The burden of chronic disease is becoming increasingly apparent among Inuit in the Canadian Arctic. Compared with all of Canada, life expectancy is 13 years lower and stroke mortality is two times higher among Inuit( 1 ), and the prevalence of self-reported heart disease is three times higher among Aboriginal populations (i.e. First Nations, Metis and Inuit/Inuvialuit)( 2 ). Behavioural risk factors such as smoking and diet might play a central role in chronic disease development and mortality among Inuit( Reference Nielson, Storm and Gaudette 3 – Reference Hopping, Mead and Erber 5 ). The effects of smoking on cardiovascular and respiratory diseases, stroke and certain cancers are well recognized( Reference Ockene and Houston Miller 6 , 7 ). Similarly, diets high in energy and fat and low in micronutrients have been linked to diseases such as obesity, CVD and cancer( Reference Popkin 8 ). Both diet and smoking induce physiological changes, such as increased endothelial damage, oxidized low-density lipoproteins and atherosclerosis, that increases risk for development of these diseases( Reference Davis, Katz and Wylie-Rosett 9 – Reference Erhardt 11 ). In addition to its direct effect on tissues, smoking can contribute to unbalanced nutrient profiles through a combination of altered taste preferences, metabolism and demand of certain nutrients, such as folate, β-carotene, Se, Ca and vitamin C( Reference Davis, Katz and Wylie-Rosett 9 , Reference Dallongeville, Marécaux and Fruchart 12 , Reference Northrop-Clewes and Thurnham 13 ). Clustering of multiple behavioural risk factors such as smoking, low levels of physical activity, high alcohol consumption and poor diet, especially low intake of fruits and vegetables, may further increase the risk of chronic disease and the adversity of associated health outcomes. Diets of smokers in several populations have been shown to differ from those of non-smokers and have included more processed foods, meat and dairy and fewer fruits and vegetables( Reference Margetts and Jackson 14 ). Subsequently, higher intake of saturated fat and lower intakes of micronutrients such as vitamin C, vitamin A, folate and fibre have been reported among smokers( Reference Dallongeville, Marécaux and Fruchart 12 , Reference Palaniappan, Starkey and O'Loughlin 15 ).
There is limited health research overall among Inuit and, to the authors’ knowledge, specifically there are no previous studies that have explored dietary intake differences among Inuit exclusively by smoking status. Previous research among Inuit in Nunavut, Canada, highlighted inadequate intakes of dietary fibre, Ca, folate and vitamins A, D and E( Reference Hopping, Mead and Erber 5 ). According to the Aboriginal Peoples Survey in 2006, smoking prevalence among Inuit in Nunavut was 64 %, and the highest prevalence was among residents 20–24 years of age (73 %)( 16 ). The high prevalence of chronic disease and lower life expectancy among this population warrant the exploration of known chronic disease risk factors to create targeted disease prevention efforts. The purpose of the present study was to compare dietary intake and quality between adult Inuit smokers and non-smokers in Nunavut, Canada.
Methods
The survey instrument and data collection protocol have been described elsewhere( Reference Sharma 17 , Reference Sharma 18 ). In brief, a validated quantitative FFQ (QFFQ) designed specifically for this population was administered between June 2008 and October 2008 to Inuit adults in three isolated communities in Nunavut( Reference Sharma 17 – Reference Pakseresht and Sharma 19 ). Households were randomly selected from three communities using housing maps provided by the local government. One individual per household was recruited to participate, namely the main food shopper and/or preparer, and these were typically women. Children (<19 years) as well as pregnant and breast-feeding women were excluded due to their altered and changing dietary requirements. Participants reported the consumption amount and frequency of 150 items during a 30 d period by using three-dimensional food models and choosing from eight categories ranging from ‘never’ to ‘two or more times per day’( Reference Sharma 18 ). A food composition table specific for this population was developed using the Canadian food composition tables within NutriBase, Clinical Nutrition Manager version 7·17 (CyberSoft Inc., Phoenix, AZ, USA), supplemented with data from the Canadian Nutrient File( 20 ).
The non-nutrient-dense foods (NNDF) category comprised the following foods: butter/margarine, jam/marmalade, sugar/honey, non-dairy coffee whitener, hash browns/potato patties/French fries, gravy, salad dressing including mayonnaise and dips, ice cream, cakes/muffins, pies, cheesecake, chocolate bars, potato chips, crackers, cookies, hard candy, popcorn, granola bars, sweetened drinks and unsweetened drinks.
Traditional food items were defined as those obtained through subsistence practices, such as hunting and fishing, and were grouped into the following three categories: (i) traditional land foods (caribou, polar bear, musk ox and the organs of these animals); (ii) traditional sea foods (seal, seal liver, muktuk (whale skin and fat)) and various kinds of wild local fish (e.g. char and trout); and (iii) traditional sky foods (goose and ptarmigan).
The mean and standard deviation of daily energy and nutrient intakes were calculated for all participants. The energy intake requirement was defined using the Institute of Medicine's( 21 ) estimated amount of kilojoules needed to maintain energy balance for men or women aged 31–50 years at the very low physical activity–sedentary level because of previously documented low physical activity levels among this population( Reference Kuhnlein, Receveur and Soueida 22 , Reference Bjerregaard and Young 23 ). Nutrients of interest were selected based on published effects of smoking on decreased antioxidant potential and dietary risk factors for chronic disease. To compare the nutrient intakes of smokers with non-smokers, nutrient densities per 4184 kJ (1000 kcal) were determined by dividing each participant's daily nutrient intakes by their energy intake (kcal), multiplied by 4184. As nutrient densities were not normally distributed, the non-parametric Wilcoxon rank-sum test was used to determine differences in nutrient densities between smokers and non-smokers. Smokers were defined as consuming more than one cigarette daily. All smokers were treated the same, regardless of pack-years, due to small sample size.
Dietary adequacy was calculated using the Estimated Average Requirements (EAR) based on the gender- and age-specific (19–30 years, 31–50 years, 51–70 years, >70 years) recommendations( 24 ). Where the EAR was not available, as for dietary fibre, vitamin D, vitamin K, pantothenic acid, K, Na and Ca, the Adequate Intake (AI) was used, as recommended( 21 ). Due to the small sample size per age group, the number and percentage of participants not meeting the recommendations for selected nutrients were first determined by age-specific EAR and then pooled for all age groups.
Participants who reported extreme energy intake >20 920 kJ (>5000 kcal; n 3) were excluded from the analysis. No participant reported an energy intake of <2092 kJ (<500 kcal). All analyses were stratified by gender and smoking status. Data were analysed using the SAS statistical software package version 9·2 (SAS Institute, Inc., Cary, NC, USA). All tests and P values were two-sided and considered statistically significant at α = 0·05. Institutional Review Board approval was obtained from the Committee on Human Studies at the University of Hawaii and the Office of Human Research Ethics at the University of North Carolina at Chapel Hill. The Nunavut Research Institute licensed the present study.
Results
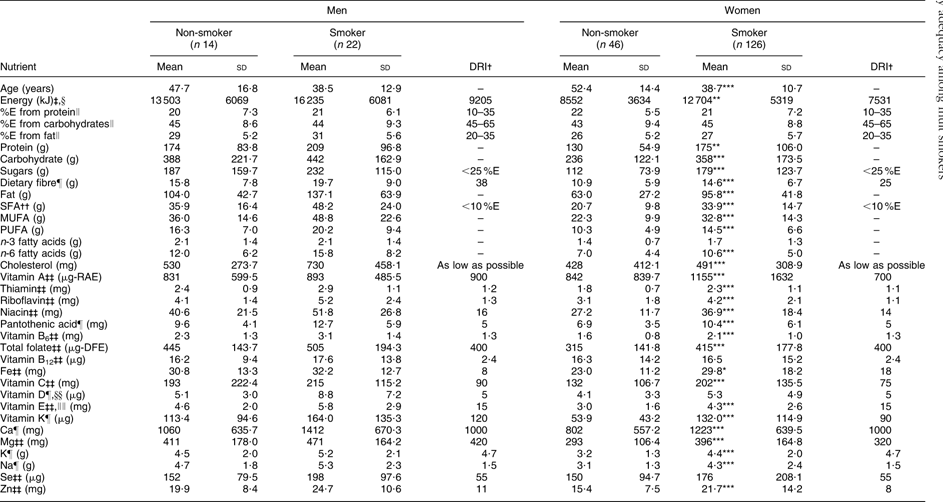
The prevalence of smoking among men and women in the present study was 61 % and 73 %, respectively. Men who reported smoking (n 22) consumed on average 16 235 kJ (3880 kcal) daily, which is 2732 kJ (653 kcal) more than men who did not smoke (n 14) and 7030 kJ (1680 kcal) above the Dietary Reference Intake (DRI) for energy (9205 kJ/2200 kcal daily). Female smokers (n 126) consumed on average 12 704 kJ (3036 kcal) daily compared with non-smoking women (n 46) who consumed an average of 8552 kJ (2043 kcal) daily. Daily energy intake was 5173 kJ (1236 kcal) and 1021 kJ (243 kcal) above the DRI (7531 kJ/1800 kcal) for female smokers and non-smokers, respectively. The mean (sd) number of cigarettes smoked daily among male and female smokers was 8·9 (6·2) and 8·0 (4·9), respectively (data not shown). Average daily energy and nutrient intakes did not differ between male smokers and non-smokers, and all men regardless of smoking status had average intakes below the DRI for fibre, vitamin A and vitamin E (Table 1). Average daily energy and nutrient intakes were higher among female smokers compared with non-smokers for all nutrients (P < 0·05) except n-3 fatty acids, vitamin A, vitamin D and Se. Average intakes of fibre, K and vitamin E were below recommendations for all females.
Table 1 Daily intakes of energy and selected nutrients among adult Inuit in Nunavut, Canada, by gender and smoking status, 2008
| Men | Women | |||||||||
| Non-smoker | Smoker | Non-smoker | Smoker | |||||||
| (n 14) | (n 22) | (n 46) | (n 126) | |||||||
| Nutrient | Mean | sd | Mean | sd | DRI† | Mean | sd | Mean | sd | DRI† |
| Age (years) | 47·7 | 16·8 | 38·5 | 12·9 | – | 52·4 | 14·4 | 38·7*** | 10·7 | – |
| Energy (kJ)‡,§ | 13 503 | 6069 | 16 235 | 6081 | 9205 | 8552 | 3634 | 12 704** | 5319 | 7531 |
| %E from protein∥ | 20 | 7·3 | 21 | 6·1 | 10–35 | 22 | 5·5 | 21 | 7·2 | 10–35 |
| %E from carbohydrates∥ | 45 | 8·6 | 44 | 9·3 | 45–65 | 43 | 9·4 | 45 | 8·8 | 45–65 |
| %E from fat∥ | 29 | 5·2 | 31 | 5·6 | 20–35 | 26 | 5·2 | 27 | 5·7 | 20–35 |
| Protein (g) | 174 | 83·8 | 209 | 96·8 | – | 130 | 54·9 | 175** | 106·0 | – |
| Carbohydrate (g) | 388 | 221·7 | 442 | 162·9 | – | 236 | 122·1 | 358*** | 173·5 | – |
| Sugars (g) | 187 | 159·7 | 232 | 115·0 | <25 %E | 112 | 73·9 | 179*** | 123·7 | <25 %E |
| Dietary fibre¶ (g) | 15·8 | 7·8 | 19·7 | 9·0 | 38 | 10·9 | 5·9 | 14·6*** | 6·7 | 25 |
| Fat (g) | 104·0 | 42·7 | 137·1 | 63·9 | – | 63·0 | 27·2 | 95·8*** | 41·8 | – |
| SFA†† (g) | 35·9 | 16·4 | 48·2 | 24·0 | <10 %E | 20·7 | 9·8 | 33·9*** | 14·7 | <10 %E |
| MUFA (g) | 36·0 | 14·6 | 48·8 | 22·6 | – | 22·3 | 9·9 | 32·8*** | 14·3 | – |
| PUFA (g) | 16·3 | 7·0 | 20·2 | 9·4 | – | 10·3 | 4·9 | 14·5*** | 6·6 | – |
| n-3 fatty acids (g) | 2·1 | 1·4 | 2·1 | 1·4 | – | 1·4 | 0·7 | 1·7 | 1·3 | – |
| n-6 fatty acids (g) | 12·0 | 6·2 | 15·8 | 8·2 | – | 7·0 | 4·4 | 10·6*** | 5·0 | – |
| Cholesterol (mg) | 530 | 273·7 | 730 | 458·1 | As low as possible | 428 | 412·1 | 491*** | 308·9 | As low as possible |
| Vitamin A‡‡ (μg-RAE) | 831 | 599·5 | 893 | 485·5 | 900 | 842 | 839·7 | 1155*** | 1632 | 700 |
| Thiamin‡‡ (mg) | 2·4 | 0·9 | 2·9 | 1·1 | 1·2 | 1·8 | 0·7 | 2·3*** | 1·1 | 1·1 |
| Riboflavin‡‡ (mg) | 4·1 | 1·4 | 5·2 | 2·4 | 1·3 | 3·1 | 1·8 | 4·2*** | 2·1 | 1·1 |
| Niacin‡‡ (mg) | 40·6 | 21·5 | 51·8 | 26·8 | 16 | 27·2 | 11·7 | 36·9*** | 18·4 | 14 |
| Pantothenic acid¶ (mg) | 9·6 | 4·1 | 12·7 | 5·9 | 5 | 6·9 | 3·5 | 10·4*** | 6·1 | 5 |
| Vitamin B6‡‡ (mg) | 2·3 | 1·3 | 3·1 | 1·4 | 1·3 | 1·6 | 0·8 | 2·1*** | 1·0 | 1·3 |
| Total folate‡‡ (μg-DFE) | 445 | 143·7 | 505 | 194·3 | 400 | 315 | 141·8 | 415*** | 177·8 | 400 |
| Vitamin B12‡‡ (μg) | 16·2 | 9·4 | 17·6 | 13·8 | 2·4 | 16·3 | 14·2 | 16·5 | 15·2 | 2·4 |
| Fe‡‡ (mg) | 30·8 | 13·3 | 32·2 | 12·7 | 8 | 23·0 | 11·2 | 29·8* | 18·2 | 18 |
| Vitamin C‡‡ (mg) | 193 | 222·4 | 215 | 115·2 | 90 | 132 | 106·7 | 202*** | 135·5 | 75 |
| Vitamin D¶,§§ (μg) | 5·1 | 3·0 | 8·8 | 7·2 | 5 | 4·1 | 3·3 | 5·3 | 4·9 | 5 |
| Vitamin E‡‡,∥∥ (mg) | 4·6 | 2·0 | 5·8 | 2·9 | 15 | 3·0 | 1·6 | 4·3*** | 2·6 | 15 |
| Vitamin K¶ (μg) | 113·4 | 94·6 | 164·0 | 135·3 | 120 | 53·9 | 43·2 | 132·0*** | 114·9 | 90 |
| Ca¶ (mg) | 1060 | 635·7 | 1412 | 670·3 | 1000 | 802 | 557·2 | 1223*** | 639·5 | 1000 |
| Mg‡‡ (mg) | 411 | 178·0 | 471 | 164·2 | 420 | 293 | 106·4 | 396*** | 164·8 | 320 |
| K¶ (g) | 4·5 | 2·0 | 5·2 | 2·1 | 4·7 | 3·2 | 1·3 | 4·4*** | 2·0 | 4·7 |
| Na¶ (g) | 4·7 | 1·8 | 5·3 | 2·3 | 1·5 | 3·1 | 1·3 | 4·3*** | 2·4 | 1·5 |
| Se‡‡ (μg) | 152 | 79·5 | 198 | 97·6 | 55 | 150 | 94·7 | 176 | 208·1 | 55 |
| Zn‡‡ (mg) | 19·9 | 8·4 | 24·7 | 10·6 | 11 | 15·4 | 7·5 | 21·7*** | 14·2 | 8 |
DRI, Dietary Reference Intake; %E, percentage of energy; RAE, retinol activity equivalents; DFE, dietary folate equivalents.
Mean values were significantly different from those of non-smokers of the same gender: *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001.
†The DRI are presented using Adequate Intake/RDA for men and women aged 31–50 years, Acceptable Macronutrient Distribution Ranges and the recommendation on saturated fat intake by Joint WHO/FAO( 21 , 46 ).
‡1 kcal = 4·184 kJ.
§Estimated kilojoules needed to maintain energy balance for men or women aged 31–50 years at the very low physical activity–sedentary level.
∥Acceptable Macronutrient Distribution Range.
¶Adequate Intake.
††Recommendation on saturated fat intake by Joint WHO/FAO( 46 ).
‡‡RDA.
§§As cholecalciferol in the absence of adequate exposure to sunlight.
∥∥As α-tocopherol.
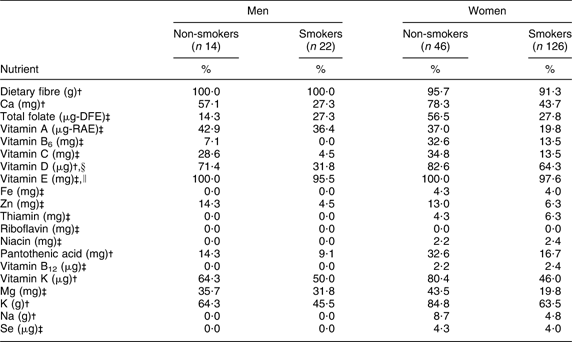
The majority of participants were below the DRI for dietary fibre (90–100 %) and vitamin E (90–100 %), regardless of smoking status or gender. Between 20 % and 50 % of male smokers were below the DRI for Ca, folate, Mg, K and vitamins A, D and K, and more than 50 % were below the DRI for fibre and vitamin E; however, the proportion of male smokers below the DRI for these nutrients was lower compared with non-smokers, with the exception of folate (Table 2). Considering female smokers, between 20 % and 50 % were below the DRI for Ca, folate, Mg and vitamins A and K, and more than 50 % were below the DRI for fibre, K and vitamins D and E, although these proportions were lower compared with non-smoking females (Table 2).
Table 2 Percentage of adult Inuit below the Dietary Reference Intakes by gender and smoking status, Nunavut, Canada, 2008
| Men | Women | |||
| Non-smokers | Smokers | Non-smokers | Smokers | |
| (n 14) | (n 22) | (n 46) | (n 126) | |
| Nutrient | % | % | % | % |
| Dietary fibre (g)† | 100·0 | 100·0 | 95·7 | 91·3 |
| Ca (mg)† | 57·1 | 27·3 | 78·3 | 43·7 |
| Total folate (μg-DFE)‡ | 14·3 | 27·3 | 56·5 | 27·8 |
| Vitamin A (μg-RAE)‡ | 42·9 | 36·4 | 37·0 | 19·8 |
| Vitamin B6 (mg)‡ | 7·1 | 0·0 | 32·6 | 13·5 |
| Vitamin C (mg)‡ | 28·6 | 4·5 | 34·8 | 13·5 |
| Vitamin D (μg)†,§ | 71·4 | 31·8 | 82·6 | 64·3 |
| Vitamin E (mg)‡,∥ | 100·0 | 95·5 | 100·0 | 97·6 |
| Fe (mg)‡ | 0·0 | 0·0 | 4·3 | 4·0 |
| Zn (mg)‡ | 14·3 | 4·5 | 13·0 | 6·3 |
| Thiamin (mg)‡ | 0·0 | 0·0 | 4·3 | 6·3 |
| Riboflavin (mg)‡ | 0·0 | 0·0 | 0·0 | 0·0 |
| Niacin (mg)‡ | 0·0 | 0·0 | 2·2 | 2·4 |
| Pantothenic acid (mg)† | 14·3 | 9·1 | 32·6 | 16·7 |
| Vitamin B12 (μg)‡ | 0·0 | 0·0 | 2·2 | 2·4 |
| Vitamin K (μg)† | 64·3 | 50·0 | 80·4 | 46·0 |
| Mg (mg)‡ | 35·7 | 31·8 | 43·5 | 19·8 |
| K (g)† | 64·3 | 45·5 | 84·8 | 63·5 |
| Na (g)† | 0·0 | 0·0 | 8·7 | 4·8 |
| Se (μg)‡ | 0·0 | 0·0 | 4·3 | 4·0 |
DFE, dietary folate equivalents; RAE, retinol activity equivalents.
†Adequate Intake used for comparison.
‡Estimated Average Requirement used for comparison.
§As cholecalciferol in the absence of adequate exposure to sunlight.
∥As α-tocopherol.
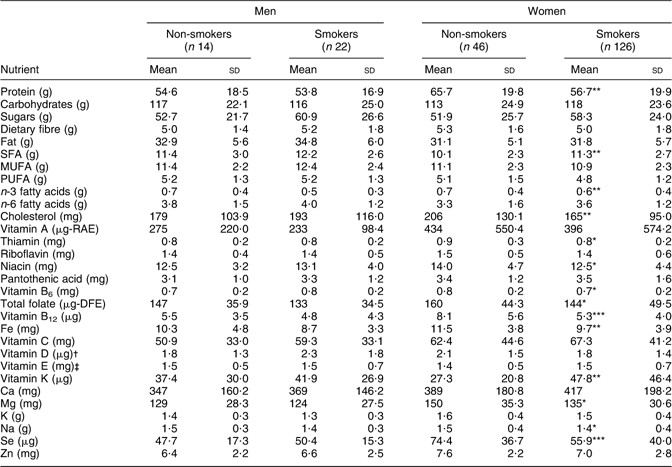
No significant differences were seen in nutrient intake density (per 4184 kJ/1000 kcal) between male smokers and non-smokers (Table 3). Female smokers had significantly lower intake densities of thiamin, niacin, vitamin B6, folate, Mg, Na (P ≤ 0·05), protein, n-3 fatty acids, cholesterol, Fe (P ≤ 0·01), vitamin B12 and Se (P ≤ 0·001), and significantly higher intake densities of saturated fat and vitamin K (P ≤ 0·01; Table 3).
Table 3 Nutrient density per 4184 kJ (1000 kcal) among adult Inuit by gender and smoking status, Nunavut, Canada, 2008
| Men | Women | |||||||
| Non-smokers | Smokers | Non-smokers | Smokers | |||||
| (n 14) | (n 22) | (n 46) | (n 126) | |||||
| Nutrient | Mean | sd | Mean | sd | Mean | sd | Mean | sd |
| Protein (g) | 54·6 | 18·5 | 53·8 | 16·9 | 65·7 | 19·8 | 56·7** | 19·9 |
| Carbohydrates (g) | 117 | 22·1 | 116 | 25·0 | 113 | 24·9 | 118 | 23·6 |
| Sugars (g) | 52·7 | 21·7 | 60·9 | 26·6 | 51·9 | 25·7 | 58·3 | 24·0 |
| Dietary fibre (g) | 5·0 | 1·4 | 5·2 | 1·8 | 5·3 | 1·6 | 5·0 | 1·8 |
| Fat (g) | 32·9 | 5·6 | 34·8 | 6·0 | 31·1 | 5·1 | 31·8 | 5·7 |
| SFA (g) | 11·4 | 3·0 | 12·2 | 2·6 | 10·1 | 2·3 | 11·3** | 2·7 |
| MUFA (g) | 11·4 | 2·2 | 12·4 | 2·4 | 11·1 | 2·3 | 10·9 | 2·3 |
| PUFA (g) | 5·2 | 1·3 | 5·2 | 1·3 | 5·1 | 1·5 | 4·8 | 1·2 |
| n-3 fatty acids (g) | 0·7 | 0·4 | 0·5 | 0·3 | 0·7 | 0·4 | 0·6** | 0·4 |
| n-6 fatty acids (g) | 3·8 | 1·5 | 4·0 | 1·2 | 3·3 | 1·6 | 3·6 | 1·2 |
| Cholesterol (mg) | 179 | 103·9 | 193 | 116·0 | 206 | 130·1 | 165** | 95·0 |
| Vitamin A (μg-RAE) | 275 | 220·0 | 233 | 98·4 | 434 | 550·4 | 396 | 574·2 |
| Thiamin (mg) | 0·8 | 0·2 | 0·8 | 0·2 | 0·9 | 0·3 | 0·8* | 0·2 |
| Riboflavin (mg) | 1·4 | 0·4 | 1·4 | 0·5 | 1·5 | 0·5 | 1·4 | 0·6 |
| Niacin (mg) | 12·5 | 3·2 | 13·1 | 4·0 | 14·0 | 4·7 | 12·5* | 4·4 |
| Pantothenic acid (mg) | 3·1 | 1·0 | 3·3 | 1·2 | 3·4 | 1·2 | 3·5 | 1·6 |
| Vitamin B6 (mg) | 0·7 | 0·2 | 0·8 | 0·2 | 0·8 | 0·2 | 0·7* | 0·2 |
| Total folate (μg-DFE) | 147 | 35·9 | 133 | 34·5 | 160 | 44·3 | 144* | 49·5 |
| Vitamin B12 (μg) | 5·5 | 3·5 | 4·8 | 4·3 | 8·1 | 5·6 | 5·3*** | 4·0 |
| Fe (mg) | 10·3 | 4·8 | 8·7 | 3·3 | 11·5 | 3·8 | 9·7** | 3·9 |
| Vitamin C (mg) | 50·9 | 33·0 | 59·3 | 33·1 | 62·4 | 44·6 | 67·3 | 41·2 |
| Vitamin D (μg)† | 1·8 | 1·3 | 2·3 | 1·8 | 2·1 | 1·5 | 1·8 | 1·4 |
| Vitamin E (mg)‡ | 1·5 | 0·5 | 1·5 | 0·7 | 1·4 | 0·5 | 1·5 | 0·7 |
| Vitamin K (μg) | 37·4 | 30·0 | 41·9 | 26·9 | 27·3 | 20·8 | 47·8** | 46·4 |
| Ca (mg) | 347 | 160·2 | 369 | 146·2 | 389 | 180·8 | 417 | 198·2 |
| Mg (mg) | 129 | 28·3 | 124 | 27·5 | 150 | 35·3 | 135* | 30·6 |
| K (g) | 1·4 | 0·3 | 1·3 | 0·3 | 1·6 | 0·4 | 1·5 | 0·4 |
| Na (g) | 1·5 | 0·3 | 1·4 | 0·3 | 1·5 | 0·4 | 1·4* | 0·4 |
| Se (μg) | 47·7 | 17·3 | 50·4 | 15·3 | 74·4 | 36·7 | 55·9*** | 40·0 |
| Zn (mg) | 6·4 | 2·2 | 6·6 | 2·5 | 7·6 | 2·2 | 7·0 | 2·8 |
RAE, retinol activity equivalents; DFE, dietary folate equivalents.
Mean values were significantly different from those of non-smokers of the same gender: *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001.
†As cholecalciferol in the absence of adequate exposure to sunlight.
‡As α-tocopherol.
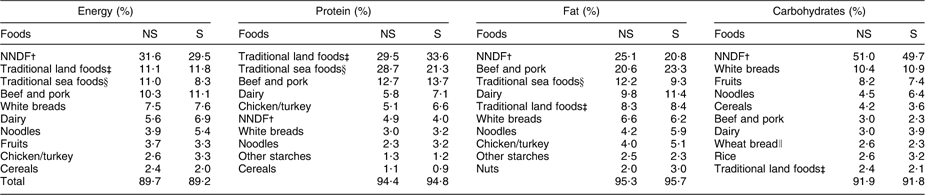
Among men and women combined, the primary contributors to energy, protein and carbohydrates were the same regardless of smoking status (Table 4). Comparing smokers with non-smokers, NNDF contributed less to energy (−2·1 %), fat (−4·3 %) and carbohydrates (−1·3 %). The contribution of traditional foods to energy was lower (−2·0 %) among adults who smoked compared with non-smokers. Smokers had similar contributions of dairy to energy (6·9 % v. 6·3 %) and fruits to energy (3·3 % v. 3·7 %).
Table 4 Top ten food sources of energy and selected nutrients among Inuit in Nunavut, Canada, by smoking status, 2008
| Energy (%) | Protein (%) | Fat (%) | Carbohydrates (%) | ||||||||
| Foods | NS | S | Foods | NS | S | Foods | NS | S | Foods | NS | S |
| NNDF† | 31·6 | 29·5 | Traditional land foods‡ | 29·5 | 33·6 | NNDF† | 25·1 | 20·8 | NNDF† | 51·0 | 49·7 |
| Traditional land foods‡ | 11·1 | 11·8 | Traditional sea foods§ | 28·7 | 21·3 | Beef and pork | 20·6 | 23·3 | White breads | 10·4 | 10·9 |
| Traditional sea foods§ | 11·0 | 8·3 | Beef and pork | 12·7 | 13·7 | Traditional sea foods§ | 12·2 | 9·3 | Fruits | 8·2 | 7·4 |
| Beef and pork | 10·3 | 11·1 | Dairy | 5·8 | 7·1 | Dairy | 9·8 | 11·4 | Noodles | 4·5 | 6·4 |
| White breads | 7·5 | 7·6 | Chicken/turkey | 5·1 | 6·6 | Traditional land foods‡ | 8·3 | 8·4 | Cereals | 4·2 | 3·6 |
| Dairy | 5·6 | 6·9 | NNDF† | 4·9 | 4·0 | White breads | 6·6 | 6·2 | Beef and pork | 3·0 | 2·3 |
| Noodles | 3·9 | 5·4 | White breads | 3·0 | 3·2 | Noodles | 4·2 | 5·9 | Dairy | 3·0 | 3·9 |
| Fruits | 3·7 | 3·3 | Noodles | 2·3 | 3·2 | Chicken/turkey | 4·0 | 5·1 | Wheat bread∥ | 2·6 | 2·3 |
| Chicken/turkey | 2·6 | 3·3 | Other starches | 1·3 | 1·2 | Other starches | 2·5 | 2·3 | Rice | 2·6 | 3·2 |
| Cereals | 2·4 | 2·0 | Cereals | 1·1 | 0·9 | Nuts | 2·0 | 3·0 | Traditional land foods‡ | 2·4 | 2·1 |
| Total | 89·7 | 89·2 | 94·4 | 94·8 | 95·3 | 95·7 | 91·9 | 91·8 | |||
NS, non-smokers; S, smokers; NNDF, non-nutrient-dense foods.
†Including: butter/margarine, jam/marmalade, sugar/honey, non-dairy coffee whitener, hash browns/potato patties/French fries, gravy, salad dressing including mayonnaise and dips, ice cream, cakes/muffins, pies, cheesecake, chocolate bars, potato chips, crackers, cookies, hard candy, popcorn, granola bars, sweetened drinks, sugar-free drinks (e.g. sugar-free Kool-Aid™, Kraft Foods Inc., Northfield, IL, USA), unsweetened juice, coffee, tea, artificial sweetener, and carbonated drinks (regular and diet).
‡Including: caribou, musk ox, polar bear and their organs.
§Including: seal, seal liver, muktuk (whale skin and fat) and several wild, local fish (e.g. char and trout).
∥Wheat bread was among the top ten contributors of carbohydrates for non-smokers. Among smokers, this value corresponds to vegetables.
Discussion
The present study supplements the limited literature available on nutrition of adult Inuit by exploring dietary intake differences by smoking status. The smoking prevalence among men and women in our study was consistent with a recent Statistics Canada survey( 16 ). Overall, the comparison of dietary intake and quality by smoking status varied by gender. There were no significant differences between male smokers and non-smokers in average daily energy and nutrient intakes or dietary quality; however, nutrient intakes were significantly higher and diet quality significantly lower for a majority of nutrients comparing female smokers and non-smokers. The energy intake among all participants was high; however, this was anticipated because this population is known to have high energy requirements due to the challenging climate. The QFFQ contains a large number of food items that make a substantial contribution to energy intake( Reference Pakseresht and Sharma 19 ). Despite the possible overestimation of energy intake, the QFFQ used in the present study was developed specifically for this population and has been shown to be a valid measure of dietary intake. As discussed in the Methods section, extreme intakes (<2092 kJ/<500 kcal and >20 920 kJ/>5000 kcal) were excluded. The high energy intake levels are also reflected by high rates of overweight and obesity in this population( Reference Hopping, Mead and Erber 5 , Reference Sharma 18 ).
A lower proportion of smokers did not meet the recommendations for all nutrients of interest, with the exception of fibre and folate among males. Therefore, non-smokers appeared to have fewer dietary inadequacies than smokers. Smokers had higher energy intake but consumed fewer nutrient-dense, traditional foods which might have contributed to inadequate nutrient intakes. The number of smokers with nutrient intakes below recommendations may not be very different from non-smokers in this population group; however, it is worth noting the increased demand tobacco and its metabolites places on certain nutrients, especially antioxidants such as vitamin C, vitamin A and β-carotene( Reference Northrop-Clewes and Thurnham 13 ).
Decreased antioxidant intake, particularly vitamins A and C( Reference Northrop-Clewes and Thurnham 13 ), have been documented among smokers in other populations and high levels of smoking-associated oxidative stress subsequently increase the antioxidant demand( Reference Schectman, Byrd and Hoffman 25 ). Contradicting these observations from previous literature, smokers in the present study had higher average daily intakes and similar nutrient densities compared with non-smokers, and fewer participants were below the DRI for these nutrients. Even so, between 20 % and 36 % of smokers were below the DRI for vitamin A, a nutrient which may have depleted serum concentration in smokers( Reference Handelman, Packer and Cross 26 , Reference Alberg 27 ) and might be associated with decreased risk of anaemia( Reference Lynch 28 , Reference Fishman, Christian and West 29 ), which has been documented as highly prevalent among Inuit( Reference Jamieson and Kuhnlein 30 ).
A high proportion of smokers had nutrient profiles which might increase the risk of adverse cardiovascular outcomes. Average daily intake of Mg was above the DRI for male and female smokers; however, between 20 % and 32 % of all smokers were below recommended intakes. Mg is involved in a wide range of physiological mechanisms( Reference Saris, Mervaala and Karpannen 31 ) and low serum concentrations have been associated with CVD( Reference Saris, Mervaala and Karpannen 31 , Reference Touyz 32 ) and diabetes( Reference Simmons, Joshi and Shaw 33 ). Between 46 % and 64 % of smokers were below the recommended K intake. K is inversely related to the risk of hypertension and high K mitigates the blood-pressure-raising effects of Na( Reference Adrogue and Madias 34 ). This is notable among smokers as Na intakes were three to four times higher than the DRI. High Na, energy, saturated fat and cholesterol intakes combined with low K and Mg intakes might increase the risk of obesity, atherosclerosis, hypertension and CVD among smokers in this population( Reference Simmons, Joshi and Shaw 33 , Reference Appel 35 ).
Differences in dietary quality between female smokers and non-smokers was evident in the lower intake density of several nutrients among smokers, many of which are consistent with the lower intake of traditional foods among this group( Reference Kuhnlein, Chan and Leggee 36 ). These differences cannot be attributed to smoking alone, as female smokers were significantly younger than non-smokers and age has been correlated with traditional food intake( Reference Kuhnlein, Receveur and Soueida 22 , Reference Hopping, Erber and Mead 37 ). Stratifying respondents by demographic factors beyond gender was outside the scope of the present study. Certainly a wide range of demographic factors contribute to smoking and dietary intake; however, the primary aim of the study was to characterize the dietary intake by smoking status, regardless of factors that might have contributed to a participant's smoking status. Considering dietary patterns, the high contribution of NNDF and low contribution of traditional food to energy intake was consistent with the ongoing nutritional transition in this population( Reference Sharma 18 ). Traditional foods such as caribou, musk ox and marine mammals are rich in protein, polyunsaturated fats, vitamins A and D, several B vitamins and Fe( Reference Kuhnlein, Chan and Leggee 36 , Reference Kuhnlein, Barthet and Farren 38 ). The lower dietary quality documented among female smokers reinforces previous recommendations( Reference Kuhnlein and Receveur 39 ) of promoting nutrient-rich diets, primarily from traditional food sources.
The present study is not without limitations. Targeted participants for the study included the main food shopper/preparer of the household who are mostly women, thus there was a lower amount of male participants. This likely contributed to the lack of difference seen between male smokers and non-smokers and limits the generalizability of these results to the male population. The small overall sample size prohibited in-depth exploration and inferential statistical comparisons of dietary intake differences by level of smoking and behaviours that affect dietary intake, smoking and chronic disease risk, such as physical activity( Reference Heath 40 ) or alcohol consumption( Reference Piasecki, McCarthy and Fiore 41 – Reference Rehm, Room and Graham 43 ). Previous literature( Reference Stringhini, Sabia and Shipley 44 , Reference James, Nelson and Ralph 45 ) has discussed dietary intake differences based on socio-economic status (SES), including a study among this population( Reference Hopping, Erber and Mead 37 ) which documented higher consumption frequency of traditional foods, fruits and vegetables among Inuit men and women with higher material style of life scores, a proxy for SES. Despite these limitations, the present study was able to provide a general overview of the dietary intake differences between Inuit smokers and non-smokers. Although it provided mixed results on the dietary adequacy varying by gender and smoking status, our study highlights nutrients affected by smoking and the potential increased risk for diet-related chronic diseases. Further exploration of the differences in dietary adequacy among Inuit by smoking status should consider disaggregating participants as only smokers, only alcohol consumers and participants who use both substances to further understand the effects of smoking and alcohol use on dietary adequacy.
Conclusions
Adult smokers in the present study consumed fewer nutrient-dense, traditional foods and had increased energy intake which likely contributed to fewer dietary inadequacies compared with non-smokers. Monitoring the dietary adequacy of smokers could prove useful for preventing diet- and smoking-related chronic diseases, especially in populations undergoing a nutrition transition and that experience an increased risk of CVD and aero-digestive cancers. The study highlights the need for comprehensive and community-based healthy lifestyle programmes that address the effects of food choice, dietary intake, physical activity and smoking on overall health. Further, studies exploring dietary adequacy of smokers may help to identify potential policy interventions to create supportive environments that enable individual behaviour change. In addition, a multidimensional approach to the reduction of risk for chronic disease in this population may be more effective in addressing clustered risk factors such as smoking and poor diet.
Acknowledgements
The project was supported by American Diabetes Association Clinical Research award 1-08-CR-57. All authors declare they have no conflicts of interest. S.E.R. interpreted the data and drafted the manuscript. C.R. participated in the acquisition and interpretation of the data. S.S. was responsible for the conception and design of the study. All authors critically reviewed and approved the final manuscript. The authors extend special thanks to Ms Eva Erber for performing the statistical analysis.






