Vitamin D deficiency and insufficiency constitute a global health problem(Reference Holick1). Subclinical vitamin D deficiency, characterised by low serum concentrations of 25(OH)D, the primary form of vitamin D in the bloodstream, is prevalent in both developed and developing nations(Reference Lips2). In the USA, the prevalence of vitamin D insufficiency stands at 40·9 %, while the prevalence of severe and moderate vitamin D deficiency stands at 2·6 % and 22·0 %, respectively. A higher rate of severe and moderate vitamin D deficiency was observed among non-Hispanic black Americans(Reference Cui, Xiao and Ma3). While vitamin D plays a critical role in regulating Ca and P balance and maintaining bone health, its deficiency has also been linked to heightened inflammation, autoimmune disorders and dysregulation of the immune system across various chronic diseases(Reference Clark and Mach4,Reference Dhawan and Priyanka Choudhary5) .
The emergence of the COVID-19 pandemic in 2019 has ignited considerable debate about the potential role of vitamin D in both preventing and treating COVID-19. Numerous studies have reported a strong association between lower vitamin D levels (serum 25(OH)D) and unfavourable COVID-19 outcomes and prognosis(Reference AlSafar, Grant and Hijazi6–Reference Baktash, Hosack and Patel13). However, some studies have failed to establish a definitive connection between vitamin D status and COVID-19 severity and mortality(Reference Ferrari, Locatelli and Faraldi14–Reference Al-Jarallah, Rajan and Dashti16).
The inconsistencies in findings regarding the impact of vitamin D on COVID-19 severity may stem from methodological variations. In most studies, blood samples were collected from COVID-19 patients upon hospital admission to measure vitamin D concentrations. Subsequently, COVID-19 patients were categorised based on the severity of their illness, and vitamin D levels were compared to determine whether severe COVID-19 cases were more likely to exhibit vitamin D deficiency(Reference AlSafar, Grant and Hijazi6,Reference De Smet, De Smet and Herroelen17–Reference Sulli, Gotelli and Casabella24) . However, it is important to note that vitamin D concentrations in these studies were measured after patients had contracted the virus, and the SARS-CoV-2 infection itself might directly influence vitamin D levels(Reference Smolders, van den Ouweland and Geven25).
In some studies, vitamin D concentration was determined before SARS-CoV-2 infection. For instance, vitamin D levels were analysed in relation to the severity of COVID-19 in 348 598 patients from the UK Biobank(Reference Hastie, Pell and Sattar26). Additionally, a Mendelian randomisation study involving 443 734 individuals included 401 460 participants from the UK Biobank(Reference Butler-Laporte, Nakanishi and Mooser27). Several meta-analyses have also incorporated a substantial number of cases from the UK Biobank, comprising a significant portion of the analysed patient data(Reference Liu, Sun and Wang28,Reference Dissanayake, de Silva and Sumanatilleke29) . Another large retrospective study encompassed a cohort of over 190 000 US COVID-19 patients, with vitamin D concentrations obtained from the preceding 12 months(Reference Kaufman, Niles and Kroll11). Nonetheless, the vitamin D measurements in these aforementioned studies were taken well before SARS-CoV-2 infection, potentially not serving as the most accurate indicators of pre-infection vitamin D status.
An ideal approach would involve assessing vitamin D levels in individuals immediately prior to SARS-CoV-2 infection and subsequently examining the clinical severity of COVID-19 and its outcomes in these patients. However, obtaining blood samples immediately before infection was virtually impractical during the pandemic, as infections often occurred unexpectedly. On 7 December 2022, the Chinese Center for Disease Control and Prevention modified its epidemic prevention policy, relaxing the stringent ‘zero-COVID’ policy that had been in place for nearly 3 years(Reference Huang, Gao and Wang30). Subsequently, a major outbreak of Omicron infections transpired in late December and January 2023. The peak of positive cases was observed on December 22, with a gradual decline throughout late January 2023(Reference Wu, Zhou and Tang31). Hospitalisations due to COVID-19 reached their zenith on January 5, while the number of deaths peaked on January 4 and subsequently declined by 89·9 % by 30 January 2023(32). Remarkably, in Henan Province, home to nearly 100 million people, the provincial government reported on 9 January 2023 that a staggering 89 % of the provincial population had been infected(Reference Huang, Gao and Wang30).
In this study, we conducted a retrospective analysis of pre-infection serum vitamin D data obtained from elderly participants (aged 60 and above) within a health management centre. These individuals were screened within 3 months prior to the onset of their illness. Our analysis sought to explore the associations between vitamin D concentrations and the incidence rate, reoccurrence rate and severity of Omicron COVID-19 within this cohort.
Methods
Study design and participants
In this retrospective study, we initially identified 309 individuals aged 60 years or older who underwent vitamin D testing within 3 months before contracting COVID-19 between 19 September 2022 and 19 January 2023 from the database of the Physical Examination Center of a large tertiary hospital in Taizhou of Zhejiang Province. We conducted a telephone questionnaire survey, obtained verbal informed consent and assessed the severity of symptoms during and after COVID-19 occurred. And the ethical approval had been approved (Fig. 1).

Fig. 1 Flow chart diagram for selection of participants
Measurement of the serum vitamin D concentrations
Serum concentrations of 25(OH)D, 25(OH)D2 and 25(OH)D3 were determined using liquid chromatography-tandem MS (AB Sciex QTrap® 3200 Tandem Mass Spectrometer), which was referred to as the ‘gold standard’ method for measuring vitamin D status in human samples(Reference El-Khoury, Reineks and Wang33–Reference Alexandridou, Schorr and Stokes35). The total 25(OH)D concentrations were calculated as the sum of 25(OH)D2 and 25(OH)D3. Participants were categorised into vitamin D deficiency (< 20 ng/ml), insufficiency (20 ng/ml to < 30 ng/ml) and sufficiency (≥ 30 ng/ml) groups, following established criteria(Reference Holick36).
Timeline of main events in the method
From 19 September 2022 to 19 January 2023, all COVID-19-negative participants underwent vitamin D measurement. From mid-January 2023 to mid-March 2023, the first to the last initial COVID-19 cases occurred. From the beginning of April 2023 to mid-June 2023, the first to the last recurrent COVID-19 case occurred. Continuous use of vitamin D was defined as more than 3months before the end of the initial COVID-19 cases (mid-March) (Fig. 2).

Fig. 2 Timeline of the main events in the method
Definition of smoking status and vitamin D supplement use
Participants who had a history of smoking and continued to smoke were classified as cigarette smokers. Those who regularly consumed vitamin D supplements for more than 3 months were considered vitamin D supplement users.
Definition of COVID-19-related comorbidities
Chronic obstructive pulmonary disease, hypertension, diabetes, chronic kidney disease and hyperlipidaemia were defined as individuals with past medical history or correspond to ICD10-CM codes (chronic obstructive pulmonary disease: J44·901; hypertension: I10.X02; diabetes: E11; chronic kidney disease: N18·905; hyperlipidaemia: E78·501). CVD was defined as participants who had a history of cardiovascular or cardiac disease. The cerebrovascular disease was defined as participants who had suffered a stroke and brain infarction. The others were referred to as asthma, bronchiectasis, pulmonary tuberculosis, cancer and immunodeficiency diseases.
Classification of COVID-19 severity
Based on the Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 9th Edition)(37), the severity of COVID-19 was categorised into level 0 to level 4. Among them, levels 0–1 are mild cases, level 2 belongs to moderate cases, level 3 is severe cases and level 4 is critical cases. Level 0: asymptomatic patients; level 1: the clinical symptoms are mild, including fever ≤ 38°C, fatigue, anosmia and ageusia lasting for less than 7 d, and there is no evidence of pneumonia in chest radiology; level 2: patients have fever > 38°C at least 3 d and respiratory symptoms lasting from 7–14 d. Chest radiology suggests pneumonia; level 3: patients meeting any of the following: rapid progression of clinical symptoms, with > 50 % progression in the lung lesions in chest radiology or with unstable blood pressure or with SpO2 < 93 % on room air in resting status or required oxygen uptake or hormone therapy; level 4: severe respiratory failure requiring mechanical ventilation or shock or admission to the intensive care unit or death.
Definition of COVID-19 and reoccurrence cases
COVID-19 illness was identified based on COVID-19 nucleic acid self-testing kits, positive laboratory tests (PCR or antigen tests), positive antibody results or a positive COVID-19 diagnosis (corresponding to U07·1 ICD10-CM code). Reoccurrence was defined as individuals recovering from a previous infection and contracting COVID-19 again within 3 months using the same testing methods.
Data collection
Demographic information, including age, gender, BMI, bone mineral density, laboratory parameters and COVID-19-related comorbidities, was collected from the Physical Examination Center database and confirmed in the hospital information system. Three months after the onset of COVID-19, a follow-up telephone survey was conducted by the Physical Examination Center to collect information on smoking status and vitamin D supplementation. Additionally, the severity of COVID-19 disease was assessed based on predefined criteria. Primary parameters included COVID-19 occurrent details, general symptoms, duration of infection, vaccination status, maximum fever temperature, fever duration, SpO2 levels, oxygen supplementation, mechanical ventilation, hormone therapy, pneumonia imaging, blood pressure stability and persistent symptoms. Vaccination rates, hospitalisation rates, intensive care unit admissions, deaths and reoccurrence rates within 3 months were also documented.
Statistics
Continuous variables were presented as mean (sd) and compared using ANOVA. Discrete variables were expressed as numbers (percentages) and compared between groups using Pearson’s χ 2 test and Fisher’s exact test. Binary logistic regression was employed to identify factors associated with COVID-19 incidence and reoccurrence rates. Ordinal logistic regression was used to investigate the association between vitamin D supplementation and the severity of COVID-19. All analyses were conducted using IBM SPSS Statistics software (version 26·0), and significance was defined as P < 0·05.
Results
Comparison of clinical characteristics among different vitamin D groups
Of the initial 309 subjects, eighty-seven individuals were excluded due to denial (n 12), unavailability (n 48) or lack of contact information (n 27) during the telephone follow-up (Fig. 1). Thus, the final study cohort consisted of 222 participants. The mean age was 68 ± 6 years (range, 61–89 years), with 58 % being female. Participants were categorised into vitamin D deficiency (n 30), insufficiency (n 108) and sufficiency (n 84) groups. There were no significant differences among the three groups in terms of age, gender, BMI, bone mineral density, smoking status or major COVID-19-related comorbidities, including chronic obstructive pulmonary disease, hypertension, diabetes, CVD, chronic kidney disease, cerebrovascular disease, hyperlipidaemia and others (all P > 0·05). However, a higher proportion of participants in the deficiency and insufficiency groups reported taking vitamin D supplements (P < 0·01). The proportion of vaccination rates among the three groups was relatively high: 99 % of individuals in the vitamin D insufficiency group claimed to have been vaccinated, with a higher vaccination rate compared with the deficiency group (97 %) and sufficiency group (96 %), and there was no significant difference in vaccination rate among the groups (P = 0·41). Serum concentrations of 25(OH)D and 25(OH)D3 differed significantly among the three groups (P < 0·01), while other laboratory parameters showed no significant differences (all P > 0·05). (Table 1)
Table 1 Comparison of general characteristics in participants with different serum vitamin D concentrations

Vit-D, vitamin D; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; WBC, white blood cell count; NEUT, neutrophil count; LYMPH, lymphocyte count; MONO, monocyte count; EO, eosinophil count; BASO, basophil count; NEUT%, neutrophil ratio; LYMPH%, lymphocyte ratio; MONO%, monocyte ratio; EO%, eosinophil ratio; BASO%, basophil ratio; PLT, platelet; NLR, neutrophil:lymphocyte ratio; MLR: monocyte:lymphocyte ratio; PLR: platelet:lymphocyte ratio; ESR: erythrocyte sedimentation rate; CRP, C-reactive protein; GLU, glucose; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
P-values were determined using the Pearson χ 2 test†, Fisher exact test‡ or ANOVA§.
Effect of serum vitamin D concentrations on COVID-19 incidence, severity and reoccurrence rate
The COVID-19 incidence was higher in the vitamin D deficiency group compared with the insufficiency and sufficiency groups (83 % v. 69 % v. 58 %, P = 0·03). Furthermore, the vitamin D deficiency group also exhibited a higher reoccurrence rate of COVID-19 (28 %) compared with the insufficiency group (16 %) and sufficiency group (8·2 %) (P = 0·02). However, no significant difference was observed in COVID-19-related hospitalisation rates among the groups (P = 0·30). (Table 2)
Table 2 Comparison of incidence, hospitalisation and reoccurrence of COVID-19 among participants with different concentrations of vitamin D
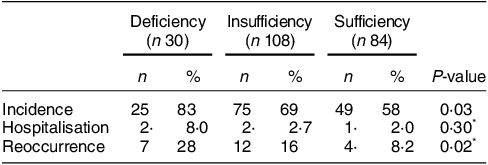
Reoccurrence: reoccurrence numbers/incidence.
* Fisher exact test.
In addition, the three groups also differed in the severity scores of COVID-19 (P = 0·003). The proportion of mild cases (level 0 and level 1) was highest in the vitamin D sufficiency group (69 %) and lowest in the vitamin D deficiency group (30 %). In contrast, the severe and critical cases (level 3 and level 4) were higher in the vitamin D deficiency group (35 %) than the vitamin D insufficiency group (19 %) and vitamin D sufficiency group (10 %). (Fig. 3)

Fig. 3 Comparison of severity of COVID-19 in vitamin D deficiency, insufficiency and sufficiency groups. (a) Mild cases: level 0–1. (b) Moderate case: level 2. (c) Severe case: level 3. (d) Critical case: level 4. Fisher’s exact test. **: P = 0·003
Association between vitamin D supplementation and incidence and reoccurrence rate of COVID-19 using binary logistic regression analysis
The dependent variables were incidence (v. no incidence) or reoccurrence (v. no reoccurrence). Vitamin D supplementation was selected as the independent variable based on the results from ANOVA with a P-value < 0·05. Before data analysis, the assumption of multicollinearity was tested, and there was no collinearity. The statistical results showed that vitamin D supplementation was not statistically significant (P > 0·05). Thus, vitamin D supplementation was not a significant predictor of COVID-19 incidence and reoccurrence rate in the general population (Table 3).
Table 3 Binary logistic regression results of vitamin D supplementation correlates to the incidence and reoccurrence rate of COVID-19

*2 Log-likelihood = 274·72; Cox and Snell R2 = 0·03; Nagelkerke R2 = 0·04; Hosmer and Lemeshow test: x2 = 5·69, P = 0·68. †2 Log-likelihood = 138·68; Cox and Snell R2 = 0·04; Nagelkerke R2 = 0·08; Hosmer and Lemeshow test: x2 = 2·47, P = 0·96. aVariable(s) entered in step 1: vitamin D supplementation. 25(OH)D, 25(OH)D3.
Ordinal logistic regression results of the relationship between vitamin D supplementation and COVID-19 severity
The five-level ordinal COVID-19 outcomes were the dependent variable, and vitamin D supplementation was the independent variable of interest. The predictor variable of this model was also selected according to the results of ANOVA (P < 0·05). The results of ordinal logistic regression analysis showed that vitamin D supplementation had no association with the severity of COVID-19 in individuals (P > 0·05) and was not considered a risk factor for the severity of disease (Table 4).
Table 4 Results of vitamin D supplementation correlates to the severity level of COVID-19: ordinal logistic regression model

2 Log-likelihood = 426·49; goodness of fit: Cox and Snell R2 = 0·03; Nagelkerke R2 = 0·03; McFadden R2 = 0·009; test of parallel lines: P = 0·49.
Discussion
In this study, we observed a higher incidence rate of COVID-19 among individuals with vitamin D deficiency compared with those with vitamin D insufficiency or sufficiency. Moreover, individuals with vitamin D deficiency who contracted COVID-19 were more likely to experience severe/critical cases. Additionally, within the group of individuals who had contracted the virus, those with vitamin D deficiency had a significantly higher reoccurrence rate compared with those with vitamin D insufficiency or sufficiency.
Unlike previous studies that primarily focused on hospitalised COVID-19 patients, our study examined a cohort of elderly individuals who underwent routine medical examinations shortly before potentially contracting COVID-19. Most studies involving hospitalised COVID-19 patients have consistently reported an association between lower serum vitamin D concentrations and increased COVID-19 severity and mortality(Reference De Smet, De Smet and Herroelen17,Reference Vanegas-Cedillo, Bello-Chavolla and Ramirez-Pedraza21,Reference Radujkovic, Hippchen and Tiwari-Heckler23,Reference Sulli, Gotelli and Casabella24) . However, studies from Saudi Arabia and Kuwait did not find a significant association between serum 25(OH)D levels and disease severity or mortality in hospitalised COVID-19 patients(Reference AlKhafaji, Al Argan and Albaker15,Reference Al-Jarallah, Rajan and Dashti16) . It is important to note that in these studies, vitamin D concentrations were measured after patients were already infected, potentially influenced by the systemic inflammatory response induced by COVID-19 itself(Reference Smolders, van den Ouweland and Geven25). In contrast, our study focused on individuals aged 60 years and older, with a mean age of 68 years. In studies that did not support the relationship between vitamin D and COVID-19, younger patients were typically included, such as those aged 4–60 years(Reference AlKhafaji, Al Argan and Albaker15) or with a mean age of 49 ± 17 years(Reference Al-Jarallah, Rajan and Dashti16). To investigate the association between vitamin D levels and COVID-19 more effectively, it is reasonable to assess vitamin D concentrations in the general population just before infection, as we did in our study.
Prior research using data from the UK Biobank did not find a significant link between 25(OH)D levels and COVID-19 susceptibility, severity, hospitalisation or mortality(Reference Hastie, Pell and Sattar26,Reference Butler-Laporte, Nakanishi and Mooser27,Reference Hastie, Mackay and Ho38,Reference Ma, Zhou and Heianza39) . However, it is worth noting that the 25(OH)D serum samples analysed in the UK Biobank studies were collected years before the patients’ infections and may not accurately represent their pre-infection vitamin D status. In contrast, a study of a diverse cohort of over 4599 veterans with positive COVID-19 tests found that serum 25(OH)D concentrations measured 15–90 d before testing were independently associated with COVID-19-related hospitalisations and mortality in an inverse dose–response relationship(Reference Seal, Bertenthal and Carey9). Our study aligns with this approach, as we measured pre-infection serum 25(OH)D concentrations in individuals who were not COVID-19 patients but rather local residents undergoing routine annual medical examinations.
Furthermore, our study was conducted during a period when the Omicron variant was responsible for a significant COVID-19 outbreak in late December 2022 and January 2023(Reference Wu, Zhou and Tang31,32) . The infection rate was exceptionally high during this period, with approximately 89 % of the provincial population of nearly 100 million people infected(Reference Huang, Gao and Wang30). Approximately 23 months after the first reported case of COVID-19, the Omicron variant was first reported in Africa in November 2021, and by early 2022, the Omicron variant was already the dominant strain worldwide, accounting for 99·7 % of the sequences registered from 23 February to 24 March 2022(Reference Rana, Kant and Huirem40). Therefore,iCOVID-19 mutant strains in the studies before November 2021 were COVID-19 mutant strains like alpha, beta and delta variants(Reference AlSafar, Grant and Hijazi6–Reference Kaufman, Niles and Kroll11,Reference Baktash, Hosack and Patel13–Reference Al-Jarallah, Rajan and Dashti16) . However, the effect of vitamin D levels on Omicron COVID-19 has not yet been reported.
Regarding vitamin D supplementation, we observed a higher percentage of individuals taking vitamin D supplements for at least 3 months in the vitamin D insufficiency and deficiency groups. However, binary and ordinal logistic regression models indicated that vitamin D supplementation was not significantly associated with COVID-19 severity in our study. Conflicting reports exist regarding the effectiveness of vitamin D supplementation in reducing the risk of COVID-19 infection and its severity. In a double-blind and parallel randomized controlled trial (RCT) study of highly exposed individuals, Villasis-Keever et al. found that the risk of SARS-CoV-2 infection was lower in those treated with vitamin D supplements with an elevated serum level of 25(OH)D(Reference Villasis-Keever, Lopez-Alarcon and Miranda-Novales41). While some studies have suggested a lower risk of infection with elevated serum 25(OH)D levels and a reduced risk of SARS-CoV-2 infection with habitual vitamin D supplement use(Reference Ma, Zhou and Heianza39), others, such as the CORONAVIT trial, did not find a reduction in the risk of acute respiratory infections or COVID-19(Reference Jolliffe, Holt and Greenig42). It is important to note that individuals who consume vitamin D supplements may not necessarily have significantly elevated vitamin D levels in circulation, as was the case in our study. Furthermore, the definition of a normal serum vitamin D concentration remains a subject of debate, with some arguing that a serum concentration of 30 ng/ml is the minimum for adequate immunity, while others suggest that a range of 40–60 ng/ml may be more functionally adequate(Reference Wolsk, Harshfield and Laranjo43,Reference Mirzakhani, Litonjua and McElrath44) . In our study, the protective effect of vitamin D was based on serum vitamin D concentrations rather than the regular intake of vitamin D supplements.
Our study has several limitations, including a limited sample size, imprecise measurement of vitamin D supplement dosages, the frequency of vitamin D intake, the potential for variations in vitamin D concentrations within 3 months before major disease onset, overlapping periods of vitamin D supplementing and COVID-19 contraction and the fact that participants in routine health screenings may not fully represent the local community.
In conclusion, our study suggests that serum vitamin D levels shortly before the major Omicron COVID-19 outbreak were associated with the incidence, severity and reoccurrence rate of COVID-19 in the elderly population.
Acknowledgements
We appreciate the assistance provided by the health management centre and clinical laboratory staff in data collection. We greatly appreciate the participants for their time and effort in participating in our research. We also want to thank Tao-Hsin Tung for his assistance in data analysis. The individuals listed in this statement have agreed.
Financial support
This study was supported by the Medical and Health Science and Technology Program Project of Zhejiang Province for Dun Hong (grant no.: 2023KY396 and 2020PY030) and Funds of Taizhou Famous Medical Workshop.
Conflict of interest
There are no conflicts of interests.
Authorship
J.C. contributed to clinical data collection, data analysis and drafting and editing of the paper. F.L. contributed to clinical data collection. B.S. contributed to laboratory data collection. H.X. contributed to clinical data collection. Y.C. contributed to laboratory data collection. Q.H. contributed to clinical data collection. A.X. contributed to clinical data collection. T.T. contributed to data analysis and revision of the paper. D.H. contributed to study concept and design and critical revision of the paper. All authors reviewed and approved the final version, and no other person made a substantial contribution to the paper.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ethics Committee of Taizhou Hospital of Zhejiang Province (K20230732). Written or verbal informed consent was obtained from all subjects/patients. Verbal consent was witnessed and formally recorded.










