Chronic low-grade inflammation has been implicated in many chronic diseases, including cancer(Reference Zhou, Yang and Nie1), CVD(Reference Alfaddagh, Martin and Leucker2) and type 2 diabetes(Reference Tsalamandris, Antonopoulos and Oikonomou3). Diet has the potential to influence systemic inflammatory status(Reference Barrea, Muscogiuri and Frias-Toral4). For instance, n-3 fatty acids from fish can have anti-inflammatory and immune function effects(Reference Ghasemi Fard, Wang and Sinclair5). Dietary fibre can also have anti-inflammatory effects, possibly mediated by the intestinal microbiome(Reference Hills, Pontefract and Mishcon6). In contrast, SFA have pro-inflammatory effects(Reference Candido, Valente and Grzeskowiak7). Thus, a person’s diet can contain a mix of pro- and anti-inflammatory foods and nutrients.
The overall inflammatory potential of a person’s diet can be summarised by calculating a summary score, such as the Dietary Inflammatory Index (DII)(Reference Shivappa, Steck and Hurley8). The underlying basis for this index consists of evidence of associations between specific foods or nutrients and systemic inflammation. A growing number of studies have investigated associations between DII and mortality(Reference Brlek and Gregorič9–Reference Shivappa, Steck and Hussey14). A recent umbrella review of previous systematic reviews, evaluating health outcomes associated with adherence to different diet indices, indicated that a pro-inflammatory diet, as measured by the DII, was associated with increased all-cause and CVD mortality(Reference Brlek and Gregorič9). Similarly, a meta-analysis of previous studies suggested that a more pro-inflammatory diet, measured by DII scores, was associated with higher cancer mortality(Reference Fowler and Akinyemiju10–Reference Shivappa, Steck and Hussey14).
Previous studies have predominantly included subjects from Europe and North America—evidence from Australia is still very limited. A cohort study of older women in Western Australia showed increased CVD mortality among those with a more pro-inflammatory diet(Reference Bondonno, Lewis and Blekkenhorst15). Similar associations were seen in a study of older adults in Melbourne, particularly those of Northern European ancestry(Reference Hodge, Bassett and Dugué16), but more evidence from general population samples in Australia is needed.
We therefore investigated associations between the inflammatory potential of the diet and mortality in a community-based sample of Australian adults, including consideration of the timing of deaths in relation to age. Based on prior evidence, we hypothesised that a pro-inflammatory diet, as measured by the DII, would be positively associated with mortality, including CVD and cancer mortality.
Methods
Study sample
We used data from the Nambour Skin Cancer Prevention Study, a prospective cohort study in Queensland, Australia. Participants aged 20–69 years were randomly selected from the electoral roll (voting is compulsory in Australia) for a survey of skin cancer prevalence in 1986. In 1992, 1621 of the original survey participants were enrolled in a skin cancer prevention trial (1992–1996). In a two-by-two factorial design, participants were randomly allocated to either daily or discretionary sunscreen use and daily β-carotene supplement or placebo group(Reference Green, Williams and Neale17,Reference Green, Battistutta and Hart18) .
Dietary assessment
We used dietary intake data collected using a self-administered, semi-quantitative FFQ completed by Nambour Study participants in 1992, 1994 and 1996. The FFQ was initially created for the Nurses’ Health Study in the USA and was adapted for the Nambour Study to ensure the list of foods appropriately represented the Australian diet(Reference English, Cashel and Bennett19). The FFQ was validated in this study population which indicated exact agreement with weighed food records between 30 % and 50 % for almost all nutrients and for black tea (63 %)(Reference Marks, Hughes and van der Pols20,Reference Marks, Hughes and van der Pols21) . Participants were asked to record their usual intake of 129 foods or food groups during the previous 6 months. A common serving size was specified, and participants were asked to estimate the frequency at which they ate the given amount of food. The nine response options for frequency ranged from ‘never’ to ‘4+ times per day’. Seasonal variation was accounted for by asking participants to state their consumption frequency of seasonal foods to which a weighting factor was applied based on typical duration of availability. Food intake in grams was calculated by multiplying the standard serving size of each food by the consumption frequency, expressed as a proportion of daily use. Nutrient intakes were calculated using the Australian food composition tables, NUTTAB95(22). Participants with energy intakes outside the normal ranges (500–3500 kcal/d for women and 800–4000 kcal/d for men)(Reference Willett23) were excluded. Nutrient intake from supplements was analysed using a specially designed supplement database(Reference Ashton, Ambrosini and Marks24). Nutrient intakes from both foods and supplements were included in the present analysis.
To assess the inflammatory potential of the dietary intakes of our study participants, we calculated DII scores using the DII and methods developed by Shivappa and colleagues(Reference Shivappa, Steck and Hurley8). The DII was created by applying a weighted scoring algorithm based on published literature on the relationship between forty-five food parameters and six inflammatory biomarkers. The DII comprises forty-five weighted scores, each summarising the inflammatory effect of one food parameter. To calculate a DII score for an individual’s diet, we first calculated the energy-adjusted mean intake for each food parameter (except for tea intake) from each of the three FFQ, which was then averaged for each individual. This was converted to a z-score using standardised values from eleven nutritional databases. The z-score was then converted to a percentile using the PROBNORM function in SAS (SAS Institute Inc.), centred and multiplied by the relevant literature-derived inflammatory effect score. The results for individual food parameters were summed to provide a DII score for the participant(Reference Shivappa, Steck and Hurley8). The resulting DII scores for all participants represent a continuum from anti- to pro-inflammatory diets, with lower DII scores indicating a less inflammatory diet and higher scores indicating a more inflammatory diet.
For our analysis, thirty-one of the possible forty-five food parameters that comprise the DII were available and were used to calculate DII scores (including ethanol, β-carotene, carbohydrate, cholesterol, energy, fat, fibre, iron, magnesium, monounsaturated fat, niacin, n-3 fatty acid, n-6 fatty acid, protein, polyunsaturated fat, riboflavin, saturated fat, selenium, thiamine, retinol equivalents, vitamin C, vitamin D, vitamin E, flavan-3-ols, flavones, flavonols, flavanones, anthocyanidins, isoflavones, black tea and zinc). The literature supports the calculation of DII scores based on between twenty-two and thirty-six food parameters(Reference O’Neil, Shivappa and Jacka25,Reference Graffouillere, Deschasaux and Mariotti26) .
Outcome assessment
The outcomes of interest were mortality due to all causes and mortality due to CVD, cancer or other causes (referred to as other-cause mortality hereafter, which included unclassified death events). Mortality and cause of death data were obtained for all participants from 1992 to 31 March 2022 from the National Death Index of Australia and the Queensland Registry of Births, Deaths and Marriages. The cause of death was classified per the WHO International Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Mortality due to CVD was defined by the presence in the death certificate of an ICD-10 code in the range I25–I29 (CHD) and I60–I69 (stroke) and cancer mortality by a code in the range C00–C97. Causes of death not otherwise classified as CVD or cancer were analysed collectively as other causes. Additionally, a subject could be classified as having died from multiple causes, including CVD and cancer.
This study was approved by the Human Research Ethics Committee of the QIMR Berghofer Medical Research Institute, and all participants gave written informed consent.
Statistical analysis
Cox proportional hazard regression was performed to calculate hazard ratios (HR) and 95 % CI. Continuous DII scores were ranked from lowest to highest and divided into four sex-specific groups; the least inflammatory DII scores were in quartile 1, and the most inflammatory DII scores were in quartile 4. HR were estimated for higher compared with the lowest quartile group. To test for linear trends, we assigned an ordinal number ranging from 1 (lowest quartile) to 4 (highest quartile) and modelled this value as a continuous variable. We also examined DII scores as a continuous variable, wherein the estimated HR indicated the increased risk per one-unit increase in DII score.
In models for cause-specific mortality, each death is attributed exclusively to only one cause, creating a ‘competing risk’ situation where the assumption that subjects will experience an event of interest if followed up for long enough, no longer holds due to the occurrence of another event(Reference Andersen, Geskus and de Witte27). To correct for bias from competing risks in our analysis, we fit standard proportional sub-distribution hazard models using the %PSHREG macro in SAS(Reference Kohl, Plischke and Leffondre28).
All analyses were adjusted for age using age as the time scale and for the randomised intervention groups (daily sunscreen and/or β-carotene groups). Additionally, all models were adjusted for smoking status and medical conditions (self-reported diabetes, hyperlipidaemia, hypertension, angina, heart attack, stroke, cancer and medication use for cardiac disorders or diabetes). We also adjusted models for sex by including this covariate in the modelling of continuous DII scores. For quartile analyses, sex-specific quartiles were calculated when DII scores were categorised. Additional adjustments for other potential confounding variables, including education, BMI, physical activity and dietary supplement use, did not alter results markedly. The proportional hazard assumption for models on all-cause mortality was assessed using the ASSESS option of PHREG in SAS. Weighted Schoenfeld-type residuals were used to evaluate possible violation of the proportional sub-distribution hazard assumption by the cause-specific mortality models using the %PSHREG macro in SAS version 9.4(Reference Kohl, Plischke and Leffondre28). In models that considered DII scores as a continuous variable, the proportionality of hazards was not met; thus, we further assessed models in attained age groups ≤ 55, 56–65, 66–75, 76–85 and 86–96 years old.
Restricted cubic spline regression using the %RCS_reg macro was used to check for possible non-linearity of the association between DII analysed as a continuous variable and mortality in the Cox proportional hazard model, with three knots located at the 5th, 50th and 95th percentiles(Reference Desquilbet and Mariotti29). Results of the restricted cubic spline analysis showed no evidence of a non-linear association between continuous DII scores and all-cause mortality (P = 0·36), CVD mortality (P = 0·99), cancer mortality (P = 0·70) and other-cause mortality (P = 0·61).
The cause of death for thirty-three recent deaths was unspecified in the National Death data. In the main analyses, we classified these cases as other causes. However, in a sensitivity analysis, we excluded these deaths from the analysis of other causes of death.
P < 0·05 was considered statistically significant (two-sided). All analyses were carried out using SAS software version 9.4.
Results
Of the 1621 Nambour Study participants, 1529 completed at least one FFQ. Overall, 1032 (68 %) participants completed three valid FFQ, 284 (19 %) completed two FFQ and 213 (14 %) participants completed one FFQ. Participants with missing information on whether they suffered from a medical condition were excluded (eighty-nine), resulting in a sample of 1440 study participants for the present analysis. A comparison of baseline characteristics of included and excluded participants indicated that those who were included were somewhat older (49·6 ± 13·1 years v. 46·7 ± 13·5 years, respectively; P = 0·006) and more often used dietary supplements (54 % v. 35 %, respectively, P < 0·001), but they were otherwise the same as those excluded.
Participant characteristics at baseline, by DII quartile, are presented in Table 1. The mean (±sd) DII score was –0·08 ± 2·15, and scores ranged from –6·04 to +6·65. A one-unit increment of DII scores corresponded with approximately 8 % of the total range of DII scores in this population. Participants in the lowest quartile, thus with the least inflammatory diet, were older, had a slightly higher BMI, were more likely to have a medical condition, were more likely to be physically active, were less likely to be smoker and were more likely to use a dietary supplement (Table 1). There were no associations between DII categories and education level, BMI or the randomised trial treatment groups (β-carotene supplement or daily sunscreen use).
Table 1 Participant characteristics by Dietary Inflammatory Index (DII) quartile group at baseline, Nambour study, 1992 (n 1440)

BMI, Basal Metabolic Index; Q1–4, quartile groups 1–4; IQR: interquartile range.
* P from ANOVA.
† P from Pearson χ 2.
‡ P from Mann–Whitney U test.
§ Participants responded yes to the presence of a medical condition if they had been told by a doctor or nurse that they had at least one of the following, diabetes, high cholesterol, high triglycerides, high blood pressure, angina, heart attack, stroke or cancer, or were taking medication for a cardiac disorder or diabetes.
In total, 488 participants died between 1992 and 2022, including 188 deaths from CVD, 151 from cancer and 170 from other causes. DII categories showed a significant, positive association with all-cause mortality. Participants in DII quartile groups 2, 3 and 4 were more likely to die compared with participants in quartile 1, independent of confounders (HRQ2 v. Q1 = 1·38; 95 % CI 1·09, 1·75; HRQ3 v. Q1 = 1·42; 95 % CI 1·10, 1·83; HRQ4 v. Q1 = 1·55; 95 % CI 1·19, 2·03; P < 0·001) (Table 2). The association with continuous DII scores showed a similar relationship (Table 3), with a one-unit increase in DII corresponding with a 12 % increased risk of all-cause mortality (adjusted HR per one-unit increment of DII score = 1·12; 95 % CI 1·05, 1·19). This association was stronger among middle-aged participants (aged ≤ 55 years, 1·25; 95 % CI 1·11, 1·41; and aged 56–65 years, 1·18; 95 % CI 1·10, 1·19) and gradually attenuated among older-aged participants (Table 3).
Table 2 Associations between Dietary Inflammatory Index and mortality, Nambour study, 1992–2022
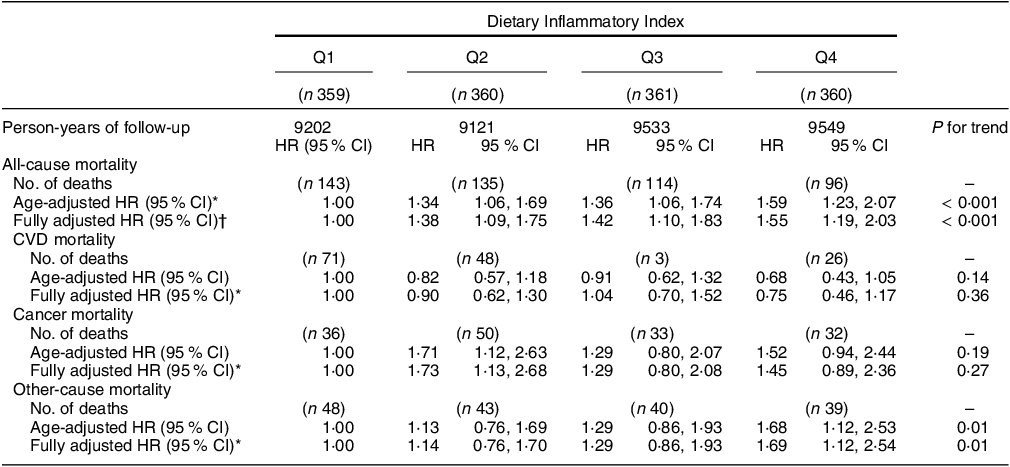
Q1–4, quartile groups 1–4; HR, hazard ratio.
* Adjusted for age only.
† Adjusted for age, medical conditions, smoking status and randomised trial treatment groups (β-carotene and/or daily sunscreen use). Other covariates such as dietary supplement use, BMI and physical activity did not confound these estimates.
Table 3 Risk of all-cause and cause-specific mortality in relation to Dietary Inflammatory Index (as a continuous variable), overall and at different age-at-death categories, Nambour study, 1992–2022

HR, hazard ratio; SHR, sub-distribution hazard ratio.
* Adjusted for age (by using age as the time scale) and sex, medical conditions, smoking status and randomised trial treatment groups (β-carotene and/or daily sunscreen use).
† The number of deaths indicated in each age category is the number of deaths up to that age, for example, all deaths in participants up to age 55 years for the ≤ 55 year group, all deaths that occurred in participants aged 56–65 years for the 56–65 year category, etc.
There was no significant association between DII categories and CVD mortality (HRQ4 v. Q1 = 0·75; 95 % CI 0·46, 1·17; P = 0·36) or when DII scores were considered as a continuous variable (adjusted HR per one-unit increment of the DII score = 0·96; 95 % CI 0·87, 1·06). However, a one-unit increase in continuous DII was associated with a 36 % (HR, 1·36; 95 % CI 1·04, 1·78) increased risk of CVD mortality among those younger than 55 years of age; this association was attenuated among older-aged participants (e.g. aged 56–65 years, 1·14; 95 % CI 0·97, 1·35) (Table 3).
There was no association between a pro-inflammatory diet and risk of death from cancer (HRQ4 v. Q1 = 1·45; 95 % CI 0·89, 2·36; P = 0·27), and there was no trend of associations across the quartile groups (Table 2). When DII was treated as a continuous variable (Table 3), the data suggested an increased risk of cancer deaths for persons with higher DII scores (adjusted HR per one-unit increment of the DII score = 1·08; 95 % CI 0·98, 1·19). Similar to CVD mortality, the risk of cancer mortality was particularly increased among middle-aged participants (aged ≤ 55 years, 1·20; 95 % CI 1·02, 1·40; and aged 56–65 years, 1·11; 95 % CI 1·00, 1·23) and not among older-aged participants.
Participants with the most pro-inflammatory DII scores were more likely to die from other causes (HRQ4 v. Q1 = 1·69; 95 % CI 1·12, 2·54), but there was no association with lower DII categories (Table 2). A one-unit increase in continuous DII score was associated with an increase in mortality risk of 12 % from other causes (adjusted HR per one-unit increment of DII score = 1·12; 95 % CI 1·02, 1·22), and this association was also particularly apparent among middle-aged participants (Table 3). Exclusion of deaths with unknown cause from the analyses did not change these results (data not shown). Exclusion of the variable medical condition, which may be a mediator of associations between DII and mortality, did not substantially change results. For example, the estimate for overall mortality per unit increase in DII was HR 1·10, 95 % CI 1·04, 1·17, when medical condition was removed from the model, compared with HR 1·12, 95 % CI 1·05, 1·19, for the fully adjusted model (Table 3).
Discussion
Our results indicated that a more pro-inflammatory diet was associated with increased all-cause mortality. This association was strongest for deaths among younger (middle)-aged participants compared with older-aged participants. The cause-specific mortality analyses showed similar patterns of associations, with a more pro-inflammatory diet being associated with increased mortality due to other causes and with increased deaths due to CVD and cancer among middle-aged participants (≤ 55 years and ≤ 65 years, respectively).
These findings are broadly consistent with previous evidence(Reference Brlek and Gregorič9), including two other Australian studies(Reference Bondonno, Lewis and Blekkenhorst15,Reference Hodge, Bassett and Dugué16) , thus supporting the notion that a more pro-inflammatory diet likely increases overall mortality, independent of other risk factors. No previous studies have assessed the timing of this association in terms of age at death, but our data suggest that this association is strongest for deaths that occur in middle age compared with old age. We cannot determine the exact reasons as to why these associations differed by age at death; however, it is possible that a pro-inflammatory diet may cause an acceleration of underlying disease mechanisms such as systemic inflammation, atherosclerosis and other common pathophysiology associated with chronic diseases and death, making this effect more easily detectable in middle age. Moreover, in old age, a multitude of different factors impact the probability of dying, including a person’s history of smoking, physical activity and obesity(Reference O’Doherty, Cairns and O’Neill30–Reference Borodulin, Karki and Laatikainen32), making it more difficult to tease out the influence of dietary factors. It is also possible that dietary exposures earlier in life are more influential and critical in terms of longer-term risk of disease and death. These findings are in line with and corroborate global food-based dietary guidelines including those in Australia that promote consumption of fruits and vegetables with their anti-inflammatory health benefits throughout the life course(33).
Among cause-specific deaths, the inflammatory potential of diet was not associated with CVD deaths overall; it was only associated with deaths that occurred among those younger than 56 years of age when the DII score was treated as a continuous variable. A prior meta-analysis of CVD mortality outcomes indicated significant positive associations between DII scores and CVD mortality(Reference Ji, Hong and Chen34); however, the sample size in our study was relatively small compared with previous studies, which may have restricted our ability to detect statistically significant relationships (e.g. there were 2399 CVD deaths out of 33 747 participants in a previous Swedish study(Reference Shivappa, Harris and Wolk35)). Also, different dietary assessment methods were used in previous studies, and DII scores were calculated using a varying number of food parameters out of the possible forty-five, which may explain some of these differences.
Our results suggested that a more pro-inflammatory diet may be associated with cancer deaths, but this was also not statistically significant in the overall analyses. When we considered DII scores as continuous variable, these were associated with cancer deaths in those younger than 65 years (similar to our observation for CVD deaths). Inflammation is a consistent feature of the cancer microenvironment(Reference Elinav, Nowarski and Thaiss36), and several complex, inflammation-induced, cancer-promoting pathways have been identified(Reference Grivennikov, Karin and Terzic37,Reference Yu, Pardoll and Jove38) . While we could not analyse associations with different cancer types, the literature indicates that the strongest associations with a pro-inflammatory diet are generally for digestive tract cancer mortality(Reference Deng, Shivappa and Tang11,Reference Shivappa, Blair and Prizment12,Reference Tabung, Steck and Ma39) , and specific analyses of obesity-related cancers would also be of interest in future studies.
A more pro-inflammatory diet was associated with increased other-cause mortality in our study, both when DII scores were considered in quartile groups and as continuous variable. Again, this association was particularly seen in relatively younger-aged participants (before 75 years of age) compared with older age. While the causes of death included in this category are varied, there are a number of death causes within this group that are particularly associated with inflammation, including respiratory diseases such as pneumonitis, pneumonia, chronic obstructive pulmonary disorder and chronic bronchitis(Reference Varraso, Willett and Camargo40). Inflammation is also thought to contribute to Alzheimer’s disease development(Reference Gardener, Rainey-Smith and Martins41,Reference Huang, Jin and Zhou42) . Thus, there are different possible disease mechanisms and death causes that could explain this association with other-cause mortality. The distribution of causes of death in this other-cause mortality group in our study was comparable with those reported in national data(43).
This study has strengths and limitations. The data were collected from a randomly selected community-based sample of Australian adults, thereby increasing the generalisability of the results. The use of repeated FFQ and the long follow-up are also the strengths of this study. We used a prospective study design, which reduced bias and possible reverse causation associated with some other study designs. Unlike many other studies, we adjusted for competing risks, which could otherwise influence results. Our study included a long follow-up period (1992–2022), and repeated measures of usual dietary intake were used to estimate DII scores where possible, thus ensuring that a more reliable estimate of long-term nutritional habits could be obtained; however, change in dietary habits over time may have occurred. The DII is an evidence-based index validated against multiple biomarkers in previous studies(Reference Shivappa, Steck and Hurley8), and while we did not use a more recently developed energy-adjusted version of DII(Reference Hebert, Shivappa and Wirth44), our nutrient intake estimates were adjusted for energy within our study population before calculating z-scores, thereby removing potential confounding by energy intake. Due to the relatively small study population, we used relatively broad cause of death groupings to optimise statistical power by maximising the number of deaths that could be considered in each group (the main determinant of statistical power in these analyses), which may have hidden associations with individual disease outcomes. However, associations were apparent in some of the sub-groups with a relatively small number of deaths.
In conclusion, our findings generally support the notion that a pro-inflammatory diet is associated with increased mortality, in particular among middle-aged adults. Our results support the promotion of anti-inflammatory diets to help promote longevity.
Acknowledgements
We thank the Nambour Skin Cancer Study participants for their interest and support. We acknowledge Professor Adele Green for her longstanding leadership of the Nambour Study and for providing support for these data analyses. We also acknowledge Professor Gail Williams and Associate Professor Geoff Marks for their involvement in research design and data collection of the original Nambour trial.
Financial support
This study was supported by the National Health and Medical Research Council of Australia (data collection).
Conflict of interest
The authors declare no conflict of interest.
Authorship
J.C.v.d.P., M.C.B.H., K.M., A.M., I.K.W.: conceptualised these analyses and were responsible for the methodology and statistical analysis. A.M., J.C.v.d.P., I.K.W.: wrote the first manuscript drafts. All authors provided critical revisions of the manuscript and approved the final manuscript.
Ethics of human subject participation
The Nambour Study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (HREC). Written informed consent was obtained from all subjects/patients.






