To improve health benefits for mothers and infants, the WHO and UNICEF recommend the initiation of breast-feeding within an hour after birth, exclusive breast-feeding for the first 6 months of life and continued breast-feeding throughout the second year of life or beyond, with safe and adequate complementary feeding( 1 ). However, only 39 % of 0- to 5-month-old infants in low-income countries were reported to be exclusively breast-fed and an estimated 800 000 children die each year before their fifth birthday due to suboptimal breast-feeding( Reference Black, Victora and Walker 2 ). Furthermore, studies show that mothers will breast-feed their babies optimally when they receive appropriate information( Reference Dulon, Kersting and Bender 3 ) and health workers’ support( Reference Abada, Trovato and Lalu 4 ). A recent study found that knowledge, programme and facility support for breast-feeding mothers at the workplace in urban areas in Indonesia increased exclusive breast-feeding practices almost sixfold( Reference Basrowi, Sulistomo and Adu 5 ).
In contrast, information and promotional materials produced by breast-milk substitute companies( Reference Shealy, Li and Benton-Davis 6 ) and the distribution of free samples of breast-milk substitutes have a detrimental impact on breast-feeding( Reference Howard, Howard and Lawrence 7 ). A study conducted in health facilities in rural areas in Central Java, Indonesia, also found that mothers in these areas were more likely to discontinue exclusive breast-feeding when health providers suggested the use of infant formula( Reference Susiloretni, Hadi and Prabandri 8 ).
In 1981, the World Health Assembly (WHA) adopted the International Code of Marketing of Breast-milk Substitutes (‘the Code’) as a recommendation outlining the minimum international standard to support breast-feeding and to protect mothers from commercial pressure to feed breast-milk substitutes to their babies( 9 ). In 2010, WHA Resolution 63·23 called on Member States to develop or strengthen legislative, regulatory or other effective measures to control the marketing of breast-milk substitutes in order to give effect to the Code and relevant subsequent WHA resolutions. In addition, this Resolution also called on manufacturers and distributors to comply fully with their responsibilities under the Code and the WHA resolutions. Despite being a member of the WHA, Indonesia is one of the countries that has only partially adopted the Code. Currently, there are two pieces of national legislation that incorporate some of the principles of the Code into national regulations: (i) Health Law No. 36/2009, which provides some degree of protection to exclusive breast-feeding in the health-care system; and (ii) a Government Decree (PP 33/2012) to implement elements of the 2009 Health Law, which covers exclusive breast-feeding for babies, restriction of advertisements for breast-milk substitutes for infants under 6 months old and making the establishment of nursing rooms for mothers at workplaces mandatory (see Table 1). However this Decree contains weaknesses, including the scope of the decree itself, which is limited to protecting exclusive breast-feeding for the first 6 months of life, and it regulates marketing only within the health system. Moreover, the decree allows breast-milk substitute advertisements in printed health media, after prior approval from the Minister of Health.
Table 1 Articles on breast-feeding in Indonesian Health Law no. 36/2009 (‘National Health Law 2009’) and the scope of the International Code of Marketing of Breast-milk Substitutes (‘the Code’)

The current study was conducted in November 2012 to February 2013 by university-based researchers with support from the Ministry of Health of the Republic of Indonesia. The study surveyed compliance with the Code in each of the six provinces on Java island in Indonesia (Greater Jakarta, Banten, West Java, Central Java, Yogyakarta, East Java) using the Interagency Group on Breastfeeding Monitoring Protocol (‘the Protocol’)( 10 ). Earlier work in Bangladesh, Poland, South Africa and Thailand using a previous version of the Protocol was published in 1998( Reference Taylor 11 ) and in Toga and Burkina Faso in 2003( Reference Aguayo, Ross and Kanon 12 ).
Methods
As described in the Protocol( 10 ), the current study was designed as a cross-sectional survey. Multistage cluster sampling was applied to select three samples: (i) health facilities (public and private) serving at least ten pregnant women or mothers of young infants daily, on at least 2d/week; (ii) health workers at participating health facilities; and (iii) pregnant women and mothers of infants under 6 months of age.
In addition to the criteria set down in the Protocol, the sample of health facilities in each province consisted of one state or public hospital in the capital city of each province, one randomly selected private maternity health facility in the capital city of each province, and one randomly selected state or public hospital in one selected district nearby the capital city. The Protocol requires random selection of the women( 10 ). However, because it was rare for the health facilities to have a list of patients, in the present survey the selection of the women was modified to the use of consecutive sampling using an interval of 3 or 5 (depending on the average number of visits at the day of data collection). The selection of health workers (doctors, nurses, midwives and administration staff) was done purposively based on their having direct contact with pregnant women and mothers of infants below 6 months of age, as well as their availability for the interview. Thus, the survey sampled three health facilities, health workers (three to five per facility) and 140–150 women in each province. This resulted in a total sample of eighteen health facilities, seventy-seven health workers and 874 women in the six provinces.
The survey also used a qualitative method to assess violations of the Code on labels of breast-milk substitutes. Promotional activities or materials related to breast-milk substitutes, bottles and teats were observed in three modern stores (e.g. hypermarket, supermarket and shops) within a radius of about 2 km around each selected health facility. In addition, a qualitative analysis of printed and television (TV) advertisements of breast-milk substitutes, bottles and teats was undertaken to capture violations of the Code. As described in the Protocol, all information collected from shops and other public domains was gathered within a period of one month of the survey (i.e. November–December 2012) to reflect the products and information that were available during the time of the study. For the print advertisements, five media were selected from available newspapers, tabloids and magazines based on the following criteria: (i) targeting pregnant women and mothers of young infants; (ii) among the top five media with the highest circulation in its category; and (iii) available in the study areas during the study period. All relevant advertisements published by the selected media were gathered and analysed. In addition, twenty-seven TV commercials for growing-up milk for children aged 12–24 months aired by national TV stations in November 2012 were gathered.
Data collection procedures and data analyses
Based on the Protocol, the tools consisted of Form 1 (Questionnaire for pregnant women and mothers), Form 2 (Questionnaire for health workers), Form 3 (Checklist for information materials and posters), Form 4 (Checklist for pharmacies and retail outlets), Form 5 (Checklist for labels and inserts) and Form 6 (Checklist for advertisements). Prior to use, all tools were discussed for their applicability to the Indonesian setting. Modifications of the wording, order and additional opening questions were done prior to translation into the Indonesian language.
Six field supervisors and twelve data collectors, all with experience in research fieldwork, were specifically hired for the survey. The field supervisors were given a 3d training to enable them to train the data collectors on the background, objectives and procedures of the study and to familiarize them with the tools.
Respondents, both women and health workers, were interviewed to capture any Code violations; the questions were classified into the categories of ‘breast-milk substitutes, bottles and teats’ and ‘information and advice’ received by pregnant women, mothers of young infants and health workers through promotion either at or outside the health facilities. All information collected from women and health workers related to the period of 6 months preceding the interview.
The observations at the health facilities were mostly done during interviews with the health workers and during the selection of the women. Whenever possible, evidence of the observations was documented by photographs (mostly done candidly), especially to fill in the Form 3 questionnaire. In some locations, samples of materials were collected through a formal request; while in others, the materials were simply picked up from the displays at the retail outlets.
The analysis of labels and promotional materials was carried out through visits to selected stores in the Greater Jakarta area in October 2012, as well as the observation during our study at the selected hospitals. The analysis was grouped based on the product category: (i) formula milk for less than 6 months; (ii) follow-on milk for 6–12 months or 6–18 months; (iii) growing-up milk for 12–36 months; and (iv) baby food/drink for 6 months and older.
The monitoring of advertisements for breast-milk substitutes aimed to capture violations of the Code; their mere existence classifies any advertisement for breast-milk substitutes as a Code violation. The study specifically looked at the tagline or title of the advertisements, visualizations and pictures, and messages or statements written in the advertisements.
One data coder was employed to verify all completed questionnaires and four data-entry clerks were recruited, trained and tasked to number, register and enter all of the data. Data entry of questionnaires for women/mothers and health workers was done using the statistical software package SPSS version 15. Due to limited resources, only single data entry was done. Prior to analysis, one author (J.F.) performed the data cleaning. Data analyses were mainly univariate to estimate the magnitude of the violations of the Code. Because participants could give multiple answers, the denominators change throughout the results.
Ethical clearance from the Ethical Committee of the Faculty of Medicine, Universitas Indonesia was obtained prior to the conduct of the survey. Written informed consent was obtained from all subjects involved in the study.
Results
Pregnant women and mothers of young infants
A total of 874 women in six provinces were surveyed. The sample consisted of 492 women with infants younger than 6 months (56 %) and 382 pregnant women (44 %). Sixty-three per cent of the pregnant women were in their third trimester, and 80 % of the infants were younger than 3 months. The women’s age range was wide, from 16 to 52 years old, with 40 % of them working, 43 % graduated from senior high school and 64 % living as nuclear families (Table 3). These characteristics are typical as the women were recruited from hospital-based facilities.
Table 3 Characteristics of interviewed women (n 874) in all six provinces, Java, Indonesia, November–December 2012
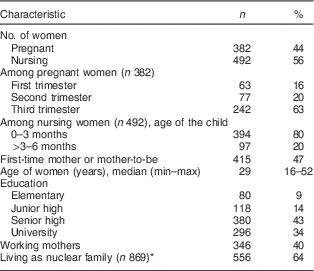
* Due to missing values.
Information and advice
Out of the 874 women interviewed, 20 % (n 175) reported receiving advice to use feeding bottles and teats, and to use breast-milk substitutes to feed their infants under the age of 6 months (Table 4). The sources of this advice were partners/parents/relatives/friends (n 143), health workers (n 99), representatives from companies (n 17) and other sources such as books, TV and magazines (n 15), while the small remainder received the advice from pharmacists (n 8). Information and advice the women received included the use of infant formula when breast milk has not fully come in and the superior nutritional content of formula compared with breast milk. Some of the advice and information was given along with a suggestion of using a certain brand. When asked whether they were advised to use a particular brand or company, 118 out of 172 (69 %) women confirmed this.
Table 4 BreakdownFootnote * of information and advice to use breast-milk substitutes received from different sources among interviewed women (n 874) in all six provinces, Java, Indonesia, November–December 2012
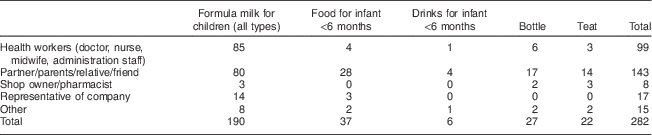
* Data presented are number (n).
Promotion
All women were asked whether or not they had seen promotional materials related to breast-milk substitutes. Although it was hard to recall the types of infant formula advertisements, 72 % of the women reported that they had seen or read promotional materials on infant formula, 68 % on follow-on formula, 76 % on growing-up milk, 36 % on complementary foods for infants under 6 months of age, and 2 % had seen promotional materials on drinks for infants under 6 months of age. In addition, 56 % had seen promotional materials on feeding bottles and 42 % on teats.
TV was identified as the most frequent place where women had seen the promotional materials for infant formula (84 %), follow-on formula (85·5 %), growing-up formula (92 %), complementary foods for infants under the age of 6 months (86·5 %) and drinks for infants under the age of 6 months (50 %). However, responses might not be accurate, since mothers relied on their memory to recall the brands or the products of the promotional materials. Other places where women observed promotional materials for breast-milk substitutes were at shops/pharmacies and in magazines. Moreover, some women identified health facilities as places where they saw promotional information about all types of formulas (26/629 for infant milk, 9/593 for follow-on milk and 6/666 for growing-up milk). In addition, shops or pharmacies were identified as the most frequent places where respondents found promotional materials on feeding bottles (263/492, 53·5 %) and teats (220/365, 60 %).
Samples and gifts
Out of 874 women, 15 % confirmed that they had received free samples of breast-milk substitutes, most often by health workers and representatives from companies. Health workers most often gave samples of infant formula (37/48, 77 %). Women who were given all types of breast-milk substitutes mentioned company representatives as the most common source of these samples (11/48 for infant formula, 6/6 for follow-on milk, 8/13 for growing-up milk and 3/6 for foods).
Women most often received samples of infant formula (48/132) and milk for pregnant women (60/132). Among the latter, thirty-four women reported receiving these samples from company representatives and eighteen received them from health workers. Furthermore, health facilities and pharmacies/shops were the top two places mentioned in terms of where the samples were received most frequently (Table 5).
Table 5 BreakdownFootnote * of responses about the location where women received the sample among interviewed women (n 874) in all six provinces, Java, Indonesia, November–December 2012
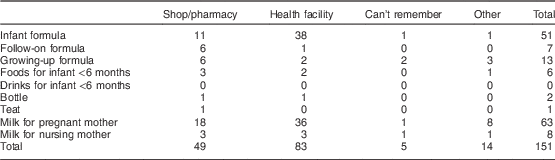
* Data presented are number (n).
As many as 139 women (16 %) had received bibs, nappies, toys or other gifts. Interestingly, nearly the same proportion (132 women, 15 %) mentioned receiving ‘other’ types of gift(s) such as a mug, T-shirt, pen, bag, lunch box, drinking bottle, food container, calendar, clock, book, baby apparel, baby oil, baby wash, baby cutlery, baby blanket, DVD for children, towel, sandals, phone voucher, umbrella, tissue, detergent and cooking oil. The sources of these gift(s) were health workers (50 %), company representatives (30 %) and shop owners or pharmacists (12 %). When further asked about the place where they received the gifts, women mentioned health facilities such as hospitals and midwives’ clinics, but also supermarkets, baby shops, or even delivery to their homes or offices.
Health facilities
Eighteen health facilities in six provinces were visited and seventy-seven health workers were interviewed. Most of these were midwives (39 %), nurses (31 %) and doctors (25 %), while the rest (4 %) were pharmacists, head of wards and administration staff. The majority worked in obstetrics clinics (42 %) and 34 % were working in other units/departments (Table 6).
Table 6 Characteristics of health workers (n 77) in all six provinces, Java, Indonesia, November–December 2012
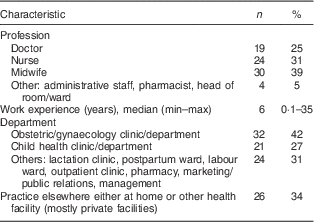
Visits
Out of the seventy-seven health workers interviewed, 23 % reported that they had received between one and six visits from company representatives in the past 6 months. The purposes of these visits were provision of product information (as reported by fifteen health workers) and proposal to collaborate with the health facilities (mentioned by three health workers).
Two of all the interviewed health workers reported having received gifts from breast-milk substitute companies over the past 6 months. Gifts were given directly to the individual health worker and not to the health facilities. Each gift carried the brand name of specific products. Gifts included refrigerator magnets, thermometers and growth charts.
Four of the eighteen participating health facilities mentioned that they had received free materials or equipment from two different formula companies in the past 6 months. These gifts included leaflets, posters/calendars, stationery, growth charts, bags, baby boxes, incubators and (surprisingly) contraceptives. These free materials were reported to carry the brand name of breast-milk substitute products. Six health workers working at the health facilities under study reported that in the past 6 months they received free samples of infant formula, bottles, teats and drinks or foods for infants under 6 months old, milk for pregnant women and special formula milk, while five others said that they were unaware of hospital receipt of any free samples. Most of the health workers answered ‘no’ when asked whether the health facilities had received any free or low-cost supplies of formula milk over the past 6 months, while nine stated they did not know.
Awareness of the Code
Less than half of the health workers interviewed (45 %) were aware of the Code. Their awareness captured the importance of breast-feeding support, regulations and sanctions for health workers and health facilities if they promote the use of breast-milk substitutes to mothers, and regulations on the labelling of breast-milk substitutes. In addition, 52 % of the health workers were aware of the national measures that implement the Code in Indonesia.
Labels
The study analysed labels of forty-four brands of breast-milk substitutes from nineteen producers to measure compliance with the provisions of the Code and subsequent relevant WHA resolutions, as well as to measure the scale of violations, if found. The breakdown of the list of products includes twenty-seven milk brands (thirteen infant formulas, six follow-on formulas and eight growing-up milks) from nine producers, six foods (baby porridge and biscuits) and two beverages from four producers, as well as five bottles and four teats from six producers. The labels were analysed based on Article 9 of the Code.
The most common labelling violations included statements or visuals that discouraged breast-feeding and the absence of mention or consideration of local climate in relation to the expiration date of the products. In addition, all infant formula brands violated the Code by placing pictures or text related to babies or other pictures that idealized the use of breast-milk substitutes. Other common violations found in the infant formula brands included use of the terms ‘humanized’ or ‘maternalized’; failing to mention the benefits and superiority of breast-feeding (only mentioning that ‘breast-milk is the best’, not ‘breast-feeding is the best’); and failing to include statements that the product should be used only on the advice of a health worker as to its need and proper use.
Three infant formula brands (23 %) clearly provided a warning against the hazards of inappropriate preparation.
Some of the bottles and teats were imported and did not contain information in Indonesian language. In addition, they were sold in small packages and the information on the packaging was written in small font sizes, making it difficult to see and read.
Promotional materials in outlets, health facilities and television advertisements
Promotional materials were observed in eighteen retail outlets in six provinces. Leaflets were the most common type used by producers of growing-up milk brands, bottles and teats. Other types of promotions included gifts (found in fifteen of eighteen stores), special displays (thirteen stores), shelf tags (eight stores), discounts (eight stores), coupons (three stores) and posters (three stores). In fourteen stores, more than one type of promotional material was found. Further, some brands deployed sales promotion girls in eight of eighteen stores, mostly in supermarkets. These sales people were usually identified as ‘nutrition consultants’.
In addition, observations on promotional and informational materials were made during visits to the health facilities. Sixty per cent of promotional materials observed mentioned brand names and these were mostly growing-up milk brands. Posters were the most common type of promotional materials (found in fifteen of eighteen (83 %) health facilities), followed by growth charts (four health facilities), health records (two health facilities), and leaflets, brochure racks and flipcharts for health workers (one health facility). The materials were strongly associated with the brands through taglines, colours, and other visuals or wording. Some hospitals covered the company or brand logos on the materials to avoid violating Articles 6·3, 6·8 and 4 of the Code.
A total of twenty-seven TV commercials for growing-up milk (>12 months) of nine brands and thirty-four print advertisements of fourteen brands violated Article 5·1 of the Code as they directly reached potential and existing customers through the advertisements and promotional materials.
Discussion
Compliance with the Code within health facilities
In the present study, some women reported receiving samples and gifts from a health professional at a health facility, which violates Articles 5·4, 6·2 and 7·4 of the Code. Although the percentage is small, samples and gifts from health workers contribute to confusing messages to new mothers about whether they should continue to breast-feed or give up and feed formula to their babies( Reference Rosenberg, Eastham and Kasehagen 13 ). Several studies reported that women who received samples or gifts of breast-milk substitutes are more likely to exclusively breast-feed their babies for shorter periods than women who did not receive any breast-milk substitute samples or gifts( Reference Susiloretni, Hadi and Prabandri 8 , Reference Rosenberg, Eastham and Kasehagen 13 – Reference Mehdi and Wagner-Rizvi 15 ). Moreover, a number of women who received samples or gifts at health facilities also received advice and information from health workers to use breast-milk substitutes to complement breast milk when breast-milk production is low, or to increase the weight or nutritional status of their baby. Such advice may convey the message to mothers that the samples or gifts would be beneficial to the health of their babies. This practice violates Article 7·1, which states that health workers ‘should encourage and protect breast-feeding’( 9 ).
Almost three-quarters of the women who participated in the study reported that they have seen promotional materials for breast-milk substitutes at health facilities. This shows that companies were using health facilities as a place to promote their products, which is in conflict with Articles 5·2, 5·4 and 5·5 of the Code( 9 ). The impact of seeing promotional materials for breast-milk substitutes has been documented through extensive research. Mendoza( Reference Mendoza 16 ) noted that breast-milk substitute promotional materials have detrimental effects on breast-feeding. Another study by Sobel et al.( Reference Sobel, Iellamo and Raya 17 ) found that mothers who have seen breast-milk substitute promotions are less likely to breast-feed over a longer period.
Furthermore, three companies were found to violate Articles 4·3, 6·2, 6·6 and 6·8 of the Code( 9 ) by distributing gifts, equipment and supplies to at least four health facilities in the study sites, where the brand names of the breast-milk substitutes were attached to each gift and piece of equipment. While incentives to health workers are forbidden by the Code because they can influence a health worker’s attitude towards the promotion of particular products rather than encouraging breast-feeding( Reference Chren and Landefeld 18 ), the current study found that representatives from breast-milk substitute companies visited some health workers and offered to collaborate with health facilities (violation of the subsequent WHA resolutions dealing with conflict of interest). The Code outlines that health facilities should not be used for the purpose of promoting breast-milk substitutes, feeding bottles and teats, yet numerous violations of the Code have been found in health facilities during the current study.
Compliance with the Code
The present study observed violations of the Code in the labelling of breast-milk substitutes, feeding bottles and teats, and promotion and advertising of these products to the public. According to the Code, labels must provide the necessary information about the appropriate use of the product, so as not to discourage breast-feeding (Article 9·1) and the container and the label must have a clear, conspicuous, and easily readable and understandable message in the appropriate language (Article 9·2). Manufacturers and distributors of infant formula should also ensure that the label does not contain the terms ‘humanized’, ‘maternalized’ or other similar terms( 9 ).
The most common labelling violations included texts or visuals that discouraged breast-feeding, not mentioning the consideration of local climate with regard to the expiration date, and the placement of pictures related to babies or other pictures that idealized the use of breast-milk substitutes. Moreover, all TV commercials for growing-up milk (>12 months) and print advertisements observed violated Article 5·1 of the Code( 9 ). These advertisements were part of promotional campaigns for breast-milk substitutes in Indonesia, which violates Article 13 of the Indonesian Consumer Protection Law No. 8/1999 on promising rewards in the form of goods and/or other services; and Article 38 of the Indonesian Government Regulation No. 69/1999 on Food Labels and Advertising.
Findings from the present study may not be generalized to other places in Indonesia because the local situation at the health facilities and the awareness of the Code could be different. The magnitude of violations of the Code by manufacturers may vary from place to place or country to country. However, these typical violations to the Code may occur in a similar fashion elsewhere since the present study identified major international breast-milk substitute companies as violators of the Code.
The need to reinforce the implementation of the Code
Overall, the current study shows that violations to the Code were found inside and outside health facilities. Although some violations were relatively rare, this does not mean that the magnitude of the violations is small. The fact that 72 % of women reported having seen promotion of breast-milk substitutes at health facilities demonstrates that health facilities were in a weak position to reinforce the Code. Increasing awareness of the Code among health workers, manufacturers and the general public should be included in the regular government agenda.
Furthermore, despite the fact that Indonesia has two national pieces of legislation that incorporate some of the principles of the Code to regulate the marketing of breast-milk substitutes, there is no adequate monitoring mechanism that leads to sanctions and enforcement remains a big challenge. The need to strengthen and reinforce the Code and national measures is a key element of a comprehensive strategy to protect, promote and support breast-feeding in Indonesia. Moreover, national regulations must be able to regulate all forms of promotion of breast-milk substitutes, bottles and teats. A study by Berry et al.( Reference Berry, Jones and Iverson 19 ) confirmed that mothers see toddler milk advertisements as a de facto infant formula advertisement. Although the Code (Article 4·3) allows manufacturers to donate informational or educational materials, the companies often use these materials to indirectly market their products to mothers. Therefore, to anticipate such practices, the government needs to ensure that there are no loopholes which can be used by manufacturers to develop new and subtle strategies to promote breast-milk substitutes, bottles and teats. The government is also responsible for ensuring that all health-related legislation is accompanied by effective education for health workers and regular monitoring. The results from the present study can be used as a baseline for continued surveillance by the government.
On the other hand, manufacturers have an obligation to comply with the standards in the Code. They should not contact mothers or women, either directly or indirectly, to promote their breast-milk substitutes, bottles or teats. They should not approach and promote the use of breast-milk substitutes among health workers and mothers of young infants. In terms of labelling, manufacturers must provide the necessary information and warnings in accordance with the Code. Furthermore, according to Article 5 of the Code, there should be no advertising or other forms of promotion to the general public of breast-milk substitutes, including infant formula, other milk products, foods and beverages, including complementary foods, feeding bottles and teats.
Strengths and limitations of the study
The current study was the first that measured compliance with the Code in Indonesia and its large sample size and wide geographical coverage underscore its importance, particularly considering the sociodemographic characteristics of the population. However, the study was hospital-based which introduces potential selection bias. Participants of the study were recruited on a voluntary basis and from the population attending selected hospitals; hence they may not be representative of the general population. Furthermore, selected health workers who participated in the study may have changed their practices since they were being observed. Another study limitation is the potential recall bias as the mothers and health workers were interviewed about breast-milk substitutes over a period of 6 months. Thus, there is the risk of potential inaccuracy in recalling the categories and/or brands of the breast-milk substitutes or the source of information.
Acknowledgements
Acknowledgements: The authors wish to thank the Ministry of Health of the Republic of Indonesia, Directorate of Community Nutrition, for supporting the conduct of the survey. They are also indebted to Dr Damayanti D. Soekarjo for her help on proofreading and editing the final version of the manuscript. Financial support: The study was funded by UNICEF Indonesia through consultancy work of the second (J.F.) and the third (V.A.P.) authors. Conflict of interest: UNICEF Indonesia had no role in the design, analysis or writing of this article. UNICEF Indonesia provided training for J.F. to undertake the survey using the Interagency Group on Breastfeeding Monitoring Protocol. Authorship: J.F. and V.A.P. contributed to the conception and design of the study, acquisition of the data, analysis and interpretation of the data. I.H. was involved in interpretation of the data and prepared the first draft of the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committee, the Faculty of Medicine, Universitas Indonesia. Written informed consent was obtained from all subjects involved in the study.










