Malnutrition is a major public health concern in low- and middle-income countries (LMIC). It has remained the highest-ranking risk factor for disability since 1990, accounting for 20 % of disability-adjusted life years in 2017(1). In Ethiopia, undernutrition is prevalent: 22 % of women and 33 % of men are underweight, 38 % of children <5 years of age are stunted, and 10 % are wasted(2). Although nationally only 8 % of women and 3 % of men are overweight, larger urban–rural disparities exist. In 2016, women’s overweight prevalence was 21 % in urban areas and 4 % in rural areas, whereas men’s overweight was 12 % and 1 %, respectively. The Addis Ababa region had the highest overweight prevalence: 29 % among women and 21 % among men(2). Despite progress over the past three decades, undernutrition remains the primary risk factor for 24 % of all deaths in Ethiopia and 63 % of deaths among children <5 years of age(1).
At the household level, the most common double burden of malnutrition (DBM) definitions encompass women of reproductive age and children <5 years of age(Reference Davis, Oaks and Engle-Stone3). A 2019 Lancet DBM Series defined four types of household-level DBM covering these populations: (1) concurrent child overweight and stunting; (2) overweight woman and at least one wasted child; (3) overweight woman and at least one stunted child; and (4) underweight woman and at least one overweight child(Reference Popkin, Corvalan and Grummer-Strawn4). In sub-Saharan Africa (SSA), total household-level DBM (i.e. presence of any of these four forms) prevalence ranges from a low of 6·02 % in Ethiopia to a high of 26·96 % in Sao Tome and Principe(Reference Popkin, Corvalan and Grummer-Strawn4). However, national household-level DBM estimates may mask within-country disparities. In SSA, women’s overweight and obesity are growing faster in urban than in rural settings(Reference Bixby, Bentham and Zhou5), suggesting household-level DBM may differ across geographical settings. In fact, the odds of an overweight woman and a stunted child in the same household are 1·24 higher in urban and peri-urban areas than in rural areas across thirty SSA countries(Reference Jones, Acharya and Galway6).
In Ethiopia, no studies to date have comprehensively examined household-level DBM and its correlates, apart from the 2019 Lancet DBM Series. This Series supplied nationally representative estimates of the prevalence of four forms of household-level DBM but did not provide urban and rural estimates separately(Reference Popkin, Corvalan and Grummer-Strawn4). No other national or sub-national estimates of other forms of household-level DBM exist. Prior studies have focused on describing the population-level burden of malnutrition and its associated factors. Using nationally representative Demographic and Health Survey (DHS) data, most prior studies aimed to identify the prevalence and factors associated with women’s or children’s underweight, overweight and obesity(Reference Yeshaw, Kebede and Liyew7–Reference Gutema, Chuka and Kondale11). Only one of these studies included adult men and examined the population-level prevalence and correlates of adult underweight and overweight(Reference Gutema, Chuka and Kondale11).
According to the WHO framework on the drivers of DBM, DBM is attributable to a diverse set of biological, environmental, behavioural, and social and demographic determinants(12). More recently, the biological determinants, such as epigenetics and early-life nutrition, have been highlighted(Reference Wells, Sawaya and Wibaek13). Given the lack of empirical studies of household-level DBM, its correlates in Ethiopia remain largely unknown. Farah et al. showed that socio-economic (i.e. low household wealth) and biological (i.e. male gender, older age, small birth size and no vitamin A supplementation in the past 6 months) factors are associated with concurrent overweight and stunting in children 6–24 months of age(Reference Farah, Nour and Endris14). Studies from other SSA countries have also found women’s short stature, age, and education, and household size as socio-economic and demographic factors associated with concurrent child overweight and stunting(Reference Keino, Plasqui and Ettyang15). With respect to other forms of household-level DBM, evidence is lacking. Only two studies from West Africa have identified socio-economic and demographic factors associated with overweight woman and stunted child(Reference Dembélé, Sossa Jérôme and Saizonou16,Reference Senbanjo, Senbanjo and Afolabi17) , and one study from Benin showed that socio-economic status and dietary diversity were the primary associated factors of overweight woman and wasted or stunted child(Reference Bouzitou, Fayomi and Delisle18). To our knowledge, no studies have examined the correlates of overweight woman and wasted child, and underweight woman and overweight child in SSA. Likewise, no studies on household-level DBM in SSA have included adult men. Further, behavioural (e.g. diet) and environmental (e.g. food supply and cost, or cultural and social aspects) factors associated with DBM are also understudied.
Therefore, we sought to describe household-level DBM and its associated factors among urban and rural Ethiopian households with men and women of reproductive age, and children <5 years of age. To our knowledge, this is the first study to provide estimates of household-level DBM including men and to identify the associated factors of multiple forms of household-level DBM across urban and rural settings in Ethiopia. In addition, based on the associated factors we identified, we suggest some potential double-duty actions to address DBM in the country.
Methods
Data and participants
We used data from two cross-sectional studies conducted in an urban setting – the capital Addis Ababa (January–February 2018), and a rural setting – Kersa district (June–September 2019). The primary objectives of the two studies were to characterise protein source food production, access, and consumption and to quantify the health and environmental impacts of optimising Ethiopian diets. The two studies employed similar multistage sampling strategies, inclusion and exclusion criteria, and quantitative questionnaires. In Addis Ababa, five out of the ten sub-cities into which Addis Ababa is subdivided were randomly selected at the first stage. Within each of the five randomly selected sub-cities, two districts were chosen at random at the second stage. Within each district, approximately 240 households were screened. Households with at least one woman of reproductive age (18–49 years) and a child aged 6–59 months were invited to participate in the study. If more than one woman of reproductive age lived in the household and was present at the time of the interview, one was randomly selected for the interview using a random number generator. If a selected woman had more than one child aged 6–59 months, one child was selected for the interview using a random number generator. The women we interviewed were the biological mother of the child (96 %), grandmothers (1·9 %), aunts (1·43 %) or another female household member (0·58 %). When present, the woman’s adult male partner (≥18 years) was also interviewed. If he was not present, another adult man was interviewed, and if more than one man was present, one was randomly selected. Thus, the women and men we interviewed were not always the biological parents of the child, and the men were not always the partners/husbands of the interviewed women. A total of 1050 households participated in the Addis Ababa study.
Kersa is a predominantly rural agricultural district is Eastern Ethiopia. At the first stage, the twenty-four kebeles (lowest administrative unit) from the Kersa Demographic Surveillance and Health Research Centre (KDS-HRC) of Haramaya University(Reference Assefa, Oljira and Baraki19) were stratified into rural (n 21) and urban (n 3) kebeles. The three urban kebeles represent small towns, which have 24-h electricity supply(Reference Assefa, Oljira and Baraki19). Electricity supply was the only criterion used to classify Kersa kebeles into urban and rural. As compared to Addis Ababa, Kersa district is still considered rural given the limited availability of electricity and that most inhabitants are farmers(Reference Assefa, Oljira and Baraki19). At the second stage, ten out of the twenty-one rural kebeles and two out of the three urban kebeles were randomly selected. Households with at least one woman of reproductive age (18–49 years old), a child 6–59 months of age and an adult man (≥18 years) were randomly selected from the KDS-HRC database. Pregnant women were excluded from the study. If more than one target individual was present at the time of the interview, one was randomly selected using the same procedures as in the Addis Ababa sample. Most women (99·4 %) we interviewed were the biological mothers of the child. However, since adult men could be randomly selected, they were not always the biological fathers of the interviewed children or the partner/husband of the interviewed woman. A total of 1197 households participated in the Kersa study. In both Addis Ababa and Kersa, the overall number of clusters at the first stage and the number of households at the second stage were based on logistical and financial constraints.
Identical quantitative household questionnaires were used in both studies to collect information on socio-economic and demographic characteristics, household food security, food supply shortages, food expenditures, and participants’ diets. Trained fieldworkers directly assessed participants’ height and weight. Both adults and children were barefoot and wore light clothing for the assessment. Height was measured to the nearest 0·1 cm and weight to the nearest 0·1 kg. Adult height and height for children 24–59 months of age was measured using a Seca stadiometer. Length of children <24 months of age was measured using a length board. Weight was measured using a Seca digital scale.
Participants’ diet was assessed using a non-quantitative food frequency questionnaire (FFQ), locally adapted from a semi-quantitative FFQ version validated for use among urban Tanzanian adults(Reference Zack, Irema and Kazonda20). Adult men and women were asked if they consumed each of seventy-three individual foods in the past 7 d, and if they had, they were then asked if they consumed the food in the past 24 h. The FFQ included locally available foods and varieties, and an option to specify other foods. Portion sizes and frequencies of intake were not collected. Women reported on children’s dietary intake.
Outcome measures
Adult body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. We categorised adults as underweight (BMI < 18 kg/m2), normal weight (BMI ≥ 18·5 kg/m2 and BMI < 25 kg/m2), or overweight (BMI ≥ 25 kg/m2)(21). We calculated child length/height-for-age Z-score (HAZ) and weight-for-length/height Z-score (WHZ) using the 2006 WHO Child Growth Standards(22). Stunting was defined as HAZ < -2 sd, wasting as WHZ < -2 sd and overweight as WHZ ≥ 2 sd.
Using these anthropometric indicators, we calculated fifteen forms of household-level DBM encompassing most forms of malnutrition within the same individual, pairs of individuals or multiple individuals that can be defined based on anthropometry. Within the same individual, we defined DBM as concurrent child overweight and stunting. Within pairs of individuals, we defined DBM as overweight woman or man and stunted or wasted child, underweight woman or man and overweight child, and overweight woman or man and underweight man or woman. Within households, we defined DBM as at least one overweight adult and wasted or stunted child, at least one underweight adult and overweight child, two overweight adults and wasted or stunted child, and two underweight adults and overweight child. For each DBM form, we created binary indicators where 1 = DBM form was present and 0 = otherwise.
Double burden of malnutrition associated factors
We considered sixteen DBM factors grouped along the WHO drivers of DBM framework. This framework outlines the biological (e.g. epigenetics and early-life experiences), environmental (e.g. food supply and systems, food portion size and cost, urban and built environment), behavioural (e.g. lifestyle and psychological factors), and socio-economic and demographic factors of DBM (e.g. poverty and food insecurity)(12). The biological factors we included were child age and sex, and women’s age. The environmental factors were the number of food supply shortages in the past year (range 0–13), distance to market (in km), monthly food expenditures as a proxy of food cost (total household expenditures on meat, fish, poultry, pulses, nuts and seeds, and fats and oils in the past month), religion (Christian Orthodox v. non-Christian Orthodox) and duration of residence (number of years living in Addis Ababa or Kersa). In Kersa, distance to market and food expenditures were only available for a subset of households and were therefore not considered as associated factors in this sample since doing so may introduce bias if the households with data on distance to market and food expenditures are systematically different from households without this data. Religion and duration of residence served as proxies for cultural and social practices. In Ethiopia, Orthodox Christians abstain from animal source foods and fats for 180 d throughout the year(Reference Zellelew23). Given the differential association between animal source foods consumption and overweight risk, e.g. higher egg, dairy or fish consumption does not increase overweight risk in adults, whereas higher red meat and processed meat consumption does(Reference Schlesinger, Neuenschwander and Schwedhelm24), we had no a priori hypotheses about the association between religion and household-level DBM.
Diet was the only behavioural factor we considered. We assessed women’s dietary diversity using the minimum dietary diversity for women (MDD-W) indicator(Reference Martin-Prevel, Arimond and Allemand25). We grouped foods into ten non-overlapping food groups and summed them into a dietary diversity score (DDS-W, range 0–10). We categorised women as meeting MDD if they consumed at least five food groups (DDS ≥ 5). MDD-W serves as a proxy for micronutrient adequacy(26). In the absence of similar indicators validated for use among adult men ≥18 years of age, we created the same indicators for dietary diversity and minimum dietary diversity. Children’s dietary diversity was assessed using the WHO indicators for dietary diversity score (DDS, range 0–7) and minimum dietary diversity (MDD, defined as DDS ≥ 4)(27). Although not originally designed to assess diet in children older than 24 months of age, a recent study showed that both DDS and MDD can serve as adequate proxies for micronutrient intake in children of 24–59 months of age(Reference Diop, Becquey and Turowska28). MDD showed 79 % specificity and 56 % sensitivity in correctly classifying children with probability of adequacy >80 % for eleven micronutrients(Reference Diop, Becquey and Turowska28). Given this evidence and the absence of other diet indicators validated for use among children of 24–59 months of age, we used the same indicators for all children of 6–59 months of age. All diet indicators were based on a 24-h recall. In secondary analyses, we recalculated all diet indicators based on a 7-d recall since one-quarter of Addis Ababa households reported that the day prior to the FFQ administration was a fasting day and thus the 24-h recall may potentially underestimate dietary diversity in these households.
Finally, the socio-economic and demographic factors we included were household wealth, housing floor quality (parquet or polished floor v. natural floor), sanitation, food security, household size, women’s and men’s education (primary or higher v. no or incomplete primary), and women’s employment (engaged v. not engaged in an income generating activity). To assess household wealth, we derived wealth indices separately for the full Addis Ababa sample and the full Kersa sample using principal component analysis based on items representing housing quality and asset ownership (twelve items in Addis Ababa and ten items in Kersa). The wealth quartiles were defined separately for the full Addis Ababa sample and the full Kersa sample. We then created a binary indicator for whether a household belonged to the lowest wealth quartile or to a higher wealth quintile. Household sanitation was assessed using an indicator for whether the household had access to an improved latrine. Although housing floor quality and sanitation were included in the wealth indices, we also considered them separately, since unimproved sanitation is the second most important risk factor for child stunting in Ethiopia(Reference Danaei, Andrews and Sudfeld29). Household food security was assessed using the Household Food Insecurity Access Scale(Reference Coates, Swindale and Bilinsky30).
Analytic samples
In both Addis Ababa and Kersa, we restricted the samples to households with available anthropometric measurements for all three individuals: woman of reproductive age (18–49 years), adult man (≥18 years) and child (6–59 months). In Addis Ababa, we excluded 392 households in which an adult man was not interviewed. We further excluded pregnant women (n 56) to harmonise the urban sample with the rural sample, which did not recruit pregnant women. Finally, ten children with abnormal HAZ or WHZ scores (above or below 6 sd) were also excluded. The final analytic sample in Addis Ababa consisted of 592 households. In Kersa, 335 households where at least one household member was missing anthropometric data were excluded. The final analytic sample in Kersa was 862 households. Supplemental Figures 1 and 2 provide flow charts of how the analytic samples were derived.
In each sample, we compared household and women’s demographic characteristics among households included in the analytic sample and those excluded using Wald tests. Differences were considered statistically significant at P < 0·05. Overall, there were few differences between included and excluded households in either sample. Only one statistically significant difference was observed in Addis Ababa, where excluded households were significantly smaller than included households (data not shown). As a result, we did not expect the exclusion of households from either sample to bias our results.
Statistical analysis
We used generalised linear models to assess the univariate and multivariate relationships between household-level DBM forms and the biological, environmental, behavioural, and socio-economic and demographic factors. DBM factors were investigated only for DBM forms prevalent in ≥5 % of households in either the Addis Ababa or Kersa sample. We estimated unadjusted OR (uOR) and 95 % CI from univariate models and adjusted OR (aOR) and 95 % CI from multivariate models. Since factors were selected a priori based on the WHO drivers of DBM framework, multivariate models included all factors regardless of whether they were statistically significant in univariate models. Where both a continuous and binary indicator were considered in univariate models (e.g. DDS and MDD), we entered only the continuous indicator in the multivariate model to increase power. Our assessment of the biserial correlations between all the factors included in the multivariate model indicated no presence of multicollinearity, that is, all correlations were between –0·44 and 0·52 in Addis Ababa and –0·35 and 0·43 in Kersa (data not shown). Missing values on any of the factors were imputed using mean cluster-level imputation. Standard errors were clustered at the lowest administrative level in each sample: woreda in Addis Ababa and kebele in Kersa. Associations were considered statistically significant at P < 0·05. Given the exploratory nature of the analyses, we also present p-values corrected for multiple comparisons using the Benjamini and Yekutieli method, which controls the false discovery rate under dependency(Reference Benjamini and Yekutieli31). All analyses were performed using Stata 16(32).
Results
Characteristics of the two analytic samples are shown in Table 1. Overall, Addis Ababa households had better living conditions than Kersa households. Although most households in both samples were food-secure, rural households experienced more food supply shortages in the past year than urban households. Generally, overnutrition was more prevalent in Addis Ababa, whereas undernutrition was more prevalent in Kersa. In Kersa, women’s employment and religion were not considered as associated factors due to lack of variability in the sample: 99·3 % of women were employed and only 3·4 % were Orthodox Christians. The higher frequency of women’s employment in Kersa relative to Addis Ababa was likely due to women being engaged in agricultural activities (given that Kersa is predominantly an agricultural district).
Table 1 Characteristics of the households included in the Addis Ababa and Kersa samples

The combination of overweight adult and stunted child was the most prevalent form of household-level DBM in Addis Ababa (Table 2). Overweight woman and stunted child, and concurrent child overweight and stunting were also prevalent. The remaining forms of household-level DBM occurred in <5 % of the sample, with many occurring in <10 households. In contrast, concurrent child overweight and stunting was the most prevalent form of household-level DBM in Kersa, affecting 11 % of children. Overweight adult and stunted child was the only other form of DBM affecting ≥5 % of households.
Table 2 Household-Level forms of malnutrition in Addis Ababa and Kersa
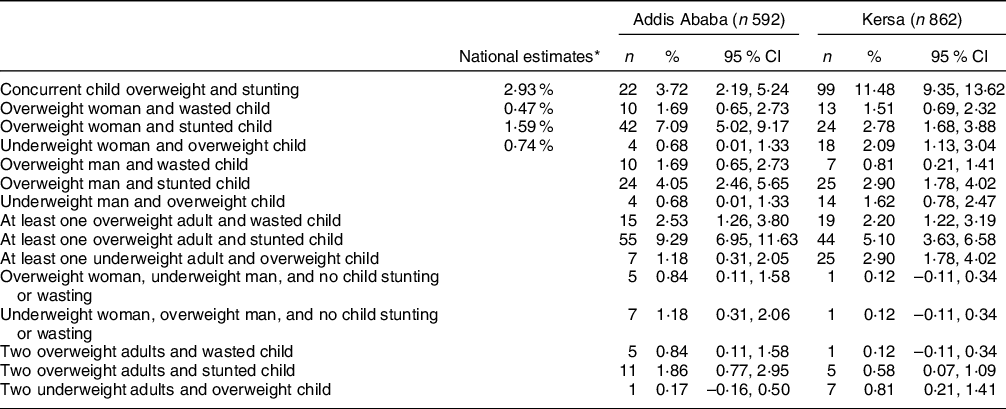
* Nationally representative estimates based on the 2016 Ethiopian Demographic and Health Survey.
Only estimates for the first four forms of household-level malnutrition available(Reference Popkin, Corvalan and Grummer-Strawn4).
In Addis Ababa, older children were less likely to be concurrently overweight and stunted (uOR 0·96 (95 % CI 0·94, 0·98)), whereas children of employed women, relative to unemployed, were more likely to be concurrently overweight and stunted 2·02 (95 % CI 1·14, 3·56) (Table 3). After correcting for multiple comparisons, only child age remained a significant factor. In the multivariate model, child age and women’s employment were similarly associated with concurrent child overweight and stunting (Table 4). However, only women’s employment was significant after correcting for multiple comparisons. In Kersa, more food supply shortages, women and men meeting MDD in the past 24 h, and improved housing floor quality were associated with lower odds of concurrent child overweight and stunting in univariate models (Table 5). In contrast, lower household wealth was associated with increased odds of this form of DBM (uOR 2·21 (95 % CI 1·58, 3·09)). After correcting for multiple comparisons, men’s MDD in the past 24 h, household wealth and housing floor quality remained significant associated factors. In the multivariate model, improved housing floor quality was associated with lower odds of concurrent child overweight and stunting, whereas low household wealth and access to an improved latrine were associated with higher odds (Table 6). However, only housing floor quality was significant after the p-value correction for multiple hypotheses testing. In the secondary analyses using a 7-d recall period for all diet indicators, diet did not predict concurrent child overweight and stunting in Addis Ababa in neither the univariate nor the multivariate analyses (see online Supplemental Tables 1 and 2). Women and men’s DDS and MDD in the past 7 d were significant associated factors of concurrent overweight and stunting in Kersa in univariate analyses (though only women’s DDS and MDD in the past 7 d remained significant after the multiple comparisons correction), but not in multivariate analyses (see online Supplemental Tables 3 and 4).
Table 3 Univariate factors associated with household-level forms of double burden of malnutrition in Addis Ababa

* P-value corrected for multiple hypothesis testing using the Benjamini–Yekutieli method to control the false discovery rate.
Table 4 Multivariate factors associated with household-level forms of double burden of malnutrition in Addis Ababa

* P-value corrected for multiple hypothesis testing using the Benjamini–Yekutieli method to control the false discovery rate.
Table 5 Univariate factors associated with household-level forms of double burden of malnutrition in Kersa

* P-value corrected for multiple hypothesis testing using the Benjamini–Yekutieli method to control the false discovery rate.
Table 6 Multivariate factors associated with household-level forms of double burden of malnutrition in Kersa

* P-value corrected for multiple hypothesis testing using the Benjamini–Yekutieli method to control the false discovery rate.
Older women’s age, longer duration of residence in Addis Ababa and larger household size were associated with higher odds of overweight woman and stunted child in Addis Ababa, though only the first two remained statistically significant after the multiple hypothesis correction (Table 3). In the multivariate model, duration of residence remained a significant factor (Table 4), though it was no longer significant after correcting for multiple comparisons. In contrast, in Kersa, we found no significant factors associated with overweight woman and stunted child in univariate models (Table 5). In the multivariate model, older child’s age was associated with higher odds of overweight woman and stunted child but did not remain significant after correcting for multiple hypothesis testing (Table 6). In the secondary analyses using the 7-d recall diet indicators, higher men’s dietary diversity was associated with higher odds of overweight woman and stunted child in the multivariate model for Addis Ababa (see online Supplemental Table 2), but in the univariate model or in Kersa.
Lastly, the univariate factors associated with overweight adult and stunted child in Addis Ababa were the same as those of overweight woman and stunted child: older women’s age, longer duration of residence in the city and larger household size (Table 3). Only women’s age and duration of residence remained significant after adjustment for multiple comparisons. In the multivariate model, longer duration of residence in the city, religion and larger household size were associated with higher odds of overweight adult and stunted child (Table 4). No factors remained significant after correction for multiple comparisons. However, in the analyses using the 7-d diet recall, higher women’s dietary diversity was associated with lower odds of overweight adult and stunted child, whereas higher men’s dietary diversity was associated with higher odds of this DBM form (see online Supplemental Table 2), though only the latter association remained statistically significant after correcting for multiple comparisons. In Kersa, older child age and higher number of food supply shortages were associated with increased odds of overweight adult and stunted child in univariate models, but estimates were no longer significant after correcting for multiple comparisons (Table 5). Likewise, in the multivariate model, older child age and higher number of food supply shortages remained associated with higher odds of this outcome, but only prior to adjusting for multiple comparisons (Table 6). None of these factors remained statistically significant after adjusting for multiple comparisons.
Discussion
We showed that similar forms of household-level DBM manifest in urban and rural Ethiopian households. Overweight adult and stunted child was the most prominent form of household-level DBM in Addis Ababa, followed by overweight woman and stunted child, and concurrent child overweight and stunting. Other forms of household-level DBM were virtually non-existent. In Kersa district, concurrent child overweight and stunting was most common affecting 11 % of households, with other forms of household-level DBM affecting <5 % of households. The primary factors associated with household-level DBM in Addis Ababa were longer duration of residence in the city, women’s employment and larger household size. In Kersa, more food supply shortages, low household wealth and improved household sanitation were factors associated with higher prevalence of household-level DBM, whereas improved housing floor quality was associated with lower prevalence.
The high prevalence of concurrent child overweight and stunting in Kersa district is of note. Several explanations are plausible. First, studies based on the developmental origin of health and disease (DOHaD) theory have shown that foetal growth restriction, low birth weight and being born small for gestational age (risk factors for childhood stunting(Reference Danaei, Andrews and Sudfeld29)) are associated with increased risk of childhood overweight and obesity(Reference Barouki, Gluckman and Grandjean33–Reference Hoffman, Reynolds and Hardy35). Second, child stunting is associated with impaired fat oxidation and decreased energy expenditures, both of which predispose to developing obesity(Reference Hoffman, Sawaya and Verreschi36,Reference Sawaya and Roberts37) . Third, Ethiopian diets, which are primarily cereal-based, high in carbohydrates, and low in protein, Zn, and vitamin A(38), could also help explain this high prevalence of concurrent child overweight and stunting. If children are receiving primarily energy-dense foods, this could help explain concurrent overweight (driven by high carbohydrate intake) and stunting (driven by low or inadequate micronutrient intake).
The household-level DBM prevalence we observed was much higher than the nationally representative Lancet estimates which showed that concurrent child overweight and stunting was the most prevalent form of household-level DBM at 2·93 %(Reference Popkin, Corvalan and Grummer-Strawn4). Malnutrition levels in our samples were generally similar to those for the Addis Ababa region and rural settings(2). The somewhat higher adult overweight prevalence and lower stunting prevalence in Addis Ababa and lower adult underweight prevalence in Kersa district (relative to national estimates) can help account for the differences between the nationally representative household-level DBM estimates and ours. Importantly, these differences in household-level DBM estimates highlight large within country disparities. As expected, household-level DBM forms involving an overweight adult were more prevalent in the urban area, in agreement with evidence from SSA(Reference Jones, Acharya and Galway6) and Ethiopia(Reference Dagnew and Asresie39).
The low prevalence of household-level DBM in Ethiopia can at least partially be attributed to more limited factors accelerating the global nutrition transition, for example, transformation of the global retail food system, increased supply of packaged and processed foods, and global diet shifts towards increased consumption of sugar-sweetened beverages (SSB), Na and fats(Reference Popkin, Corvalan and Grummer-Strawn4,Reference Popkin40) . The prohibition of foreign direct investment in food retail has eliminated the role of multinational supermarkets and led to slower roll-out of modern food retailers in the country, relative to other LMIC(Reference Assefa, Abebe and Lamoot41). Although the private retail sector in Ethiopia is growing in large urban settings, modern retail shops account for only 1–3 % of cereal, fruit, vegetable and processed foods sales in Addis Ababa(Reference Assefa, Abebe and Lamoot41). The absence of multinational food retailers has likely slowed the spread of low-cost pre-packaged processed and ultra-processed unhealthy foods and beverages that drive the rise of DBM in other LMIC(Reference Popkin, Corvalan and Grummer-Strawn4). In addition, the food supply remains high in complex carbohydrates and low in sugars and unhealthy fats. The increase in palm oil and milk as fat sources may be signalling the emergence of more highly processed foods. However, these changes are not yet representative of patterns exhibited by most LMIC that have experienced major shifts in food supply towards increasing fats and decreasing carbohydrates supply(Reference Sheehy, Carey and Sharma42). Lastly, Ethiopian diets are still low in non-essential foods and beverages. Ethiopia has the lowest Na(Reference Powles, Fahimi and Micha43) and trans fats(Reference Micha, Khatibzadeh and Shi44) consumption levels in the world, and lower SSB consumption than other LMIC(Reference Singh, Micha and Khatibzadeh45). Consumption of ultra-processed foods in Addis Ababa is also low(Reference Melesse, van den Berg and de Brauw46). In fact, Ethiopian diets are shifting towards high-value foods, for example, more expensive cereals, animal source foods, fruits and vegetables, and enset (a root crop known as false banana)(Reference Worku, Dereje and Minten47). All these factors together can help explain the low prevalence of household-level DBM in Ethiopia. However, many of these estimates of Na, trans fat and SSB consumption were derived from studies conducted nearly a decade before our data were collected. It is unclear how consumption trends have changed since then and how these changes may be associated with household-level DBM.
With respect to DBM factors, the only environmental factors associated with household-level DBM were duration of residence in Addis Ababa and food supply shortages in Kersa, both of which increased the odds of overweight adult and stunted child. The former likely worked through longer exposure to a relatively more obesogenic urban environment (albeit less obesogenic than other LMIC settings), whereas the latter by reducing consumption of nutritious foods. Of note is that despite the lower number of food supply shortages in Addis Ababa, food security was less prevalent in the Addis Ababa sample relative to the Kersa sample. This may be due to higher urban food prices: Addis Ababa residents likely purchase their food, whereas Kersa residents (who are primarily farmers) produce it. More work is needed to understand why distance to market was not associated with household-level DBM. Prior work in Ethiopia has shown that greater distance to market was associated with lower food consumption, higher food insecurity and less diverse diets, but not with women’s or children’s anthropometric outcomes(Reference Stifel and Minten48). However, this work was conducted in undernourished populations. In populations where overnutrition is more prevalent, greater distance to market could help reduce DBM by increasing energy expenditure. Future studies should be specifically designed to assess other aspects of the food environment hypothesised to determine DBM, such as food availability, prices, marketing and promotion(Reference Hawkes, Ruel and Salm49).
Importantly, adult dietary diversity was positively associated with concurrent child overweight and stunting in Kersa district, though only in univariate analyses. Women’s dietary diversity was an associated factor regardless of the recall period (both 24 h and 7 d), whereas men’s dietary diversity was an associated factor only in the primary analyses using a 24-h recall period. Although no other studies have documented the role of diet in household-level DBM in Ethiopia or SSA, our findings are generally in agreement with results from both high- and low-income settings indicating diverse diets rich in vitamin A-rich fruits and vegetables and animal source foods are protective of stunting and can reduce the burden of diet-related disease(Reference Hawkes, Ruel and Salm49), including obesity. The homogenous household diets in Kersa imply that as adults increase their dietary diversity so do their children, which protects them from concurrent overweight and stunting. In contrast, men’s dietary diversity was an associated factor for overweight woman/adult and stunted child in Addis Ababa, but only using a 7-d recall period. Several explanations for this association are plausible. Men might be allocated more nutritious foods within the household given their higher economic contribution (only 34 % of women in Addis Ababa were employed). They might purchase and consume more nutritious foods outside the home, reducing household food budget and thus increasing malnutrition risk among other household members. Alternatively, a more diversified diet may be higher in energy and micronutrients(Reference Ponce, Ramirez and Delisle50), which could translate into higher likelihood of adult overweight. Of note is that we lacked data on intra-household food distribution, out-of-home food consumption, and not all men we interviewed were biological fathers of the children or the husband/partner of the woman. Thus, we can only speculate on the exact mechanisms through which adult diet may help reduce the odds of concurrent child overweight and stunting. Moreover, the men’s dietary diversity indicator we used has not been validated for use among men and it might not adequately capture micronutrient intake. Future research should carefully assess the role of men’s diet in household-level DBM.
Our results should be interpreted with several other caveats. First, the diet assessment did not collect information on portion size, frequency of food consumption or out-of-home food consumption, all of which contribute to excess adiposity. The dietary diversity indicators we used are designed to assess maternal and child health and have not been validated against non-communicable disease risk(Reference Miller, Webb and Micha51). Their cultural appropriateness in Ethiopia has not been assessed either. Moreover, DDS and MDD have only been validated among Burkinabe children of 24–59 months of age(Reference Diop, Becquey and Turowska28), and more work is needed to assess the validity of these measures in children of 24–59 months of age in other settings. Second, our surveys were not explicitly designed to study DBM. We lacked data on other indicators used in household-level DBM definitions (e.g. anaemia, micronutrient deficiencies, diabetes(Reference Davis, Oaks and Engle-Stone3), hypertension and dyslipidaemia), and other factors associated with DBM (e.g. early-life experiences, urban and built environment, physical activity(12)). Thus, we may be underestimating the burden of malnutrition in all its forms. Factors such as physical activity may be particularly relevant in urban settings, typically characterised by high availability of cheap, energy-dense foods and reduced occupational physical activity and active transport(Reference Ford, Patel and Narayan52). Third, our sample in Kersa district included both rural and urban kebeles. Although the entire district is considered rural, the urban kebeles are small towns with permanent electricity supply and it is possible that they differ from rural kebeles in ways that might be important for DBM. In addition, including these urban kebeles may limit the generalisability of our findings to other rural areas. Fourth, data were collected in different periods in the year: the dry season in Addis Ababa and the rainy season in Kersa district. Although adult and child diets appeared similar in our samples, it is possible that this seasonality influenced other DBM factors which we did not account for in our analyses. Finally, any analysis aiming to understand all the factors associated with household-level DBM will suffer from a problem of multiplicity. To address this issue, we presented both standard P-values and P-values corrected for multiple comparisons. However, by applying this correction, we inevitably increase the risk of type II error (false-negatives). Our selection of factors was based on the WHO framework on the drivers of DBM(12), and we therefore can be confident that all factors included in our models are theoretically important. However, due to this problem of multiplicity, our small sample size, the low prevalence of some associated factors and the low prevalence of household-level DBM, we were unable to determine the direction and magnitude of the associations in our samples with sufficient statistical certainty. These statistical issues are evidenced by the opposing associations between DBM, low household wealth, improved household sanitation and improved housing floor. Although significantly negatively correlated, low household wealth and improved household sanitation were both associated with increased risk of concurrent child stunting and overweight in Kersa.
Nevertheless, our findings have implications for interventions to address both undernutrition and overnutrition. Despite the low household-level DBM prevalence, the co-occurrence of undernutrition and overweight at the population level remains a problem as evidenced by the high prevalence of women’s overweight in Addis Ababa (37·2 %), high prevalence of child stunting in both Addis Ababa and Kersa (18·6 % and 56·6 %, respectively), and high prevalence of child underweight and wasting in Kersa (33·5 % and 19 %, respectively) we observed. Examining the associated factors with population-level undernutrition and overweight was beyond the scope of this article, since multiple prior studies have assessed the national prevalence and associated factors of population-level DBM in Ethiopia(Reference Yeshaw, Kebede and Liyew7–Reference Gutema, Chuka and Kondale11). However, given the high population-level prevalence of both forms of malnutrition, double-duty actions, that is, actions which simultaneously address multiple forms of malnutrition(Reference Hawkes, Ruel and Wells53), should be targeted at the population level to help alleviate the larger population-level burden of malnutrition. Further, our findings indicate that double-duty interventions should target women and children given the higher (albeit generally low) prevalence of household-level DBM involving these individuals. Several successful double-duty interventions(Reference Hawkes, Ruel and Salm49) can help Ethiopia address DBM: breast-feeding promotion, complementary feeding education, social safety nets, nutrition-sensitive agriculture and food policy. Social and behaviour change communication (SBCC) programmes which have been shown to increased exclusive breast-feeding rates(Reference Kim, Rawat and Mwangi54) should also include messaging to reduce maternal obesity and micronutrient deficiencies, which alter the biology of lactation(Reference Wells, Sawaya and Wibaek13). Breast-feeding and complementary feeding education can also be included in the SBCC strategy of the Productive Safety Net Programme (PSNP). This safety net programme can further be leveraged to address DBM by redesigning the food transfer (e.g. to provide nutritious foods or earmark it for women and young children) and/or including messages on nutritious and healthy diets for the whole family. Likewise, the national homestead gardening programme already includes a SBCC strategy promoting breast-feeding and complementary feeding practices. Improving the take up (currently as low as 7·5 % of target households in some areas(Reference Hirvonen and Headey55)) can also serve to address DBM through the SBCC strategy but also through the direct production of healthy and nutritious foods. Lastly, additional policy changes can help support DBM prevention efforts. Specifically, the elimination of promotion of breast-feeding substitutes and formula is paramount to bolster breast-feeding promotion efforts(Reference Hawkes, Ruel and Salm49,Reference Jaacks, Kavle and Perry56,57) .
Conclusion
We showed that concurrent child overweight and stunting, and overweight woman or adult and stunted child were the most prevalent forms of household-level DBM among urban and rural Ethiopian households. Environmental, socio-economic and demographic factors emerged as the most prominent factors associated with these DBM forms. Despite the low household-level DBM prevalence we observed, there was a high burden of child undernutrition and maternal overweight at the population level. Interventions to address DBM should focus on women and young children at the population level, who experienced the highest burden. Promising double-duty actions include breast-feeding promotion, complementary feeding education, redesigning the food transfer of the national safety net programme, improving take up of the national homestead gardening programme and food policy interventions. Ethiopia has already demonstrated strong political commitment towards reducing all forms of malnutrition, which features prominently in the country’s strategic policies and plans. The National Nutrition Programme already outlines ways and initiatives to improve multisectoral coordination and implementation capacity(58), indicating that the political commitment and logistical, financial and implementation capacity for these double-duty interventions exists. Moving forward, DBM targets and double-duty initiatives should be explicitly included in national nutrition policies and programmes to help Ethiopia eliminate all forms malnutrition at the household and population level.
Acknowledgements
Acknowledgements: The authors would like to acknowledge the study participants and data collectors for their time and for making the study possible. Financial support: The Addis Ababa study was funded by the United Kingdom Economic and Social Research Council (ESRC) and Department for International Development (DFID) Joint Fund for Poverty Alleviation Research (grant number: ES/R002118/1). The Kersa study was funded by Harvard T.H. Chan School of Public Health Office for Research Strategy and Development (grant number: not applicable). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflicts of interest: There are no conflicts of interest. Authorship: L.B. conceptualised the analyses presented in this paper. Y.B., A.W.T., G.D., C.R.C. and W.W.F. designed and implemented the Addis Ababa study. A.W.T. and Y.B. led the Addis Ababa data collection activities. N.A. and W.W.F. designed the Kersa study, and N.A., E.C.H. and W.W.F. implemented it. N.A. led the Kersa data collection activities. L.B. led the data analyses and drafted the manuscript. All authors critically reviewed the manuscript, contributed to the intellectual content and approved the final manuscript. L.B. had final responsibility for submitting this article for publication. Ethics of human subject participation: Written informed consent was obtained by fieldworkers who provided information about the study to each participant. The Addis Ababa study received ethical approved by the institutional review boards of the Addis Continental Institute of Public Health (reference number ACIPH/IRB/001/2018) and the Harvard T.H. Chan School of Public Health (reference number: IRB17-1825). The Kersa study received ethical approval by the Institutional Health Research Ethical Review Board of the College of Health and Medical Sciences at Haramaya University (reference number: SHE/SIM/144/708/19) and by the institutional review board of the Harvard T.H. Chan School of Public Health (reference number: IRB19-0029).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003700









