Disease-related malnutrition (DRM) encompasses malnutrition that arises in association with both acute and chronic disease( Reference Jensen, Mirtallo and Compher 1 ). Unlike starvation-related malnutrition, disease may interfere with the ingestion or absorption of nutrients, increase energy needs or cause undernutrition due to significant dietary restrictions imposed during medical treatment( 2 ). Such DRM is a significant public health problem in both primary and secondary health-care settings, affecting an estimated 20 million people in the European Union at an annual cost of at least €120 billion( Reference Ljungqvist, van Gossum and Sanz 3 ). Such is the scale and cost of DRM that it is now the subject of pan-European initiatives aimed at tackling the lack of awareness, detection and treatment of this condition by governments, health and social care providers, health-care professionals, patients and carers( 4 – 6 ). The economic implications of DRM are significant and arise primarily through increased health and social care utilisation, since DRM increases complications of disease, delays recovery, increases length of hospital stay and increases the likelihood that care will be needed after discharge( Reference Stratton, Green and Elia 7 – Reference Stratton, King and Stroud 11 ). Malnourished individuals in the community have also been found to have more visits to general practitioners (GP), out-patient attendances and hospital admissions than well-nourished individuals( Reference Elia, Stratton and Russell 9 , Reference Martyn, Winter and Coles 12 – Reference Feldblum, German and Bilenko 14 ).
In the UK, the British Association for Parenteral and Enteral Nutrition (BAPEN) estimated the cost of DRM in 2007 to be over £13 billion, corresponding to more than 10 % of total expenditure on health and social care and more than double the cost of obesity and its co-morbidities( Reference Elia and Stratton 15 , 16 ). In the Republic of Ireland there have been few published studies on the prevalence of DRM, and none of its likely costs. This is despite an urgent need to identify areas for potential savings within the Irish health-care system, and to avoid any increase in DRM or its costs that might be predicted to occur as the proportion of the population aged 65 years or more rises from its current level of 11 % to 16 % within the next 15 years( 17 ). Against this background, the present study examines the likely scale of DRM in different care settings in Ireland and the associated resource and cost implications for the health service, providing a basis for future strategies aimed at cost reduction.
Methods
The study aimed to establish the likely costs associated with the health and social care of patients with DRM in Ireland, using the methodology developed by Elia and Stratton to determine the costs of malnutrition in the UK, published as part of a BAPEN report in 2009( Reference Elia and Stratton 15 ).
First, it was necessary to establish the prevalence of DRM in hospitals and the community as a basis for determining the proportion of health and social care resources that might be attributed to patients with DRM. Estimates were established for each care setting using age-standardised comparisons between available Irish data and large-scale UK surveys that employed validated and consistent criteria. These are described in more detail in the subsection entitled ‘Estimating the prevalence of diet-related malnutrition’.
Official publications and national data sets provided by the Department of Health and Children, the Health Service Executive, and the Economic and Social Research Institute were used to establish patient activity levels and cost information for hospital and community services in 2007. The methods for calculating total costs per service and the estimated proportion attributable to patients with DRM is outlined in the subsection entitled ‘Quantifying the proportion of health and social care used by adults with diet-related malnutrition’.
Estimating the prevalence of diet-related malnutrition
The prevalence of DRM was estimated for different health-care settings, i.e. acute hospitals, psychiatric hospitals/mental health units, hospital out-patient and primary-care (GP) clinics, and in social care, i.e. long-stay care for the elderly (long-stay hospitals, nursing and residential care homes) and for adults receiving care at home. As the prevalence of DRM is highly influenced by the criteria used to define malnutrition, estimates were based on studies which used the Malnutrition Universal Screening Tool (MUST), developed by BAPEN( Reference Elia and Stratton 15 , Reference Elia 18 ) (http://www.bapen.org.uk/musttoolkit.html). The MUST screening tool has been validated for use in adults across all health-care settings and has been shown to predict clinical outcome in different patient groups and to have fair/good to excellent agreement with other validated screening tools( Reference Stratton, King and Stroud 11 , Reference Elia 18 , Reference Stratton, Hackston and Longmore 19 ). MUST criteria were also used in the largest Irish survey of DRM in hospitals and care homes as part of the BAPEN Nutrition Screening Week Survey (NSW10) conducted in 2010( Reference Russell and Elia 20 ), allowing direct comparison of prevalence rates with large-scale UK studies. MUST comprises a five-step screening process that attributes an overall risk score to an individual (low risk = 0, medium risk = 1, high risk = ≥2) on the basis of BMI, recent unplanned weight loss and an acute disease effect. Studies using MUST equate DRM with a medium or high risk score for malnutrition, as in the present study.
Acute hospital in-patients
Findings from 1602 patients from twenty-seven Irish hospitals revealed a prevalence of DRM according to MUST criteria of 33 % (25 % high risk, 8 % medium risk)( Reference Russell and Elia 20 ). The survey (NSW10), conducted in winter as the third of four seasonal surveys coordinated by BAPEN, was the first in which Irish hospitals participated. While the results were similar to those obtained in UK hospitals, they were somewhat higher than the prevalence figures obtained from surveys in 2007 (27 %) and 2008 (28 %)( Reference Russell and Elia 21 , Reference Russell and Elia 22 ). This may reflect a seasonal effect on the type of admissions, which the BAPEN researchers intend to eliminate by amalgamating the results of four surveys to get a more complete picture of DRM over the year. To reduce the possibility that the extrapolation of results from one study to the full year might overestimate annual prevalence, a 10 % reduction has been applied to that obtained (i.e. 33 % × 0·9 = 30 %), bringing it closer to the mean of the results obtained from amalgamating the three UK surveys( Reference Russell and Elia 20 – Reference Russell and Elia 22 ). This is also within the range of prevalence rates of DRM observed in hospitals in other European countries, many of which employed similar criteria to MUST but in some cases different screening tools; 23·8 % in The Netherlands, 25 % in Scotland, 27·4 % in Germany, 27 % in Sweden and 41 % in Hungary( Reference Meijers, Schols and van Bokhorst-de van der Schueren 23 – Reference Lelovics, Bozo-Kegyes and Bonyar-Muller 27 ).
Patients at risk of DRM (using MUST criteria) have been found to have a 30 % longer length of hospital stay( Reference Elia, Stratton and Russell 9 ). Therefore, the proportion of in-patients with DRM at any time was calculated to be 36·3 %, using the equation: p × [1·3 – (0·3p)], where p equals the proportion of patients at risk of malnutrition on admission (30 %) and 1·3 is the ratio of length of stay in malnourished patients relative to non-malnourished patients. In other words, malnourished patients can be expected to account for at least 36·3 % of adult in-patient bed-days and associated hospital costs.
Psychiatric hospital/mental health unit in-patients
Psychiatric units in general hospitals and psychiatric hospitals had an average of 3314 in-patients in 2007( 28 ), of whom 20 % were in private hospitals( Reference Daly, Walsh and Moran 29 ). As there are no published data on the prevalence of DRM in Irish psychiatric in-patients, a similar prevalence to that found in the BAPEN 2007 and 2008 screening surveys in the UK (overall 19 %) has been assumed( Reference Russell and Elia 21 , Reference Russell and Elia 22 ).
Hospital out-patient and primary-care clinics
Data for the prevalence of DRM in Irish GP clinics are incomplete. A study of Irish adults aged ≥ 65 years attending a GP clinic found that 15 % had a BMI of <20 kg/m2, the-cut off value for BMI risk used in MUST, but the sample size was small (n 48) and no data were available on recent weight loss( Reference Corish, Flood and Kennedy 30 ). For hospital out-patient and primary-care (GP) clinics, a prevalence of 10 % DRM risk was used by BAPEN( Reference Elia and Stratton 15 ) although this is lower than that observed in a recent survey of a range of out-patients in two hospitals in England (n 321, mean age 54 (sd 16·7) years), which found that 15·9 % were malnourished( Reference Rust, Cawood and Walters 31 ). A recent multi-centre study in The Netherlands (n 2288, nine centres) found that 7 % of hospital out-patients were malnourished, with the highest prevalence of severe malnutrition of 17 % in oral maxillofacial surgery, 10 % in oncology, 8 % in rehabilitation and 7 % in gastroenterology and pulmonology( Reference Leistra, Neelemaat and Evers 32 ). The figure used by BAPEN for primary care refers to individuals who access the services and not all those who are registered with a GP. However, on the basis that those aged ≥65 years have been found to be at significantly higher risk of DRM than younger adults( Reference Russell and Elia 22 ) and as older people account for just 11 % of the Irish population v. 16 % of the UK population( 33 , 34 ), it is estimated that DRM in primary-care settings may be 12 % lower in Ireland. A prevalence of 9 % has therefore been assumed.
Care homes
Of 154 residents recently admitted and screened in twelve Irish nursing and residential care homes as part of the BAPEN NSW10, 32 % were malnourished (16 % high risk, 16 % medium risk)( Reference Russell and Elia 20 ). This was lower than the 37 % prevalence found in care homes in the UK as part of the same survey (23 % high risk, 15 % medium risk)( Reference Russell and Elia 20 ), and may reflect the difference in type of care homes that participated. Furthermore, the survey screened patients within 6 months of admission, despite longer stay having been found to be associated with a higher risk of malnutrition( Reference Suominen, Muurinen and Routasalo 35 ). In larger studies that screened all residents in a mixture of nursing and residential homes, risk of malnutrition was found to be 39 % (n 1176, forty-three care homes) and 32 % (n 703, nineteen care homes), respectively( Reference Parsons, Stratton and Elia 36 , Reference Cawood, Smith and Dalrymple-Smith 37 ). Thus, a prevalence of 35 % across all publicly funded long-stay beds has been assumed.
Care at home
Since home care packages are made available only to those requiring medium to high caring support to continue to live at home independently( 38 ), and as the majority in receipt of home support are aged >65 years, the prevalence of DRM is likely to be significantly higher than in a representative sample of free-living people aged >65 years.
A recent study of a group of self-selected Irish older people in receipt of meals-on-wheels at home (n 63, mean age 78·5 (sd 10·7) years) found that although only 2 % were classified as underweight on the basis of a cut-off point of BMI < 18·5 kg/m2, 38·5 % of individuals were assessed as malnourished or at-risk of malnutrition using the Mini Nutritional Assessment( Reference O'Dwyer, Corish and Timonen 39 ). This compares with a finding that 31 % of people receiving a delivered meals service in Newcastle, UK were at medium or high risk of DRM using MUST( Reference Skinner, Dugdale and Crowe 40 ), suggesting a prevalence of DRM in those in receipt of home services closer to that found on admission to hospital or care homes than to prevalence estimates for free-living older people( Reference Russell and Elia 20 , Reference Elia and Stratton 41 ). For the present analysis, a prevalence of 25 % was used for patients receiving home help and/or home care packages, similar to that assumed by the authors of the BAPEN report( Reference Elia and Stratton 15 ).
Quantifying the proportion of health and social care used by adults with diet-related malnutrition
For in-patient or residential care, costs were estimated by multiplying the total number of publicly funded bed-days, obtained from 2007 records on in-patient activity extracted from the Hospital InPatient Enquiry System (Health Research and Information Division, Economic and Social Research Institute, personal communication, 2009), by the percentage estimated to be malnourished at any time and then by the average bed-day costs obtained from estimates provided by Casemix (Economic and Social Research Institute, personal communication, 2007).
For primary-care (GP) services including general prescription costs, estimates were made of the proportion of total annual spend attributable to malnourished patients and not on activity rates, for which there was insufficient detail. For out-patient visits, the total number of attendances was multiplied by the proportion estimated to be attributable to malnourished patients and by the average cost per attendance. It was assumed that 77 % were publicly funded, in line with overall health-care expenditure split( 42 ).
Costs of in-patient care
The annual total number of in-patient bed-days for 2007, excluding those attributable to maternity patients or those aged 0 to 17 years, was 3 093 122( Reference McEnery 43 ), of which approximately 76 % were publicly funded( 44 ). Applying the average cost per in-patient day of €844 (Casemix/Economic and Social Research Institute, personal communication, 2007), this gives a total public cost of adult in-patient care in Ireland of €1·984 billion. The proportion attributable to patients with DRM was derived from earlier prevalence estimates (36·3 %), without any upward adjustment to allow for higher daily costs of patients with DRM.
Costs of long-term psychiatric care
The expenditure on long-term residential care in mental health units was €249 million( 45 ). The proportion of psychiatric-care costs was assumed to be in line with the estimated proportion of patients with DRM (i.e. 19 %).
Cost of hospital out-patients
The number of out-patient attendances in 2007 (i.e. 3 025 300( 46 )) was multiplied by the average cost per attendance (€160; Casemix/Economic and Social Research Institute, personal communication, 2007) to obtain a total cost of out-patient care in Ireland of €484 million, of which 77 % was assumed to be publicly funded (i.e. €373 million). The proportion of attendances by patients with DRM was assumed to be in line with the prevalence estimate for Irish out-patients (i.e. 9 %).
Cost of primary care
Total Primary Care Reimbursement Services (PCRS) costs for General Medical Services (GMS), including costs for prescriptions and GP services but excluding fees for dental and ophthalmic services, were €2·207 billion( 47 ). Clinical nutrition products cost €53·2 million (including fees to pharmacists and mark-ups), of which an estimated €13·2 million (PCRS and clinical nutrition manufacturer's data, unpublished results) was spent on specialised gluten-free, low-protein, in-born errors of metabolism and paediatric products, with the remainder (i.e. €40 million) comprising nutritional support products for adult DRM patients( 47 ). Hence, total GMS expenditure, excluding clinical nutritional products, was €2·154 billion (i.e. €2·207 billion – €0·053 billion). Of these costs, it has been assumed that malnourished patients use 9 % of primary-care costs, in line with earlier prevalence estimates. Although patients with DRM have been shown to have a significantly higher number of GP visits and higher prescription rates than non-malnourished patients( Reference Elia, Stratton and Russell 9 , Reference Martyn, Winter and Coles 12 ), no attempt has been made to factor this into cost estimates. This is because the majority of GP in Ireland are paid primarily on a capitation fee per person basis and there are insufficient data on the allocation of prescription costs according to age and diagnosis that would allow selective adjustment. For clinical nutrition products, 90 % of all relevant categories of products (tube, sip, specialised) have been assumed to be prescribed for patients with a medium or high risk score for DRM at the time of initial prescription, and 10 % for those at low risk. This is to account for some use of oral supplements for social reasons or due to inappropriate prescribing practice( Reference Kennelly, Kennedy and Rughoobur 48 ).
Public spend on long-stay care for elderly patients
Total public spend on long-stay nursing and residential home care was calculated from the number and cost of long-stay beds for the elderly (see Table 1). The proportion of costs attributable to patients with DRM was calculated from the estimate of DRM prevalence in long-stay facilities.
Table 1 Calculation of public expenditure on adult long-stay, residential and nursing home care in Ireland, 2007

HSE, Health Service Executive.
*Source: Parliamentary Affairs Division, Department of Health and Children, personal communication, 2009.
†Unadjusted for financing, depreciation or return on capital costs.
‡Average of cost of rural homes (€1103) and urban homes (€1352).
§A 2 % increase in Consumer Price Index for 2007 applied to 2006 average cost of €694.
Other costs not included
Costs not considered in the present analysis include those for acute day-patient care, day-care services and welfare homes, and those associated with mental health, general dental, audiology, ophthalmic elements, specialist services including rehabilitation and ambulance services.
Sensitivity check
Increases or decreases of up to 25 % were applied to prevalence estimates according to the degree of uncertainty or likely variance for each care setting.
Results
Applying estimates of the proportion of health and social care costs attributable to patients with DRM to annual expenditure data for different care settings or services, the total minimum cost associated with DRM patients was calculated to be €1·4 billion (€1·2 to €1·6 billion, applying sensitivity analyses) based on 2007 activity and costs (Table 2).
Table 2 Calculation of the costs of DRM in Ireland, 2007
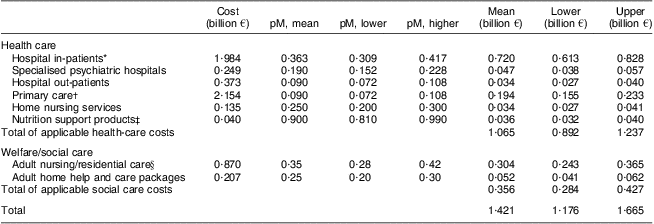
DRM, diet-related malnutrition; pM, proportion malnourished.
*Acute hospital in-patient cost excluding maternity based on bed-days × average bed-day costs × proportion publicly funded.
†Primary-care services include general medical and prescriptions but exclude general dental/ophthalmic/nutrition support services.
‡Higher and lower values (±10 %) were established to cover likely range of prescribing for those with low risk of DRM due to limited information.
§Includes long-stay hospital bed costs (public spend only) based on 90 % occupancy, and costs of contracted and sub vented private nursing home beds. Higher and lower values (±20 and ±25 %) were established to cover the likely range of DRM prevalence and to take account of the limited information on prevalence of DRM in certain care settings.
Discussion
The annual public cost of care for patients with DRM in Ireland is estimated at €1·4 billion, representing 10 % of the €13·7 billion expenditure on health care in 2007( Reference McEnery 43 ). This equates to an estimated €1·5 billion at today's prices, taking into account the higher activity and/or unit costs of hospital and community services, even after allowing for a reduction of approximately 3 % in the number of corresponding in-patient bed-days since 2007( 49 ).
It should be noted that these calculations make no distinction between the costs of treating DRM and any associated disease. This is because it is difficult to separate the two, since each can be a cause and consequence of the other( Reference Elia and Stratton 15 ).
Similar to the findings of the BAPEN calculation of the cost of DRM in the UK, this is a minimum value for several reasons( Reference Elia and Stratton 15 ). First, the costs of several services have not been taken into account (see ‘Methods’ section) and the total represents public expenditure only, which represented just 77 % of total health-care costs in 2007( 42 ). Second, we have only included costs associated with DRM in adult patients, whereas DRM affects patients of all ages. We have also excluded maternity and obstetric patients from the data set for hospital in-patients, a group that accounted for nearly 10 % of bed-days, because we had no data on DRM in this group. Third, while the daily cost of care for a malnourished patient is likely to be greater than the cost of caring for a non-malnourished individual, the model used has assumed the costs to be the same( Reference Elia and Stratton 15 ). Neither have we factored in the increased utilisation of health-care resources by malnourished compared with non-malnourished patients other than in the hospital setting. Finally, 2007 costs and activity levels have been used to obtain as complete a picture as possible, which takes no account of subsequent increases in activity levels and costs associated with hospital and community care between 2007 and 2010( 49 ).
There are several limitations of the present study. Whereas the model relies heavily on determining a reliable estimate of prevalence for each care setting using consistent methodology, large-scale Irish studies using MUST were available only for hospital in-patients, necessitating age-adjusted comparison with or extrapolation from UK studies for other settings. The reliance on prevalence data to determine the proportion of costs attributable to the care or treatment of DRM is also a limitation, since it has been well established that malnourished patients use more health-care resources than non-malnourished patients. A recurrent problem with health economic assessments of DRM is the difficulty in extricating the clinical and economic impact of DRM from that of any associated underlying disease or co-morbidity. Studies which have attempted to evaluate the additional costs of treating malnourished v. well-nourished patients have based their calculations on differences in health-care utilisation between age- and/or diagnosis-matched groups( Reference Elia, Stratton and Russell 9 , Reference Guest, Panca and Baeyens 13 ). No database of patient records exists in Ireland which would provide the necessary information for such an evaluation, which would ideally include information on patient demographics, diagnosis, co-morbidities, health-care utilisation (i.e. GP visits, out-patient visits, hospital admissions, length of stay, procedures and prescriptions) and nutritional parameters. However, a 2005 BAPEN report in the UK estimated that this represented the majority (i.e. >70 %) of the total cost of treating patients with DRM( Reference Elia, Stratton and Russell 9 ), based on the same criteria used in the present study. If the same relationship can be anticipated to hold for the corresponding health-care elements of this estimate that BAPEN considered in their 2005 report (i.e. in-patient care, long-term care, out-patient visits and nutritional support, excluding GP visits and prescription costs for which precise information was unavailable), the additional cost of care for malnourished v. well-nourished in Ireland is likely to exceed €750 million.
There is accumulating evidence of significant cost savings associated with timely nutritional intervention in both hospital and community patients. A retrospective cost analysis by Stratton et al.( Reference Stratton, Green and Elia 7 ) of nine randomized controlled trails (with or without oral nutrition supplementation) demonstrated mean cost savings of between £352 and £8179 per patient in surgical, orthopaedic, elderly and cerebrovascular accident patients, using significantly lower bed-day costs than apply currently in Ireland. Studies in community patients have also shown net savings associated with oral nutrition supplementation use v. standard care in France( Reference Arnaud-Battandier, Malvy and Jeandel 50 ), The Netherlands( Reference Nuijten and Freyer 51 ) and Germany( Reference Nuijten 52 ).
While it is not possible to determine precisely how much of the additional cost of DRM can be saved through an effective screening and intervention strategy in Ireland, this estimate can be used to indicate how much it would be worth for a health-care system to spend per patient on successful interventions.
It is estimated that there are approximately 140 000 adults with DRM in Ireland at any time. This estimate is based on age-adjusted extrapolations from UK studies in community settings (i.e. 13·9 % of those aged >65 years and 2·5 % of those aged 19–64 years( Reference Elia and Stratton 41 , Reference Ruston, Hoare and Henderson 53 )), added to the number of Irish hospital in-patients with DRM estimated from the NSW10 survey (see ‘Methods’ section). As the proportion of underweight subjects (i.e. BMI < 18·5 kg/m2) was similar between age-matched groups surveyed as part of the Irish national Survey of Lifestyle, Attitudes and Nutrition (J Lutomski, personal communication, 2009) and the National Dietary and Nutrition Survey in the UK( Reference Ruston, Hoare and Henderson 53 ), it was considered reasonable to assume the prevalence of DRM to be similar within the UK and Irish populations.
Based on these assumptions, the additional cost of DRM per adult patient (i.e. €750 million ÷ 140 000) is estimated at €5357 from which we can deduce that any spend below this figure on interventions that successfully prevent or treat DRM might be anticipated to deliver savings. Interventions that cost more may add value to the health-care system by improving the quality of health care but would require justification.
Conclusions
Given the very high costs associated with DRM, even a small percentage reduction in the prevalence or severity of DRM has the potential to deliver substantial savings within the overall health-care budget, since much DRM is preventable or treatable with nutritional interventions. While hospitals provide an opportunity to identify those at risk and initiate treatment which can be continued in the community, a screening and early intervention programme in the community may well be the most effective means of achieving cost savings. This is because malnourished patients are at significantly increased risks of complications, hospitalisation and longer length of stay once in hospital( Reference Stratton, Green and Elia 7 , Reference Sorensen, Kondrup and Prokopowicz 10 , Reference Stratton, King and Stroud 11 ), and earlier identification and appropriate intervention in the community might be expected to reduce costs by prevention of co-morbidities arising directly as a result of DRM. However, achieving overall savings requires a cross-sectoral approach to budget targeting, since it is likely that savings derived from the prevention and earlier treatment of DRM will be made in hospital and long-term care settings, but may require increases in expenditure on nutritional support from community budgets.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. There are no conflicts of interest. N.R. collected and analysed the data, adapted the methodology for use from BAPEN and wrote the paper; C.N. provided advice on methodology and guidance on health economic aspects. The authors are indebted to Professor Marinos Elia for his helpful inputs and guidance, and would like in particular to acknowledge the use of cost models and templates developed by Professor Elia and Dr Rebecca Stratton as the basis for the present study. The authors give special thanks to Fionna Page for her work in reviewing the manuscript and providing editorial assistance.




