0·01), but not for anxiety (OR: 1·62; 95 %CI: 0·94, 2·79; P trend: 0·07).
Table 2 Energy-adjusted dietary intake across tertiles of energy-adjusted dietary B-vitamins among Iranian women*, †, ‡
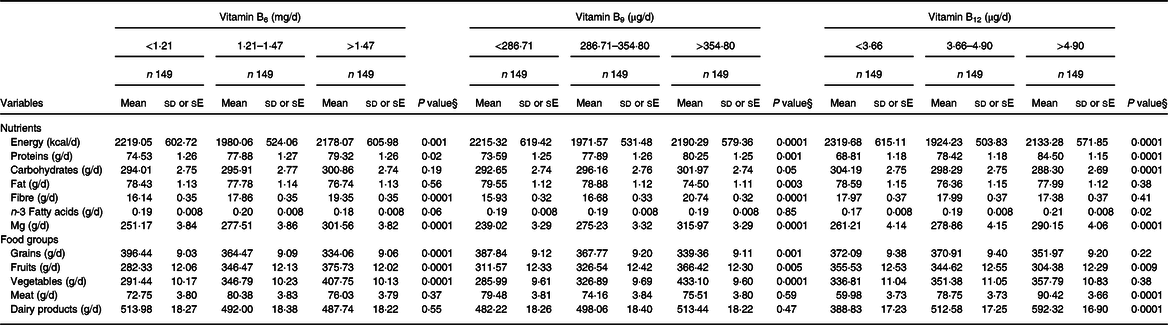
* Mean ± sd (only energy intake).
† Mean ± se (other variables).
‡ All the variables, except energy, were adjusted for energy intake.
§ Calculated using ANOVA for energy intake and multivariate ANCOVA with Bonferroni correction for other dietary variables.
Table 3 Psychological disorders by tertile of energy-adjusted dietary B-vitamin intake among Iranian women
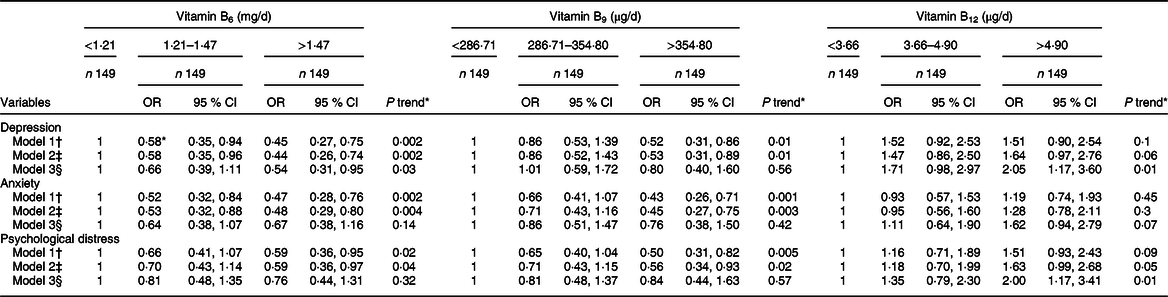
* Calculated by logistic regression.
† Model 1: Crude.
‡ Model 2: Adjusted for energy intake, age, socioeconomic status, supplement use, body satisfaction, BMI.
§ Model 3: Adjusted for energy intake, age, socioeconomic status, supplement use, body satisfaction, BMI, n-3 fatty acids, fibre, Mg.
Discussion
Dietary pyridoxine intake was inversely associated with depression, but not anxiety and psychological distress. However, higher cobalamin intake was associated with greater odds of depression and psychological distress. No relationship was found between dietary vitamin B9 with any of the psychological disorders studied. To the best of our knowledge, this is the first study to investigate the association between dietary B-vitamin intake and anxiety and psychological distress specifically in women.
Given that B-vitamins are essential for every aspect of normal brain function and that there are less than optimal levels of B-vitamins in many populations worldwide(Reference Kennedy39), we decided to focus on them in this study. There are also several reasons why we chose the subset of B6, B9 and B12 vitamins. The main driver of this decision was based on the ‘homocysteine hypothesis’. Biochemically, vitamins B6, B9 and B12 are involved in the metabolism of adenosyl methionine and methionine which are essential compounds for the production of neurotransmitters in the brain(Reference Sánchez-Villegas, Doreste and Schlatter16). Lower intake of these vitamins can result in homocysteine accumulation that can adversely affect vascular systems and subsequently increase the risk of depression(Reference Almeida, Marsh and Alfonso40,Reference Bottiglieri41) . Second, vitamin B12 deficiency is among the most prevalent nutrient deficiencies(Reference Green, Allen and Bjørke-Monsen42). Third, vitamins B6, B9 and B12 have limited dietary sources and are more likely to be deficient compared with other B-vitamins(Reference Kennedy39).
We found a significant inverse association between vitamin B6 intake and depression. In line with this finding, in the Quebec Longitudinal Study, dietary intake of vitamin B6 (>1·71 v. <1·33 mg/d) was negatively associated with risk of depression among Canadian elderly women (n 691)(Reference Gougeon, Payette and Morais43). Moreover, a prospective study showed an inverse relationship between vitamin B6 intake (from both diet + supplements: 2·4–207 v. 0·6–1·6 mg/d) and depression among US elderly (n 3503)(Reference Skarupski, Tangney and Li44). In that study, for every additional 10 mg/d of vitamin B6 intake, the risk of depression reduced by about 2 %(Reference Skarupski, Tangney and Li44). In contrast, no association was found between dietary vitamin B6 intake (men: 0·78 v. 0·47 mg/1000 kcal; women: 0·92 v. 0·66 mg/1000 kcal) and depression among Japanese adults (men: 309, women: 208; 21–67 years old)(Reference Murakami, Mizoue and Sasaki45). Moreover, there was no association between vitamin B6 intake (>1·70 v. <1·46 mg/d) and depression among elderly Dutch men (n 332)(Reference Kamphuis, Geerlings and Grobbee46). In addition, a Finnish prospective study did not show any association between dietary vitamin B6 intake (2·1–4·4 v. 0·3–1·7 mg/d) and psychological disorders among adult men (n 2682)(Reference Tolmunen, Hintikka and Ruusunen25). A systematic review indicated no treatment effect of vitamin B6 for depression, except for hormone-related depression in premenopausal women(Reference Williams, Cotter and Sabina47).
Various explanations may explain inconsistent findings across studies. First, some studies neglected to account for some confounding variables that are related to psychological disorders, such as satisfaction with body image, the average amount of time dedicated to sleeping and activities outside home, important dietary elements (fibre and n-3)(Reference Gougeon, Payette and Morais43–Reference Kamphuis, Geerlings and Grobbee46,Reference Tolmunen, Voutilainen and Hintikka48) , smoking(Reference Gougeon, Payette and Morais43,Reference Kamphuis, Geerlings and Grobbee46) and physical activity(Reference Tolmunen, Voutilainen and Hintikka48). Second, variability exists in fruit and vegetable consumption (as a rich source of B-vitamins) in different age groups across the globe(Reference Chi, Wang and Tsai49). Low fruit and vegetable consumption is more prevalent among the elderly(Reference Hall, Moore and Harper50). Third, studies used different methods to estimate dietary intake and to measure psychological status. Fourth, gender differences could be another explanation since gonadal steroids can influence mood state (Reference Nolen-Hoeksema51,Reference Laurin, Lavoie and Bacon52) and the accuracy of dietary assessment differs by gender. Men and women also differ in terms of actual food choices(Reference Beer-Borst, Hercberg and Morabia53,Reference Wardle, Haase and Steptoe54) , precision of reported dietary intakes(Reference Marks, Hughes and van der Pols55) and even self-reported preferences for foods(Reference Jensen and Holm56). Fifth, genetic variation may play a role, as people with a specific genetic make-up might be particularly sensitive to low levels of B-vitamins(Reference Mutch, Wahli and Williamson57,Reference Zeisel58) .
In the current study, we observed a positive association between dietary vitamin B12 intake with depression and psychological distress, but not anxiety. By contrast, a cross-sectional study by Sanchez-Villegas et al. observed an inverse association between dietary vitamin B12 intake (15·7 v. 5·2 µg/d) and the prevalence of depression among Spanish women (n 5459)(Reference Sánchez-Villegas, Doreste and Schlatter16). A prospective study of British women indicated an inverse association between dietary vitamin B12 (5·48 µg/d) and psychological distress(Reference Mishra, McNaughton and O’Connell59). The study by Skarupski et al. found that every additional 10 µg of vitamin B12 (from diet + supplement) was associated with 2 % reduced risk of depression among US elderly annually(Reference Skarupski, Tangney and Li44). A prospective study (with 3 years of follow-up) among Canadian elderly showed that men in the lowest tertile of dietary B12 intake (<3·16 µg/d v. >4·79) had greater risk of depression(Reference Gougeon, Payette and Morais43). Moreover, a few studies have reported null associations. For instance, no association was found between dietary cobalamin intake (men: 7 v. 2·6; women: 7·2 v. 3·8 µg/1000 kcal) and depression among Japanese adults (men: 309, women: 208; 21–67 years old)(Reference Murakami, Mizoue and Sasaki45). A cross-sectional study by Tolmunen et al. also showed no association between dietary cobalamin intake (8·7–136 v. 2·2–5·9 µg/d) and depression among Finnish men (n 2682; 42–60 years old)(Reference Tolmunen, Voutilainen and Hintikka48).
Incompatible results across studies could be due to various reasons. First, dietary patterns are not uniform across different cultures. To be more exact, the null finding in Tolmunen et al.’s study might be due to the fact that animal products such as meat and dairy (a rich source of cobalamin) play a prominent role in traditional Finnish dishes(Reference Tolmunen, Voutilainen and Hintikka48). In a cohort study, about 99 % of Finnish participants received the Recommended Dietary Allowance (RDA) of cobalamin(Reference Tolmunen, Voutilainen and Hintikka48). Second, another factor affecting this association could be poor absorption of cobalamin, particularly in older people(Reference Ho, Kauwell and Bailey60), since most of the studies that reported a negative association between cobalamin and psychological disorders have been conducted in the elderly(Reference Gougeon, Payette and Morais43,Reference Skarupski, Tangney and Li44) . A third reason relates to the use of different methods to define and assess psychological disorders (Reference Sánchez-Villegas, Doreste and Schlatter16,Reference Gougeon, Payette and Morais43–Reference Murakami, Mizoue and Sasaki45,Reference Tolmunen, Voutilainen and Hintikka48) . Dietary intake of vitamin B12 was above the RDA among participants in our study (mean: 4·58 µg/d). Therefore, consumption of foods that are rich in B12 such as meats and dairy products as well as better absorption in younger age groups might explain why we observed positive associations between this vitamin and psychological disorders in our population.
We found no relationship between dietary folate intake and psychological disorders. In line with our findings, the Chicago Health and Aging Project showed no associations between total folate intake (diet + supplement: 397–1731 v. 63–263 µg/d) and depression among 3503 US elderly (aged >65 years old)(Reference Skarupski, Tangney and Li44). Moreover, a prospective study of elderly Dutch men revealed no relationship between dietary folate intake (>194 v. <15 µg/d) and depressive symptoms(Reference Kamphuis, Geerlings and Grobbee46). Null findings in these studies could be explained in several ways. First, the prevalence of folate deficiency has fallen in the US population (16 to 1 % since 1998) due to folic acid fortification(Reference Pfeiffer, Caudill and Gunter61). Second, the association between dietary folate intake and psychological disorders might weaken as people advance in age. In support of the later idea, two studies showed a positive link between low plasma folate level and depression in middle-aged samples(Reference Morris, Fava and Jacques62,Reference Sachdev, Parslow and Lux63) , while two other studies did not find such association in the elderly(Reference Hvas, Juul and Bech64,Reference Penninx, Guralnik and Ferrucci65) . Although one study reported an inverse association between folate status and depression in the elderly, this finding appeared to be mainly due to CVD and comorbidities(Reference Tiemeier, Van Tuijl and Hofman66).
By contrast, a cross-sectional study by Tolmunen et al., conducted with Finish adults (n 2682 men; 42–60 years old), revealed that participants in the lowest tertile of folate intake (45·4–226 µg/d) had about 65 % greater risk of having depression than those in the highest tertile (269·3–587·5 µg/d)(Reference Tolmunen, Voutilainen and Hintikka48). Two studies conducted on Spanish(Reference Sánchez-Villegas, Doreste and Schlatter16) and Japanese(Reference Murakami, Mizoue and Sasaki45) adults showed associations only in men (but not women). The cross-sectional study by Sanchez-Villegas et al. found an inverse association between dietary folate intake (569·4 v. 231·9 µg/d) and the prevalence of depression among Spanish men (n 4211), but not women(Reference Sánchez-Villegas, Doreste and Schlatter16). Moreover, Murakami et al. showed that folate intake (235 v. 119 µg/1000 kcal) was associated with lower depression among Japanese men (n 309), but not women(Reference Murakami, Mizoue and Sasaki45). In these two studies, higher intake of folate in Spanish (618·50 v. 332·20 µg/d) and Japanese women (292 v. 155 µg/1000 kcal) might explain null findings in females compared to males(Reference Sánchez-Villegas, Doreste and Schlatter16,Reference Murakami, Mizoue and Sasaki45) .
These findings may suggest that even very low amounts of folate can aggravate psychological disorders. In support of this hypothesis, in the aforementioned Finnish study by Tolmunen et al., the inverse association between dietary folate intake and depression might be due to the fact that only 24 % of the participants received the recommended daily amount of folate in Finland (300 mg/d)(Reference Tolmunen, Voutilainen and Hintikka48). A higher prevalence of psychological disorders has been observed in countries where folate fortification was not compulsory, at least during the study, for instance, Japan(Reference Nanri, Mizoue and Matsushita67), France(Reference Astorg, Couthouis and de Courcy68), Finland(Reference Tolmunen, Hintikka and Ruusunen25), Norway(Reference Bjelland, Tell and Vollset69), Greece(Reference Dimopoulos, Piperi and Salonicioti70), Singapore(Reference Ng, Niti and Zaw71) and Australia before 2009(Reference Almeida, Marsh and Alfonso40). However, once a satisfactory level is achieved, further increases in the amount of folate might not result in greater reductions in the risk of mental disorders. In support of this idea, the study by Sanchez-Villegas et al. found that the estimated odds of depression in the fifth quintile (OR: 1·01; median of folate: 569·4) of folate intake was not the lowest, but was higher than that of the third (OR: 0·77; median of folate: 361·7) and fourth quintiles (OR: 0·72; median of folate: 429·7)(Reference Sánchez-Villegas, Doreste and Schlatter16).
The inverse association between dietary B-vitamin intake and psychological disorders can be understood through different mechanisms. Pyridoxine and cobalamin act as cofactors in the conversion of homocysteine to cysteine and methionine, respectively(Reference Sánchez-Villegas, Doreste and Schlatter16). Additionally, methyl folate is needed for the conversion of homocysteine to methionine as well(Reference Sánchez-Villegas, Doreste and Schlatter16). Methionine is a precursor of S-adenosylmethionine which is responsible for methylation reactions in the production of neurotransmitters, membrane phospholipids and nucleic acids(Reference Lu72). Therefore, inadequate dietary intake of B-vitamins might lead to a reduction in the production of monoamines in the brain and accumulation of homocysteine in the body, which are both likely to contribute to the development of mental disorders(Reference Bottiglieri41,Reference Almeida, McCaul and Hankey73) . The accumulation of metabolites of homocysteine (such as cysteine sulphinic acid and homocysteinic acid) might have a large impact on psychological disorders through different pathways. First, they might inhibit one-carbon methylation reactions by S-adenosylmethionine(Reference Parnetti, Bottiglieri and Lowenthal74). Second, they can negatively affect N-methyl-D-aspartate glutamate receptors in the central nervous system(Reference Parnetti, Bottiglieri and Lowenthal74). Third, they might have negative vascular effects(Reference Klerk, Verhoef and Clarke75).
There were several reasons why we prioritised studying women. First, the WHO has indicated women’s health to be an urgent priority and has noted that research on women is still limited and often unreliable. Second, according to international(3) and national(Reference Noorbala, Yazdi and Yasamy4) statistics, the prevalence of depression is much higher among women than men. Third, the overall mental health of women of reproductive age is also important because it may affect their fertility(Reference Rooney and Domar76).
To the best of our knowledge, this is the first study that investigates the relationship between dietary B-vitamin intake and psychological disorders – anxiety and psychological distress – among women. Previous epidemiological studies differ in the extent to which confounding variables were controlled. The present study went beyond that of most other studies that have been conducted on the association between B-vitamins and psychological disorders by controlling for important dietary factors (fibre and n-3), body image, number of hours spent sleeping and spent outside the home.
Several limitations should be considered when interpreting the current findings. First, we were unable to assess causality due to the cross-sectional nature of this study. Therefore, we recommend future prospective studies be carried out to understand the casual link between B-vitamins intake and psychological disorders. Second, psychological disorders can affect food consumption in favour of high-energy and energy-dense diets rather than nutrient-dense diets(Reference Gibson77). Therefore, lower dietary intake of B-vitamins, which is a feature of energy-dense diet, might be a consequence rather than a cause of depression. Although such plausible sources of bias are difficult to rule out when using a cross-sectional design, participants with a previous psychiatric disorders and depressive symptoms were excluded from our study at baseline. Apart from this, energy intake was adjusted; therefore, reverse causality is unlikely. Third, due to high within-person variation and rates of turnover regarding dietary B-vitamin intake, a valid and reliable FFQ corresponding to the last 12 months was used to measure dietary intake. However, the closed-end nature of the questionnaire could increase the possibility of under- and over-reporting, leading to misclassification. Fourth, due to the aforementioned within-person variation, it would have been better to assess blood levels of B-vitamins to support our findings. Still, dietary intake of folate and cobalamin has been shown to be positively correlated with serum levels since they can be stored within body(Reference Chew, Khor and Loh78,Reference Yang, Cogswell and Hamner79) . Fifth, although homocysteine serum level was mentioned as a possible mechanism through which low levels of B-vitamins might increase the risk of psychological disorders; however, we did not evaluate if homocysteine performed as a mediator in our study Sixth, the study population was restricted to women; however, such findings may be different among men.
Conclusion
Dietary pyridoxine intake was inversely associated with depression, but not anxiety or psychological distress. However, dietary cobalamin intake was associated with higher odds of depression and psychological distress. No relationship was found between dietary vitamin B9 intake with any of the psychological disorders studied. We recommend that future prospective studies in different populations are conducted to clarify whether B-vitamin deficiency is a cause or consequence of psychological disorders.
Acknowledgements
Acknowledgements: The authors would like to thank the participants for taking part in this study. Financial support: This study was supported by Tehran University of Medical Sciences (grant and ethics number: 98-01-161-42024). Conflict of interest: The authors declare that they have no conflict of interest. Authorship: L.A. and H.M. designed the study. H.M., M.D.M. and M.A. contributed in statistical analysis, data interpretation and manuscript drafting. P.J.S. reviewed and edited the manuscript. The final version of manuscript for submission was approved by all authors. Ethics of human subject participation: The present study was conducted in accordance with the Helsinki Declaration, and all procedures involving human subjects were approved by the ethics committee of Tehran University of Medical Sciences. All the participants gave their permission for inclusion by signing an informed consent.






