Vitamin B12 (B12, cobalamin) is a water-soluble vitamin and an essential nutrient that normally must be obtained from the diet. Metabolically, it is essential for two reactions catalysed by the enzymes methionine synthase and l-methyl-malonyl-coenzyme A mutase. The daily recommended daily intake of B12 for adults is 2 μg( Reference Herbert 1 ). In healthy individuals, nutritional B12 deficiency is unusual because total body stores in adults are about 2500 μg and daily turnover is slow( Reference Stover 2 ), meaning that reserves generally remain for up to 10 years( Reference Aaron, Kumar and Vijayan 3 ). B12 deficiency can have many causes, such as nutritional habits (strict vegetarian and vegan diets: practice of abstaining from use of animal products), intestinal malabsorption (i.e. gastritis, state after total gastrectomy), use of proton pump inhibitors and elevated requirements (hyperthyroidism)( Reference Stover 2 ).
Severe and persistent B12 deficiency has relevant adverse effects on clinical condition, namely haematological, neurological, neuropsychiatric and metabolic dysfunctions (i.e. methyl-malonyl-coenzyme A acidosis, hyperhomocysteinaemia)( Reference Aaron, Kumar and Vijayan 3 – Reference Dali-Youcef and Andrès 5 ). Mild B12 deficiency normally does not provoke clinical symptoms, but can be diagnosed by measurement of blood markers. The clinical laboratory parameters available to diagnose B12 deficiency are serum total B12, transcobalamin-bound B12 (holotranscobalamin, HoloTC: active fraction of B12), plasma homocysteine (Hcy) and methylmalonic acid (MMA)( Reference Hoey, Strain and McNulty 6 ). The metabolites Hcy and MMA can be used as indicators of B12 deficiency, but many factors other than B12 deficiency (e.g. renal failure) can increase Hcy and MMA. Furthermore, measuring MMA is complicated and expensive, requiring HPLC or GC–MS( Reference Herrmann, Obeid and Schorr 7 ). Thus algorithms for laboratory diagnosis of a B12-deficient status recommend the initial measurement of B12 and HoloTC. Clarke et al. state that HoloTC has better diagnostic accuracy than B12 (77 % v. 73 %) and that the diagnostic utility is superior in the overall population as well as in patients with renal impairment( Reference Clarke, Sherliker and Hin 8 ).
B12 deficiency is characterized by low serum concentrations of total B12 (<148 pmol/l) and HoloTC (<35 pg/l), whereas depletion shows total B12 within the grey zone (148–221 pmol/l) and HoloTC lower than the cut-off( Reference Allen 4 , Reference Clarke, Sherliker and Hin 8 , Reference Miller, Garrod and Rockwood 9 ). To date, an internationally valid consensus as to the definition of B12 deficiency has not been established, since different thresholds have been used( Reference Miller, Garrod and Rockwood 9 ). The transition from B12 depletion to deficiency is fluid. The early diagnosis of a deficient status is essential because simple B12 supplementation may reverse clinical symptoms.
B12 deficiency is prevalent primarily in elderly people, children and women of reproductive age, with prevalences ranging from 10 to 40 %( Reference Allen 4 , Reference Clarke, Sherliker and Hin 8 , Reference McLean, de Benoist and Allen 10 – Reference Garcia-Casal, Osorio and Landaeta 13 ). In general, no relationship between B12 status and geographic distribution of the population can be claimed( Reference McLean, de Benoist and Allen 10 ). The condition has the potential to be a worldwide public health problem.
The aim of the present study was to investigate the prevalence of B12 depletion and deficiency based on serum total B12 and HoloTC concentrations in a representative population of Liechtenstein. Our secondary aim was to determine factors associated with depletion and deficiency.
Materials and methods
Study population
The current retrospective study was carried out in the resident population of the Principality of Liechtenstein, without age restrictions. The study period ranged from January 2000 until December 2007. Within the study period, the population of Liechtenstein averaged 35 168 permanent residents of nearly exclusively Caucasian origin, as described elsewhere( 14 ). Corrected for migration and deaths, the reference population totals 38 839.
Serum samples of 7424 consecutive patients from child age to advanced age seeking medical attention by their physicians, referred for routine laboratory work-up in an ambulatory setting, were included in the study. Out of them, a subgroup of 1328 patients was also evaluated.
Hospitalized patients were excluded from the study. In the case of multiple determinations in the same individual, only the lowest value was kept in the database and used for further analysis.
Laboratory methods
Venous blood samples were drawn from all individuals in fasting or non-fasting state into Vacutainer tubes (BD Systems, Basel, Switzerland) or Sarstedt Monovette tubes (Sarstedt, Sevelen, Switzerland). The samples were referred to the Liechtenstein central laboratory. Serum total B12 was measured within 24 h after venepuncture. For measurement of total B12 concentrations, a competitive-binding immunoenzymatic assay employing chemiluminescence was used (Access Vitamin B12, run on two different analysers, Access2 and Unicel DxI800 instruments (Beckman Coulter, Nyon, Switzerland), whose agreement was previously compared).
In a subgroup of 1328 patients investigated during 2007, also HoloTC levels were measured on the Abbott AxSYM® immunochemical automated analyser (Abbott Diagnostics, Baar, Switzerland). The between-day CV, as evaluated by commercially available control materials, were 4·5 % (at 242 pmol/l), 5·5 % (at 360 pmol/l) and 6·3 % (at 911 pmol/l) for total B12 and 8·7 % (at 21 pmol/l) and 9·7 % (at 52 pmol/l) for HoloTC.
According to the country's validated laboratory reference values, the cut-off point for B12 deficiency was defined as serum level of total B12 <125 pmol/l( Reference Ray, Goodman and O'Mahoney 15 ). The cut-off point for B12 depletion was defined as a serum level of 125–300 pmol/l for total B12 and a serum level of <35 pg/l for HoloTC( Reference Miller, Garrod and Rockwood 9 ).
Statistical analysis
The proportion of individuals with a B12 measurement among the general population was assessed across different age strata by using the national census data controlled for cases of migration and deaths. The prevalence of individuals with B12 depletion or deficiency was calculated within the cohort. Further, the prevalence of these individuals among the general population was determined by using the adjusted national census data. Differences between proportions were assessed with the χ 2 test. Finally, a logistic regression model was applied in order to detect associations between demographic factors such as age and gender and the presence of B12 deficiency. A P value less than 0·05 was considered statistically significant.
Results
A total of 7424 patients who sought medical attention were included in the study (ambulatory setting). This cohort comprised 19·1 % of the country's entire population. The baseline characteristics of the study cohort are given in Table 1. Mean serum total B12 levels fluctuated from 199 to 226 pmol/l between the different years.
Table 1 Baseline characteristics of the study populations; ambulatory setting, Principality of Liechtenstein, January 2000–December 2007
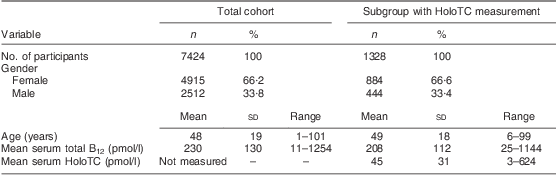
HoloTC, holotranscobalamin; B12, vitamin B12.
Remarkably, only 12·4 % of the cohort had a serum total B12 level in a reference interval where B12 deficiency is unlikely (>300 pmol/l); 74·2 % of the cohort were within the grey zone (125–300 pmol/l), exhibiting B12 depletion, while 13·4 % of the cohort showed evidence of B12 deficiency (<125 pmol/l). In the subgroup of participants with simultaneous total B12 and HoloTC determination, B12 depletion was seen in 26·4 % (i.e. B12 grey zone together with HoloTC <35 pmol/l). The mean total B12 levels and the mean HoloTC levels across the different age/gender strata are shown in Fig. 1.
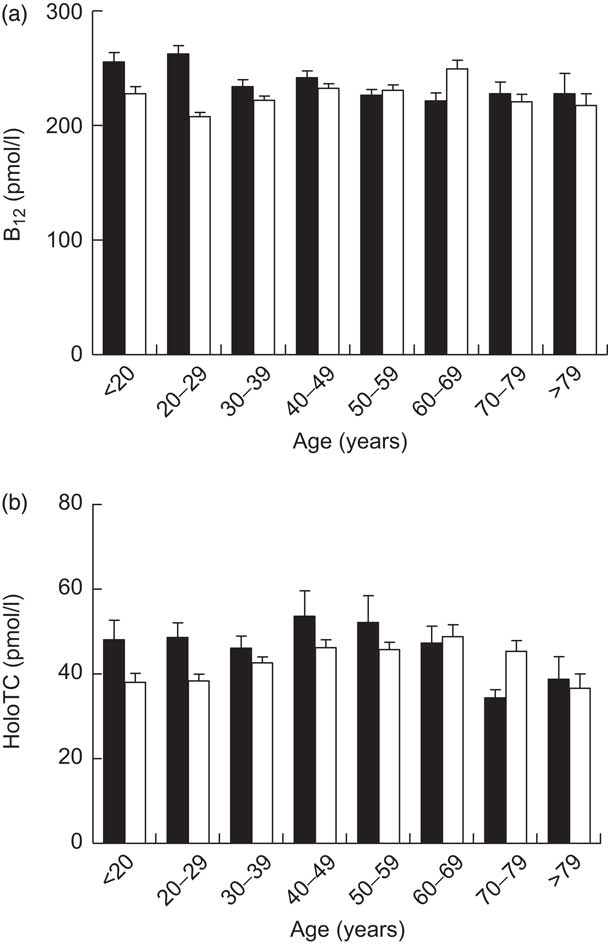
Fig. 1 Serum levels of (a) total vitamin B12 (B12) in the study cohort (n 7424) and (b) holotranscobalamin (HoloTC) in a subgroup of the cohort (n 1328), stratified according to age and gender (▪, male; □, female); ambulatory setting, Principality of Liechtenstein, January 2000–December 2007. Values are means with their standard errors represented by vertical bars
The ratio between the prevalence of B12 deficiency and depletion was investigated in both the general population and the study cohort (Table 2). Population prevalence of B12 deficiency was obtained by calculating the ratio of individuals with B12 deficiency among the reference population of 38 839. Population prevalence of B12 depletion was extrapolated from the ratio between individuals with B12 depletion and B12 deficiency in the study cohort (2:1). Taking these findings into account allowed estimation of the prevalence of B12 depletion and deficiency among the general population at nearly 8 % (Table 2).
Table 2 Prevalence of B12 depletion and deficiency in the cohort and the general population of Liechtenstein
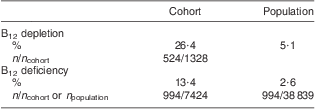
B12, vitamin B12; HoloTC, holotranscobalamin.
Serum total B12 was determined in all individuals of the cohort (n 7424). B12 depletion was measured by total B12 and HoloTC assays; the cohort with concomitant HoloTC determination comprised 1328 individuals. The prevalence of B12 depletion in the general population was extrapolated from the ratio between individuals with B12 depletion and B12 deficiency in the cohort (2:1).
Stratifying the cohort with regard to gender and age showed that the prevalence of B12 deficiency was significantly higher in females than in males (14·85 % v. 10·51 %, respectively, P < 0·001) and in persons aged 50 years and older than in those younger than 50 years (15·47 % v. 11·79 %, respectively, P < 0·001; Fig. 2). Interestingly, there was a bimodal distribution of the prevalence within females, with a first peak at childbearing age. The subgroup having both total B12 and HoloTC measurements paralleled these findings, but demonstrated higher prevalence among all age groups (Fig. 3). Surprisingly, a remarkable B12-deficient status was already seen in children.
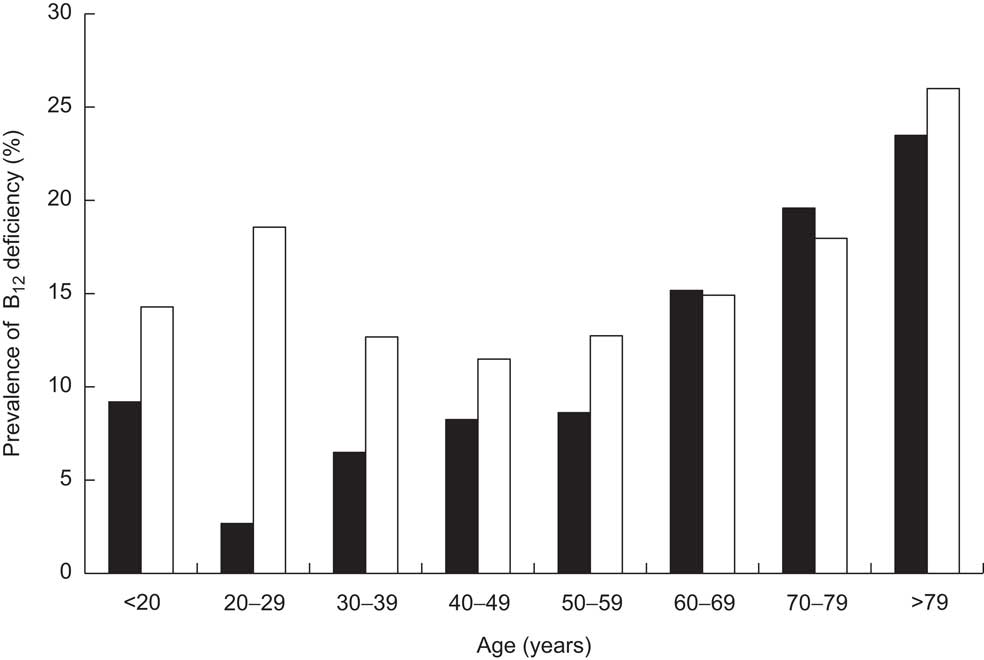
Fig. 2 Prevalence of vitamin B12 (B12) deficiency in the study cohort (n 7424), stratified according to age and gender (▪, male; □, female); ambulatory setting, Principality of Liechtenstein, January 2000–December 2007
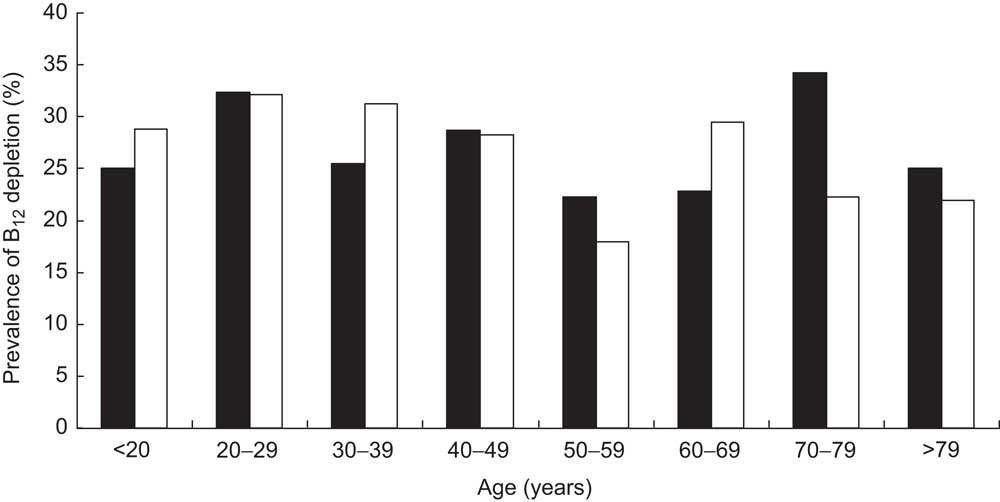
Fig. 3 Prevalence of vitamin B12 (B12) depletion (measured by total B12 and holotranscobalamin assays) in a subgroup of the cohort (n 1328), stratified according to age and gender (▪, male; □, female); ambulatory setting, Principality of Liechtenstein, January 2000–December 2007
Further calculating the prevalence of B12 deficiency within the general population revealed characteristics similar to those within the cohort: older persons and females suffer more often from B12 deficiency (Fig. 4).
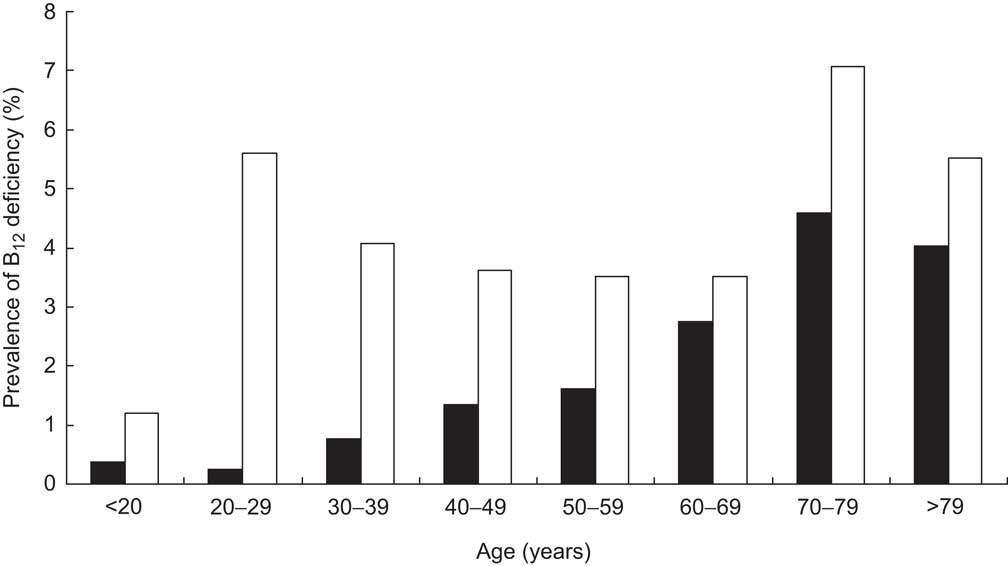
Fig. 4 Prevalence of vitamin B12 (B12) deficiency in the general population of Liechtenstein, stratified according to age and gender (▪, male; □, female)
Finally, a logistic regression model with age, gender and the interaction between age and gender as predictors of B12 deficiency found that age (OR = 1·32; 95 % CI 1·23, 1·43) and female gender (OR = 5·81; 95 % CI 3·41, 9·89) were significant predictors of the presence of B12-deficient status. Interestingly, the interaction between female gender and age was also significant (OR = 0·80; 95 % CI 0·73, 0·87), indicating that the influence of age on the frequency of B12 deficiency is stronger in women than in men.
Discussion
In the present retrospective study we found that nearly 20 % of the population had a clinical suspicion of B12 deficiency. About 40 % of the cohort had biochemical evidence of impaired B12 serum levels (26·4 % depleted and 13·4 % deficient). Within the general population of Liechtenstein, B12 deficiency is encountered in 2·6 %, whereas the prevalence of B12 depletion can be estimated at 5·1 %.
Comparing our data with other studies such as the Framingham Study( Reference Lindenbaum, Rosenberg and Wilson 11 ), which described 12 % of elderly people as suffering from B12 deficiency, we have to keep in mind that we were able to consecutively determine B12 status in persons with clinical suspicion of B12 deficiency within one entire country. Other studies have examined B12 status in individuals randomly selected from the population( Reference McLean, de Benoist and Allen 10 , Reference Miller, Garrod and Allen 16 , Reference Pflipsen, Oh and Saguil 17 ). Allen showed in a US sub-population of individuals aged ≥60 years that the prevalence of B12 deficiency was 6 % and the prevalence of B12 depletion (marginal B12 status, HoloTC not assessed) was about 20 %( Reference Allen 4 ). According to Clarke et al., in approximately 5–20 % of elderly people a B12 deficiency remains undiagnosed( Reference Clarke, Sherliker and Hin 8 ). The prevalence of subclinical functional B12 deficiency in the general population is higher than expected( Reference Herrmann, Obeid and Schorr 7 ).
There are hardly any population-wide studies about B12 depletion or deficiency. The sub-populations examined mainly are elderly people (as mentioned above), children and women of reproductive age. Children are of special interest as early B12 deficiency leads to impaired brain development and a higher risk of depression as an adult( Reference Black 18 ). Our study shows that B12-deficient status occurs at all ages, showing one peak in the third decade and another in advanced age (from age 70 years onwards). On the one hand, the capacity to absorb B12 from food decreases in older people (i.e. atrophic gastritis, intestinal dysfunction), consequently leading to a malabsorption syndrome( Reference Carmel 19 , Reference Andres, Frederici and Serraj 20 ). On the other hand, the principal reason for B12 malabsorption is the pharmacological decrease in acid secretion in the stomach, causing an impairment of protein-bound B12 absorption( Reference Stover 2 ). Drugs that decrease acid secretion comprise 3·9 % of all administered drugs in Liechtenstein (Mag. Stefan Tomaselli, Liechtenstein Office of Public Health, personal communication). However, in our database, a link between antacid use and serum total B12 concentration in an individual cannot be drawn. Accordingly, we cannot provide evidence on the epidemiological importance of antacid use as a cause of B12 deficiency.
Among young people in Madrid, Gil et al. appraised 4·8 % as being deficient, with males more likely to be deficient than females, whereas our data show a significantly higher prevalence among girls within a similar percentage of affected persons( Reference Gil, Esteban and Hernandez 21 ). This fact could be explained by the fact that in Central European countries girls show a higher rate of unhealthy dietary habits than boys( Reference Ryan 22 – Reference Chamay-Weber, Narring and Michaud 25 ).
Refsum et al. showed that total B12 and HoloTC concentrations were lower in women than in man, and they increased with age( Reference Refsum, Johnston and Guttormsen 12 ). Further analyses in that study revealed the age effect to be limited to women, and the gender differences were confined to those aged ≤45 years. In women ≤45 years of age, there was a complete shift of the HoloTC distribution towards lower concentrations of ∼20 %( Reference Refsum, Johnston and Guttormsen 12 ). Would that suggest gender as a significant predictor for B12 deficiency? In our study about 5 % of the females of this age showed a B12-deficient status. B12 deficiency among females of reproductive age has an important impact, as it can cause infertility and abortion. Additionally, the fetus may suffer neural tube defects and prematurity( Reference Molloy, Kirke and Troendle 26 ).
Data about B12 deficiency and/or depletion in men are scarce. We could not identify a single study concentrating on male individuals. In general they are mentioned among either the elderly or subgroups (i.e. alcoholics, post-gastrectomy state). In our trial men less often demonstrated a deficient B12 status, but in association with age they had a more pronounced risk of being affected (OR = 0·8). In our cohort, males over the age of 80 years suffered even more often from B12 impairment than women.
HoloTC is known to be a more sensitive and specific marker than total B12, especially for subclinical functional B12 deficiency and depletion( Reference Herrmann, Obeid and Schorr 27 ). In this context it should be kept in mind that, remarkably, B12 depletion occurs twice as often as B12 deficiency. To our knowledge there are no published studies discussing this issue. The problem in comparing different studies is that there are no internationally agreed-upon reference laboratory values for the stratification of B12 deficiency and there is no widely accepted agreement about the therapeutic implications of B12 depletion. Patients showing manifest B12 deficiency have to be treated immediately after diagnosis, as some clinical symptoms can be reversed( Reference Dali-Youcef and Andrès 5 ).
The main limitation of our study concerns the lack of performance of additional laboratory tests such as Hcy or MMA. Both are comparably expensive and laboratory-intensive tests. On the other hand, MMA concentration is considered to be the most specific and sensitive parameter for diagnosis of B12 deficiency( Reference Herrmann, Obeid and Schorr 27 ). Furthermore, we did not randomly analyse the population of Liechtenstein, either with regard to subgroups like pregnant women or individuals affected by metabolic disease. In addition, we did not assess the health status and the illness for which medical attention was sought.
Conclusions
B12 deficiency is a frequent finding in a Central European population (2·6 %), and B12 depletion is found twice as often. Female gender and age are independent, significant predictive factors of a B12-deficient state, and regular monitoring of B12 status is recommended for them. Current considerations for public health interventions to prevent B12-associated pathologies in vulnerable sub-populations are still under debate( Reference Green 28 ).
The measurement of total B12 and HoloTC concentrations are two valid and easily performed parameters for the detection of B12 deficiency. Early recognition of subclinical depletion of B12 is essential, and treatment of the deficiency is imperative, as symptoms can be reversed at an early phase and may also be preventable. Diagnosis of B12 status is important, uncomplicated, and can lead to simple treatment, preventing major disabilities.
Acknowledgements
Sources of funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector. Conflicts of interest: The authors have no potential conflicts of interest to declare. Ethics: Ethical approval was not required. Authors’ contributions: All authors have participated sufficiently, intellectually or practically, in the present study and take public responsibility for the content of the article, including the conception, design, conduct of the experiment, and data interpretation. Acknowledgements: The authors are indebted to J. Wurz for editing the manuscript.








