Iodine is an essential trace element for the synthesis of thyroid hormones and thyroxine is also critical for brain development. Iodine deficiency may cause hypothyroidism and results in severe developmental delay in infants and stillbirth in pregnant women( 1 – Reference Takeuchi, Kamasaki and Yoto 3 ). WHO, UNICEF and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) endorse the policy of iodine supplementation for pregnant and breast-feeding women in iodine-deficient countries where salt iodization is not regulated or is ineffective, in order to improve the nutritional status of iodine( Reference Andersson, de Benoist and Delange 4 ).
However, iodine deficiency and excessive exposure to iodine due to environmental or artificial factors may lead to thyroid dysfunction( Reference Pennington 5 ). Recommended by WHO, ICCIDD and UNICEF, the optimal iodine intake is 150–300 μg/d (median urinary iodine concentration (UIC): 100–199 μg/l)( 6 ). Iodine intake exceeding the optimal level will increase the risk of thyroid dysfunction. Chronic iodine intake in the form of iodide is associated with an increase in goitre and subclinical hypothyroidism due to the inhibition of thyroid hormone synthesis( Reference Stanbury, Ermans and Bourdoux 7 ). Excessive iodine intake can also trigger an immune response, resulting in autoimmune thyroiditis( Reference Zimmermann 8 ), thyroid inflammation, sensitive reaction or acute toxicity( Reference Pennington 5 , Reference Stanbury, Ermans and Bourdoux 7 ). It has been reported that excessive iodate intake has a minimal impact on thyroid hormones. Hypothyroidism or hyperthyroidism was induced in some individuals receiving iodine supplementation. Most abnormalities disappeared after 4–12 weeks( Reference Stanbury, Ermans and Bourdoux 7 , Reference Thomson, Campbell and Miller 9 ). However, in our previous study, we found that thyroid dysfunction induced by excessive iodine intake can last for a longer period. The issue regarding the safety of iodine exposure is still controversial and further studies on excessive iodine exposure are highly desired( Reference Sang, Wang and Yao 10 ).
Universal salt iodization was first introduced in China in 1996, and iodine deficiency disorders are currently under control in most areas( Reference Chen, Yan and Shu 11 – Reference Shen, Zhang and Liu 13 ). However, there are still more than ten provinces with waterborne excessive iodine. In these endemic areas, more than 30 million people are exposed to excessive iodine exposure( Reference Chen, Yan and Shu 11 – Reference Zhao, Wang and Shang 14 ). With the help of universal salt iodization, iodine intake has increased nationwide( Reference Teng, Shan and Teng 15 ). However, only a few data have demonstrated the epidemiological characteristics of populations with chronic, more than adequate or excessive iodine intake( Reference Teng, Shan and Teng 15 – Reference Li, Guan and Teng 17 ).
In order to understand the effect of excessive iodine intake on thyroid health, a cross-sectional survey was conducted in two regions with different iodine intake levels. Excessive iodine in water was 841 μg/l and 394 μg/l in Haixing County; adequate iodine in water was 13 µg/l in the same county, as control. The effect of persistent local excessive iodine intake on the prevalence of thyroid diseases was evaluated.
Methods
Participants
Haixing County, a county in Cangzhou City, is located in the south-east of Hebei Province. It is a coastal city with 0·22 million permanent residents and a total area of 960 km2. The study was carried out in April 2010. The criteria for evaluating regions with excessive waterborne iodine, or endemic areas, were according to the national standards (GB/T19380-2003) of China. According to the records of the local Center for Disease Control and Prevention, of the villages in Haixing County, Zhaogao Village and Fuzhuangzi Village were in the excessive iodine area (EIA) and Xiaoshan Village was in the adequate iodine area (AIA).
All inhabitants living in the research areas completed questionnaires concerning the history and medication of thyroid disease. Comparison of demographic characteristics between questioned inhabitants and examined participants did not reveal any significant differences (Table 1). The age of all participants was in the range of 20–50 years and these people had lived in the surveyed areas for over 5 years.
Table 1 Urinary iodine concentration and distribution in men and women aged 20–50 years who had adequate or excessive drinking water iodine, Hebei Province, People’s Republic of China, 2010

UIC, urinary iodine concentration; IQR, interquartile range; AIA, adequate iodine area; EIA, excessive iodine area; Wilcoxon, Wilcoxon rank sum test; χ 2, χ 2 test.
Research protocols were approved by the Medical Ethics Committee of Tianjin Medical University. All participants provided written informed consent after the research protocols were carefully explained to them.
Sample collection
Blood samples were collected from all participants during 08.00–12.00 hours. Serum samples were separated and stored at −20°C until analysis. Casual morning (between 08.00 and 10.30 hours) urine samples were collected from all participants in clean plastic specimen containers and stored at 4°C. The drinking water of all three villages was extracted underground and supplied by the local government through a pipe network system. Every village owns its particular underground water source. Peripheral drinking water samples were collected in five different positions such as south, north, east, west and middle of every surveyed village. Two drinking water samples were sampled from two different families in each position. The salt was not analysed because the iodized salt was not regulated in these counties.
Concentrations of iodine in drinking water and urine
The concentrations of iodine in drinking water and urine were measured by the national standard method as described previously( Reference Sang, Wang and Yao 10 ).
Thyroid hormones and autoantibodies
The contents of free thyroxine (FT4), free triiodothyronine (FT3) and thyroid-stimulating hormone (TSH) in serum from all participants were measured by an automated chemiluminescent immunoassay using diagnostic kits from Bayer Health Care, Siemens. The normal ranges were 11·5–23·5 pmol/l for FT4, 3·5–6·5 pmol/l for FT3 and 0·3–5 mIU/l for TSH( Reference Sang, Wang and Yao 10 ).
The contents of thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) in serum were measured by RIA kits from Beijing North Institution. TPOAb and TgAb assays were completed using the standard reference materials that were produced by Tianjin Nine Tripods Medical & Biological Co. Ltd( Reference Sang, Wang and Yao 10 ).
Diagnostic criteria
The diagnostic criteria for thyroid diseases were as follows: hypothyroidism (TSH>5·0 mIU/l and FT4<11·5 pmol/l); subclinical hypothyroidism (TSH>5·0 mIU/l, 11·5 pmol/l <FT4<23·5 pmol/l and 3·5 pmol/l<FT3<6·5 pmol/l); hyperthyroidism (TSH<0·3 mIU/l, and FT4>23·5 pmol/l or FT3>6·5 pmol/l); subclinical hyperthyroidism (TSH<0·3 mIU/l, 11·5 pmol/l<FT4<23·5 pmol/l and 3·5 pmol/l<FT3 <6·5 pmol/l)( Reference Sang, Wang and Yao 10 ).
Statistical analysis
All tests were two-tailed and a statistically significant difference was considered at P <0·05. Quantitative data were expressed as mean and standard deviation, except the data on urinary iodine and serum TSH that were expressed as median and interquartile range (IQR; 25th–75th percentile). R×C categorical data were analysed by the χ 2 test. The 2×2 table was analysed by Fisher’s exact test (without Bonferroni correction). Differences between the two areas for age, body weight, height, iodine intake, and serum FT3, FT4 and TSH contents were tested by using independent sample t tests, and UIC was analysed through the Kruskal–Wallis test. Statistical analyses were conducted using the SPSS statistical software package version 13·0.
Results
Participants
The concentration of iodine in drinking water from Fuzhuangzi Village, Zhaogao Village and Xiaoshan Village was 841, 394 and 13 µg/l, respectively. After being screened by questionnaires, a total of 854 inhabitants, including 348 (112 males and 236 females) in AIA and 506 (185 males and 321 females) in EIA, were investigated. The two populations were similar with respect to age, sex, economic status, accessibility to health care and smoking rate.
Concentration of urinary iodine
Median UIC was 185 (IQR 147–240) µg/l in AIA and 1152 (IQR 753–1539) µg/l in EIA. The distribution of the individual values within categories is reported in Table 1.
Thyroid hormones and autoantibodies
Mean serum FT3 concentration was significantly lower and mean serum TSH concentration was significantly higher in EIA than in AIA. Mean serum FT4 was similar in both areas (Table 2).
Table 2 Levels of thyroid hormones in men and women aged 20–50 years who had adequate or excessive drinking water iodine, Hebei Province, People’s Republic of China, 2010

FT4, free thyroxine; FT3, free triiodothyronine; TSH, thyroid-stimulating hormone; IQR, interquartile range; AIA, adequate iodine area; EIA, excessive iodine area; t test, Student’s t test; Wilcoxon, Wilcoxon rank sum test.
The total positive rates of TgAb and TPOAb were not statistically different between EIA and AIA (16·1 % v. 11·9 % for TgAb and 20·7 % v. 16·4 % for TPOAb). However, there was a significant difference in autoantibodies between genders, with the positive rates of autoantibodies in females being significantly higher than in males. Specifically, in AIA, the positive rate of TgAb in females was 6·1-fold higher than in males (22·0 % v. 3·6 %; χ 2=19·174, P=0·000) and the positive rate of TPOAb was 2·4-fold higher (25·4 % v. 10·7 %; χ 2=10·015, P=0·002). In EIA, the positive rate of TgAb in females was 2·9-fold higher than that in males (15·6 % v. 5·4 %; χ 2=11·616, P=0·001) and the positive rate of TPOAb was 1·8-fold higher (19·6 % v. 10·8 %; χ 2=6·651, P=0·010). However, the positive rates of autoantibodies were not significantly different between AIA and EIA when comparing by gender (Table 3).
Table 3 Gender ratio (number of males/number of females) and prevalence of thyroid autoantibodies in men and women aged 20–50 years stratified by adequate/excessive iodine area and gender, Hebei Province, People’s Republic of China, 2010
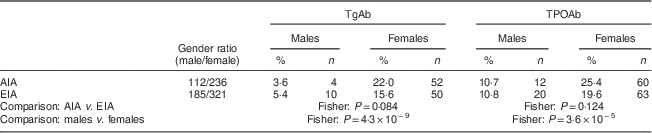
TgAb, thyroglobulin antibody; TPOAb, thyroperoxidase antibody; AIA, adequate iodine area; EIA, excessive iodine area; Fisher, Fisher’s exact test.
The percentage of positive thyroid antibodies (TgAb and TPOAb) was similar in both areas (with adequate iodine and with excessive iodine). This percentage was at least twice greater in women than in men (Table 3).
Prevalence of thyroid dysfunction
Thyroid dysfunction was two times more frequent in EIA than in AIA (20·6 % v. 10·3 %). Frequency of subclinical hypothyroidism (elevated TSH and normal FT4; 13·6 % v. 9·0 %) and subclinical hyperthyroidism (decreased TSH and normal FT4; 2·2 % v. 0 %) differed significantly between both areas. Hypothyroidism (decreased TSH and elevated FT4; 3·6 % v. 1·3 %), but not hyperthyroidism (decreased TSH and elevated FT4), was more frequent (not statistically significant) in EIA than in AIA. In men, the proportion of thyroid dysfunction was similar in AIA and EIA. By contrast, it was more than two times greater in females from EIA than in females from AIA (Table 4). As a result, there was no significant difference in thyroid dysfunction frequency by gender in AIA, but a highly significant difference in EIA (Fisher’s exact test, P=2·3×10−4).
Table 4 Prevalence of thyroid dysfunction in men and women aged 20–50 years who had adequate or excessive drinking water iodine, Hebei Province, People’s Republic of China, 2010

AIA, adequate iodine area; EIA, excessive iodine area.
More than 10 % of euthyroid participants showed at least one positive thyroid antibody test and 8·1 % were positive for both thyroid antibodies. In the hypothyroid participants, this percentage of positive anti-thyroid test was more or less double (Table 5). Due to the small number of cases, there was no statistical difference in the percentage of participants with subclinical hypothyroidism and those with isolated elevated TSH (data not shown). Only twenty-one participants were diagnosed as biological hyperthyroid or subclinical hyperthyroid among the population. In the hyperthyroid participants, the percentage of positive thyroid antibodies was similar to the hypothyroid; however, the TgAb-positive rate was obviously high but without significance because of the limited number of cases.
Table 5 Prevalence of thyroid autoantibodies in clinical categories of thyroid function among men and women aged 20–50 years who had adequate or excessive drinking water iodine, Hebei Province, People’s Republic of China, 2010
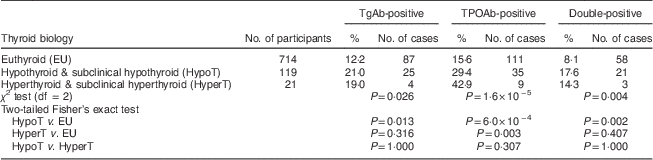
TgAb, thyroglobulin antibody; TPOAb, thyroperoxidase antibody.
Relationship between autoantibodies and hypothyroidism and subclinical hypothyroidism
As hypothyroidism and subclinical hypothyroidism accounted for a large amount of the overall prevalence of thyroid dysfunction, the relationships between hypothyroidism and subclinical hypothyroidism and positive rates of single and double autoantibodies were analysed. The positive rates of TgAb (χ 2=11·099, P=0·001) and TPOAb (χ 2=10·061, P=0·002), as well as the double-positive rate (χ 2=14·882, P=0·000), were higher in hypothyroidism than those in euthyroidism. The positive rate (χ 2=7·709, P=0·005) and double-positive rate (χ 2=4·203, P=0·040) of TPOAb in subclinical hypothyroidism was significantly higher than that in euthyroidism. The positive rates were not significantly different between hypothyroidism and subclinical hypothyroidism (Table 5).
Discussion
To understand the influence of excessive iodine intake on thyroid function and the prevalence rate of thyroid dysfunction in China, a cross-sectional investigation in three areas with adequate or excessive iodine intake was carried out. Hebei Province is located downstream of the Yellow River. The EIA in Hebei mainly were scattered around the estuary of the Yellow River. The high iodine concentration in drinking water is an important source of iodine intake by the inhabitants. The water of the surveyed villages is extracted independently underground and supplied to the inhabitants through the pipe network system. The depth of the underground water source is different, from less than 100 m to more than 400 m. So the iodine concentration is different according to the depth of source water.
The results showed that the serum levels of both FT3 and TSH were affected by the excessive iodine intake. The average level of FT3 in EIA revealed a slight decrease when compared with that in AIA, while the level of TSH exhibited a slight increase in EIA. The results suggest a link between increased iodine intake and hypothyroidism. Similar results have been reported in a randomized controlled trial in older people( Reference Thomson, Campbell and Miller 9 ). With a supplementation of 80 μg or >50 mg iodate daily for 8 weeks, ten of forty-three participants exposed to excessive iodate showed hypothyroidism at the end of the trial. A 5-year cohort study on the prevalence of thyroid dysfunction in populations with different iodine intakes such as mildly deficient (median UIC: 84 μg/l), more than adequate (median UIC: 243 μg/l) and excessive (median UIC: 651 μg/l) in China has been completed( Reference Teng, Shan and Teng 15 ). An increase in the prevalence of overt hypothyroidism, subclinical hypothyroidism and autoimmune thyroiditis with increasing iodine intake was observed. Another follow-up study in three rural Chinese communities with mild to borderline deficiency, more than adequate and excessive iodine intake, respectively, has been carried out( Reference Yang, Shan and Teng 18 ). On the contrary, a follow-up examination has been provided to these inhabitants for 13 years and the results demonstrated that chronic excessive iodine intake does not apparently increase the risk of autoimmune hyperthyroidism. In the present study, although we did not detect a significant difference in the prevalence of hyperthyroidism between AIA and EIA, we found that the prevalence of subclinical hyperthyroidism in EIA was significantly higher than that in AIA. No subclinical hyperthyroidism case was observed in AIA, and eleven inhabitants living in EIA were diagnosed as subclinical hyperthyroidism in our research.
The thyroid dysfunction spectrum of AIA and EIA was also similar. It has been reported that the prevalence of subclinical hyperthyroidism and hyperthyroidism may be increased among people with more than adequate and excessive iodine intake( Reference Teng, Shan and Teng 15 ). Similarly, high iodine intake may trigger and aggravate autoimmune thyroiditis, thus increasing the risk of clinical hypothyroidism. Moreover, the titres of autoantibodies, TgAb and TPOAb, in females are sensitive to iodine exposure. Higher levels of autoantibodies in females from AIA and EIA were observed when compared with those in males. In addition, a case–control study has reported that excessive iodine intake may trigger thyroid autoimmunity and hypothyroidism among women( Reference Alsayed, Gad and Abdel-Baset 19 ). These results indicate that women may be a susceptible population and even a high-risk population to iodine exposure.
Our data did not provide direct evidence that excessive iodine exposure could increase the risk of thyroid autoimmunity. However, the results showed a relationship between the positive rate of autoantibodies and the severity of hypothyroidism. A higher positive rate of autoantibodies was observed in hypothyroidism compared with that in subclinical hypothyroidism and euthyroidism. In Li et al.’s study, only an increased positive rate of TgAb with increased iodine intake was reported and individuals with a positive rate of TPOAb and/or TgAb had more frequent development of thyroid dysfunction( Reference Li, Teng and Shan 20 ). These results suggest that autoantibodies such as TgAb and TPOAb are effective parameters associated with the development of thyroid dysfunction, especially hypothyroidism.
Conclusion
In conclusion, excessive iodine intake (iodine concentrations in water were 394 μg/l and 841 μg/l, and median UIC was 1152 μg/l, which is larger than 300 μg/l) is not safe. Excessive iodine exposure may be a risk factor for an increased prevalence of biochemical thyroid dysfunction, especially biochemical hypothyroidism. The iodine supplementation should be tailored according to the level of iodine intake. Women may be a susceptible population to excessive iodine exposure.
Acknowledgements
Financial support: This work was supported by the National Natural Science Foundation of China (grant numbers 81273057, 30972465 and 81202201). The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: L.T. collected the data and conducted manuscript draft writing; Z.S. conducted data collection and analysis; J.S. and H.L. supervised the survey; W.C., N.Z., W.W. and G.Z. collected the data and completed the laboratory determinations; W.Z. developed the study hypothesis and instructed the data analysis and editing of the manuscript. L.T. and Z.S. contributed to this project equally. Ethics of human subject participation: Research protocols were approved by the Medical Ethics Committee of Tianjin Medical University.







