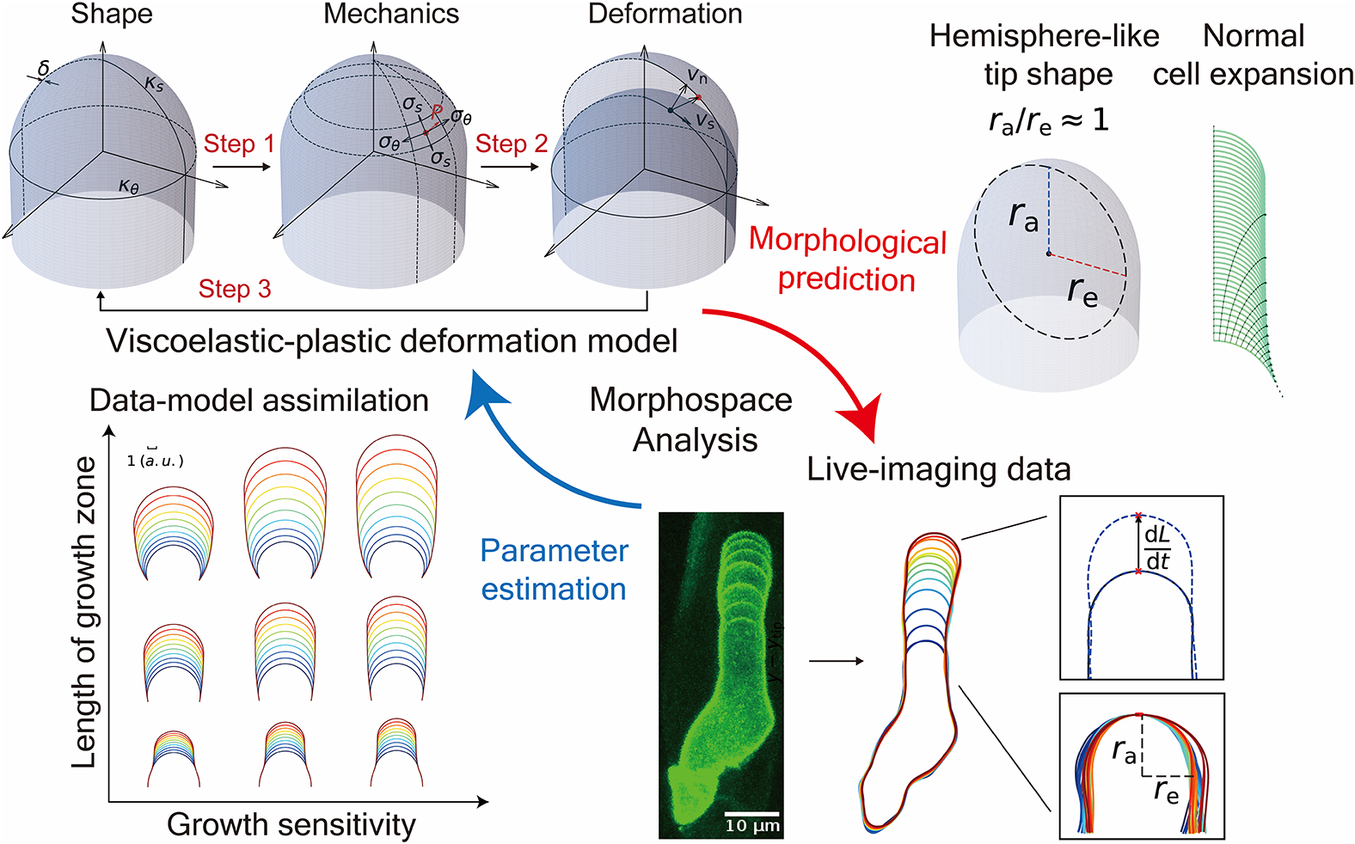
Introduction
Growth patterns in cells are divided into two types: diffuse growth, where the entire cell surface grows and tip growth, where only the tip region grows (Kropf et al., Reference Kropf, Bisgrove and Hable1998). Characteristic features of tip-growing cells include unique cell wall properties, cytoskeleton organisation and organelle activities (Rounds & Bezanilla, Reference Rounds and Bezanilla2013). Since plant cell growth is thought to be controlled by cell wall biosynthesis and orientation (Green, Reference Green1962; Ledbetter & Porter Reference Ledbetter and Porter1963), quantifying cell growth patterns is important for understanding the cell wall properties behind growth, as described more precisely below. To avoid ambiguity, we define surface growth or surface elongation as extension on the cell surface and define surface point velocity as the time derivative of the displacement of the surface point. Early studies of tip growth in Phycomyces fungi quantified surface point velocity with tips estimated to grow at 1.2–1.4 mm per hour (Castle, Reference Castle1942; Castle, Reference Castle1958), while Nitella rhizoids were found to have a linear growth velocity of 1.7 μm/min (estimated as 0.1 mm/h) at the tip dome (Chen, Reference Chen1973). During tip growth of root hairs in Medicago truncatula and pollen tubes in Lilium longiflorum, the maximal elongation zone is not located exactly at the tip but is instead located in the region slightly proximal from the tip (Shaw et al., Reference Shaw, Dumais and Long2000; Dumais et al., Reference Dumais, Long and Shaw2004; Geitmann & Dumais, Reference Geitmann and Dumais2009). These quantitative analyses indicate the importance of detailed quantification when studying tip growth in cells.
In addition to elucidating where the elongation zone is located, quantification of the direction of surface point velocity is also important. For example, imaging data from fungal hyphae with carbon particles on the cell surface revealed that some of the displacements were in directions almost perpendicular to the surface (Bartnicki-Garcia et al., Reference Bartnicki-Garcia, Bracker, Gierz, López-Franco and Lu2000). That study inferred two important aspects using a mathematical model: (1) The driving force should be turgor pressure and (2) the vesicle supply centre should be consistent with the direction almost normal to the cell surface. Viscoelastic–plastic deformation models based on this pioneering mathematical study have been extensively applied to study tip growth (Goriely & Tabor, Reference Goriely and Tabor2003; Dumais et al., Reference Dumais, Shaw, Steele, Long and Ray2006; Dumais, Reference Dumais2021). In these models, cell morphology, the mechanics on the cell surface and the deformation of the apical region are simultaneously analysed using mathematical equations. This allows the prediction of cell shape based on the history of the mechanics and deformation of the cell. These mathematical studies have prompted other studies using mechanical models (finite element methods) with modified material properties in pollen tubes (Fayant et al., Reference Fayant, Girlanda, Chebli, Aubin, Villemure and Geitmann2010) and the introduction of non-dimensional parameters that characterise cell shape independently of cell size for studying tip growth (Campàs et al., Reference Campàs, Rojas, Dumais and Mahadevan2012). Therefore, the mathematical relationships among morphology, mechanics and deformation can be used to determine the cell wall properties during cell growth.
As described above, tip growth occurs in Nitella rhizoids, fungal hyphae, root hairs and pollen tubes. We recently found that plant zygote cells also exhibit tip growth (Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023). In our previous study, we combined live imaging with the so-called normalised coordinate to show that only markers (a pollen grain expressing a sperm-specific plasma membrane) near the tip moved while other markers outside the tip region did not move. Furthermore, the elongating tip of a single zygote cell shows a characteristic hemisphere-like shape. In Arabidopsis (Arabidopsis thaliana) zygotes, microtubules (MTs) form a ring-like structure in the subapical region, which might support the hemisphere-like tip shape (Kimata et al., Reference Kimata, Higaki, Kawashima, Kurihara, Sato, Yamada, Hasezawa, Berger, Higashiyama and Ueda2016). The hemisphere-like shape and MT ring are not typical in tip-growing cells of angiosperms, but similar structures are found in the tip-growing protonema of the southern maidenhair fern (Adiantum capillus-veneris) (Murata and Wada Reference Murata and Wada1989). In this fern protonema, cellulose microfibrils are aligned in parallel to MTs, and cell division occurs. Therefore, the zygote might utilise fern-like tip growth to produce a spherical daughter cell at the tip, which develops into a globular embryo (Ueda et al., Reference Ueda, Zhang and Laux2011). Since such an approximately hemispherical shape during elongation has also been observed in the cells of fission yeast (Schizosaccharomyces pombe; Abenza et al., Reference Abenza, Couturier, Dodgson, Dickmann, Chessel, Dumais and Salas2015), the existence of a unifying mechanism for shaping a spherical tip needs to be investigated.

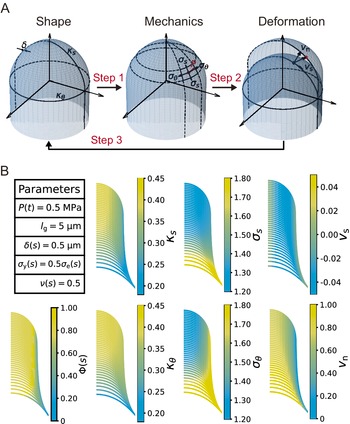
Figure 1. Schematic illustration of the viscoelastic–plastic deformation model. The model output is the cell contour of a tip-growing cell. By applying the hydraulics parameter (turgor pressure,
![]() $P$
) to the current shape with curvature
$P$
) to the current shape with curvature
![]() $\kappa$
and wall thickness
$\kappa$
and wall thickness
![]() $\delta$
, the mechanics with stress
$\delta$
, the mechanics with stress
![]() $\sigma$
are determined. By modifying the mechanical parameters of the cell wall (
$\sigma$
are determined. By modifying the mechanical parameters of the cell wall (
![]() $\nu, \Phi, \mathrm{and}\;{\sigma}_{\mathrm{y}}$
), the deformation with strain rate
$\nu, \Phi, \mathrm{and}\;{\sigma}_{\mathrm{y}}$
), the deformation with strain rate
![]() $\dot{\varepsilon}$
is determined.
$\dot{\varepsilon}$
is determined.
In this study, we reimplemented the viscoelastic–plastic deformation model described by Dumais et al. (Reference Dumais, Shaw, Steele, Long and Ray2006) to elucidate the mechanism that shapes the hemisphere-like tip. First, we obtained growth parameters in the model that matched the hemisphere-like shape described by the data. We determined that this shape results from surface point velocity almost normal to the cell surface around the tip region. Finally, we reconstructed the elongating zygote computationally using model parameters associated with turgor pressure and cell wall expansion derived from actual zygote cell contour data. Our findings shed light on the morphology and mechanics of tip growth in plant cells.
Methods
Viscoelastic–plastic deformation model
A viscoelastic–plastic deformation model was employed (Dumais et al., Reference Dumais, Long and Shaw2004; Dumais et al., Reference Dumais, Shaw, Steele, Long and Ray2006). This model comprises three steps (Figure 1). Step 1: The stress states in the meridional and circumferential directions (
![]() ${\sigma}_{\mathrm{s}}\;\mathrm{and}\;{\sigma}_{\unicode{x3b8}}$
, respectively) are determined by the mechanical equilibrium of the cell with turgor pressure
${\sigma}_{\mathrm{s}}\;\mathrm{and}\;{\sigma}_{\unicode{x3b8}}$
, respectively) are determined by the mechanical equilibrium of the cell with turgor pressure
![]() $P$
. Step 2: The strain rates in the meridional and circumferential directions (
$P$
. Step 2: The strain rates in the meridional and circumferential directions (
![]() ${\dot{\varepsilon}}_{\mathrm{s}}\;\mathrm{and}\;{\dot{\varepsilon}}_{\unicode{x3b8}}$
, respectively) are determined by the stresses applied on the wall and the mechanical properties of the cell wall (
${\dot{\varepsilon}}_{\mathrm{s}}\;\mathrm{and}\;{\dot{\varepsilon}}_{\unicode{x3b8}}$
, respectively) are determined by the stresses applied on the wall and the mechanical properties of the cell wall (
![]() $\nu, \kern0.24em \Phi \kern0.24em \mathrm{and}\;{\sigma}_{\mathrm{y}}$
), where
$\nu, \kern0.24em \Phi \kern0.24em \mathrm{and}\;{\sigma}_{\mathrm{y}}$
), where
![]() $\nu$
is the flow coupling (Dumais et al., Reference Dumais, Shaw, Steele, Long and Ray2006),
$\nu$
is the flow coupling (Dumais et al., Reference Dumais, Shaw, Steele, Long and Ray2006),
![]() $\Phi$
is the cell wall extensibility and
$\Phi$
is the cell wall extensibility and
![]() ${\sigma}_{\mathrm{y}}$
is yield stress. Step 3: The next shape is formulated using the previous cell shape and the velocity vectors (
${\sigma}_{\mathrm{y}}$
is yield stress. Step 3: The next shape is formulated using the previous cell shape and the velocity vectors (
![]() ${v}_{\mathrm{t}}\;\mathrm{and}\;{v}_{\mathrm{n}}$
), where
${v}_{\mathrm{t}}\;\mathrm{and}\;{v}_{\mathrm{n}}$
), where
![]() ${v}_{\mathrm{n}}$
is the velocity in the direction perpendicular to the surface and
${v}_{\mathrm{n}}$
is the velocity in the direction perpendicular to the surface and
![]() ${v}_{\mathrm{t}}$
is the velocity in the meridional direction, which is one of the tangential directions.
${v}_{\mathrm{t}}$
is the velocity in the meridional direction, which is one of the tangential directions.
For Step 1, the following equations were reformulated to evaluate the stress state:
where the parameter
![]() $\delta$
stands for cell wall thickness, and
$\delta$
stands for cell wall thickness, and
![]() ${\kappa}_{\mathrm{s}}$
and
${\kappa}_{\mathrm{s}}$
and
![]() ${\kappa}_{\unicode{x3b8}}$
are the curvatures in the meridional and circumferential directions, respectively.
${\kappa}_{\unicode{x3b8}}$
are the curvatures in the meridional and circumferential directions, respectively.
For Step 2, the following relationship was exploited:
for
![]() ${\sigma}_{\mathrm{e}}\ge {\sigma}_{\mathrm{y}}$
and
${\sigma}_{\mathrm{e}}\ge {\sigma}_{\mathrm{y}}$
and
![]() $K = {\left[\beta {\sigma_{\mathrm{s}}}^2+\beta {\sigma_{\unicode{x3b8}}}^2+\left(\beta -6\nu \right){\sigma}_{\mathrm{s}}{\sigma}_{\unicode{x3b8}}\right]}^{1/2}$
, where
$K = {\left[\beta {\sigma_{\mathrm{s}}}^2+\beta {\sigma_{\unicode{x3b8}}}^2+\left(\beta -6\nu \right){\sigma}_{\mathrm{s}}{\sigma}_{\unicode{x3b8}}\right]}^{1/2}$
, where
![]() ${\sigma}_{\mathrm{e}} = \left(1/\sqrt{2}\right){\left[{\left({\sigma}_{\mathrm{s}}-{\sigma}_{\unicode{x3b8}}\right)}^2+{\left({\sigma}_{\unicode{x3b8}}-{\sigma}_{\mathrm{n}}\right)}^2+{\left({\sigma}_{\mathrm{n}}-{\sigma}_{\mathrm{s}}\right)}^2\right]}^{1/2}$
(Hill, Reference Hill1998) and
${\sigma}_{\mathrm{e}} = \left(1/\sqrt{2}\right){\left[{\left({\sigma}_{\mathrm{s}}-{\sigma}_{\unicode{x3b8}}\right)}^2+{\left({\sigma}_{\unicode{x3b8}}-{\sigma}_{\mathrm{n}}\right)}^2+{\left({\sigma}_{\mathrm{n}}-{\sigma}_{\mathrm{s}}\right)}^2\right]}^{1/2}$
(Hill, Reference Hill1998) and
![]() $\beta = 2{\nu}^2-2\nu +2$
, where
$\beta = 2{\nu}^2-2\nu +2$
, where
![]() $\beta$
represents the anisotropy between the two directional stresses
$\beta$
represents the anisotropy between the two directional stresses
![]() ${\sigma}_{\mathrm{s}}$
and
${\sigma}_{\mathrm{s}}$
and
![]() ${\sigma}_{\unicode{x3b8}}$
. Using
${\sigma}_{\unicode{x3b8}}$
. Using
![]() ${v}_{\mathrm{n}}$
and
${v}_{\mathrm{n}}$
and
![]() ${v}_{\mathrm{t}}$
, the kinematic relations for cell shape can be evaluated as follows:
${v}_{\mathrm{t}}$
, the kinematic relations for cell shape can be evaluated as follows:
based on the cross-sectional radius
![]() $r(S)$
with curvilinear coordinate
$r(S)$
with curvilinear coordinate
![]() $S$
. The angle
$S$
. The angle
![]() $\varphi$
is defined as the angle between the normal vector to the surface and the axis of the cell. The normal strain rate (
$\varphi$
is defined as the angle between the normal vector to the surface and the axis of the cell. The normal strain rate (
![]() ${\dot{\varepsilon}}_{\mathrm{n}}$
) was assumed to always be zero, where thickening due to cell wall deposition (Houwink & Roelofsen, Reference Houwink and Roelofsen1954; Kataoka, Reference Kataoka1982) can be expressed in the following manner:
${\dot{\varepsilon}}_{\mathrm{n}}$
) was assumed to always be zero, where thickening due to cell wall deposition (Houwink & Roelofsen, Reference Houwink and Roelofsen1954; Kataoka, Reference Kataoka1982) can be expressed in the following manner:
where
![]() $D$
is the rate of wall deposition per unit surface area.
$D$
is the rate of wall deposition per unit surface area.
For Step 3, the following velocities in the normal (perpendicular) and meridional directions were obtained from the equations above:
where
![]() $L$
is the meridional distance between the pole and the equator. The spatial scale is based on the arbitrary unit (a.u.), which was rescaled with the typical radius scale, e.g., 5
$L$
is the meridional distance between the pole and the equator. The spatial scale is based on the arbitrary unit (a.u.), which was rescaled with the typical radius scale, e.g., 5
![]() $\unicode{x3bc}$
m.
$\unicode{x3bc}$
m.
A hemispherical tip shape is sufficient for cosine-type wall extensibility
According to Green & King (Reference Green and King1966) and under the model assumptions (Dumais et al., Reference Dumais, Long and Shaw2004; Dumais et al., Reference Dumais, Shaw, Steele, Long and Ray2006), a hemispherical cell shape is sufficient but not necessary for cosine-type wall extensibility, as described below. Considering a hemispherical cell shape with a constant radius
![]() $R$
, the allometric coefficient (anisotropy rate), defined as the rate of the strain rate in the meridional direction to that in circumferential direction, can be expressed as
$R$
, the allometric coefficient (anisotropy rate), defined as the rate of the strain rate in the meridional direction to that in circumferential direction, can be expressed as
It was shown that the meridional velocity in the curvilinear direction is proportional to the
![]() $b\mathrm{th}$
power of the sine function of the curvilinear distance
$b\mathrm{th}$
power of the sine function of the curvilinear distance
![]() $S$
as follows:
$S$
as follows:
If the surface growth around the tip is approximately isotropic on the surface, characterised by
![]() $b = 1$
, the tip shape remains hemispherical (Green, Reference Green1969). In the derivation, assuming that the deformation of the surface is infinitesimal, the strain rate in a small meridional element
$b = 1$
, the tip shape remains hemispherical (Green, Reference Green1969). In the derivation, assuming that the deformation of the surface is infinitesimal, the strain rate in a small meridional element
![]() $\Delta S$
can be expressed as
$\Delta S$
can be expressed as
 $$\begin{align*}{\dot{\varepsilon}}_{\mathrm{s}}\approx \frac{1}{\Delta S} \Delta \left(\frac{\mathrm{d}S}{\mathrm{d}t}\right) = \frac{1}{\Delta S} \Delta \left(A{\sin}^b\left(\frac{S}{R}\right)\right) = \frac{A\sin^b\left(\frac{S+\Delta S}{R}\right)-A{\sin}^b\left(\frac{S}{R}\right)}{\Delta S}.\end{align*}$$
$$\begin{align*}{\dot{\varepsilon}}_{\mathrm{s}}\approx \frac{1}{\Delta S} \Delta \left(\frac{\mathrm{d}S}{\mathrm{d}t}\right) = \frac{1}{\Delta S} \Delta \left(A{\sin}^b\left(\frac{S}{R}\right)\right) = \frac{A\sin^b\left(\frac{S+\Delta S}{R}\right)-A{\sin}^b\left(\frac{S}{R}\right)}{\Delta S}.\end{align*}$$
Let
![]() $\Delta S\to 0$
, then
$\Delta S\to 0$
, then
 $$\begin{align*}\dot{\varepsilon}_{\mathrm{s}} = \frac{\mathrm{d}\left(A{\sin}^b\left(\frac{S}{R}\right)\right)}{\mathrm{d}S} = bA{\sin}^{b-1}\left(\frac{S}{R}\right) \cos \left(\frac{S}{R}\right) \frac{1}{R}.\end{align*}$$
$$\begin{align*}\dot{\varepsilon}_{\mathrm{s}} = \frac{\mathrm{d}\left(A{\sin}^b\left(\frac{S}{R}\right)\right)}{\mathrm{d}S} = bA{\sin}^{b-1}\left(\frac{S}{R}\right) \cos \left(\frac{S}{R}\right) \frac{1}{R}.\end{align*}$$
When the tip shape remains hemispherical with
![]() $b = 1$
,
$b = 1$
,
In addition, according to Dumais et al. (Reference Dumais, Long and Shaw2004), the wall extensibility is estimated using the ratio
where
![]() $\dot{\varepsilon}_{\mathrm{s}} = \dot{\varepsilon}_{\unicode{x3b8}}$
(transversely isotropic property) and
$\dot{\varepsilon}_{\mathrm{s}} = \dot{\varepsilon}_{\unicode{x3b8}}$
(transversely isotropic property) and
![]() ${\sigma}_{\mathrm{s}} = {\sigma}_{\unicode{x3b8}}$
for a hemispherical tip. To substitute the equations for
${\sigma}_{\mathrm{s}} = {\sigma}_{\unicode{x3b8}}$
for a hemispherical tip. To substitute the equations for
![]() ${\sigma}_{\mathrm{s}}$
and
${\sigma}_{\mathrm{s}}$
and
![]() $\dot{\varepsilon}_{\mathrm{s}}$
into the above expression, the following relation can be derived:
$\dot{\varepsilon}_{\mathrm{s}}$
into the above expression, the following relation can be derived:
 $$\begin{align*}\Phi \left(\mathrm{S}\right) = \frac{\dot{\varepsilon}_{\mathrm{s}}}{\sigma_{\mathrm{s}}} = \frac{A\cos \left(\frac{S}{R}\right) \frac{1}{R}}{\frac{P}{2\delta {\kappa}_{\unicode{x3b8}}}} = \frac{2 A\delta {\kappa}_{\unicode{x3b8}}}{PR}\cos \left(\frac{S}{R}\right).\end{align*}$$
$$\begin{align*}\Phi \left(\mathrm{S}\right) = \frac{\dot{\varepsilon}_{\mathrm{s}}}{\sigma_{\mathrm{s}}} = \frac{A\cos \left(\frac{S}{R}\right) \frac{1}{R}}{\frac{P}{2\delta {\kappa}_{\unicode{x3b8}}}} = \frac{2 A\delta {\kappa}_{\unicode{x3b8}}}{PR}\cos \left(\frac{S}{R}\right).\end{align*}$$
This means that a hemispherical tip geometry is a sufficient condition for cosine-type wall extensibility. Note that cosine-type wall extensibility is not sufficient for a hemispherical shape when the transversely isotropic property is not held.
Results
The viscoelastic–plastic deformation model can simultaneously evaluate cell shape, cell wall stresses and strain rates
The viscoelastic–plastic deformation model enabled us to investigate wall stresses and deformation using only the cell shape (Figure 1, Figure 2A, see details in Methods). Specifically, we used shape information (the meridional curvature
![]() ${\kappa}_{\mathrm{s}}$
, the circumferential curvature
${\kappa}_{\mathrm{s}}$
, the circumferential curvature
![]() ${\kappa}_{\unicode{x3b8}}$
and cell wall thickness
${\kappa}_{\unicode{x3b8}}$
and cell wall thickness
![]() $\delta$
) and turgor pressure
$\delta$
) and turgor pressure
![]() $P$
to calculate the mechanical information (the meridional stress
$P$
to calculate the mechanical information (the meridional stress
![]() ${\sigma}_{\mathrm{s}}$
and the circumferential stress
${\sigma}_{\mathrm{s}}$
and the circumferential stress
![]() ${\sigma}_{\unicode{x3b8}}$
) (Step 1). We then calculated the velocity vectors (
${\sigma}_{\unicode{x3b8}}$
) (Step 1). We then calculated the velocity vectors (
![]() ${v}_{\mathrm{n}}$
and
${v}_{\mathrm{n}}$
and
![]() ${v}_{\mathrm{t}}$
) in the normal (perpendicular) and meridional directions associated with strain information (the meridional strain rate
${v}_{\mathrm{t}}$
) in the normal (perpendicular) and meridional directions associated with strain information (the meridional strain rate
![]() ${\dot{\varepsilon}}_{\mathrm{s}}$
and the circumferential strain rate
${\dot{\varepsilon}}_{\mathrm{s}}$
and the circumferential strain rate
![]() ${\dot{\varepsilon}}_{\unicode{x3b8}}$
) (Steps 2 and 3).
${\dot{\varepsilon}}_{\unicode{x3b8}}$
) (Steps 2 and 3).

Figure 2. Simultaneous evaluation of the shape, mechanics and deformation of tip-growing cells. (a) Schematic representation of the model. The mechanics variable
![]() $\sigma$
is determined from the shape data
$\sigma$
is determined from the shape data
![]() $\left(\kappa, \delta \right)$
, through mechanical equilibrium with turgor pressure
$\left(\kappa, \delta \right)$
, through mechanical equilibrium with turgor pressure
![]() $P$
. The deformation variable
$P$
. The deformation variable
![]() $\dot{\varepsilon}$
is determined from the mechanics input. (b) Evaluated shape, mechanics and deformation variables and wall extensibility.
$\dot{\varepsilon}$
is determined from the mechanics input. (b) Evaluated shape, mechanics and deformation variables and wall extensibility.
As the model includes the shape, mechanics and deformation of the cell at each time step, it is reasonable to describe all of these simultaneously, as shown in Figure 2B. Using the parameters in Figure 2B, we obtained the shape information
![]() $\left({\kappa}_{\mathrm{s}},{\kappa}_{\unicode{x3b8}}\right)$
, mechanical information
$\left({\kappa}_{\mathrm{s}},{\kappa}_{\unicode{x3b8}}\right)$
, mechanical information
![]() $\left({\sigma}_{\mathrm{s}},{\sigma}_{\unicode{x3b8}}\right)$
and deformational information
$\left({\sigma}_{\mathrm{s}},{\sigma}_{\unicode{x3b8}}\right)$
and deformational information
![]() $\left({v}_{\mathrm{n}},{v}_{\mathrm{t}}\right)$
for a typical tip-growing cell with radius 1 (a.u.). This simultaneous evaluation is important because it incorporates the mechanical and deformational events simultaneously with the corresponding shape change.
$\left({v}_{\mathrm{n}},{v}_{\mathrm{t}}\right)$
for a typical tip-growing cell with radius 1 (a.u.). This simultaneous evaluation is important because it incorporates the mechanical and deformational events simultaneously with the corresponding shape change.
Cosine-type wall extensibility results in the formation of a hemisphere-like tip shape
To further investigate the characteristic features of the model, we investigated the strain profile derived from stress input, turgor pressure and cell wall extensibility. The strain profile is defined as the curvilinear coordinate system
![]() $S,$
where
$S,$
where
![]() $S = 0$
at the tip and
$S = 0$
at the tip and
![]() $S = s$
at position
$S = s$
at position
![]() $s$
(Figure 3A). The cell wall extensibility
$s$
(Figure 3A). The cell wall extensibility
![]() $\Phi (S)$
is the degree of surface growth, as presented in Figure 3D, where the magnitude at
$\Phi (S)$
is the degree of surface growth, as presented in Figure 3D, where the magnitude at
![]() $S = 0$
is denoted by
$S = 0$
is denoted by
![]() ${\Phi}_0$
and the range of the extension zone is denoted by
${\Phi}_0$
and the range of the extension zone is denoted by
![]() ${l}_{\mathrm{g}}$
. Based on this strain profile, we explored the spatial distribution of cell wall extensibility. As the zygote maintains an approximately hemispherical shape (Figure 3A–C and Figures S1−S3), the distribution of surface growth should reflect this shape change (the current hemisphere to the next hemisphere).
${l}_{\mathrm{g}}$
. Based on this strain profile, we explored the spatial distribution of cell wall extensibility. As the zygote maintains an approximately hemispherical shape (Figure 3A–C and Figures S1−S3), the distribution of surface growth should reflect this shape change (the current hemisphere to the next hemisphere).

Figure 3. Cosine-type wall extensibility model showing a hemisphere-like shape change during cell elongation. (a) Left panel shows cell contours of a zygote with temporal colour code from blue to red with ellipse fitting (black dashed lines). Right panels show schematic illustrations of ellipse fitting and curvilinear coordinate with
![]() $S = 0$
at the tip. (b) Top panel shows the curvature profile as a function of S and that from ellipse fitting (red dashed line). Bottom panel shows the spatio-temporal kymograph of the curvature. (c) Plot of
$S = 0$
at the tip. (b) Top panel shows the curvature profile as a function of S and that from ellipse fitting (red dashed line). Bottom panel shows the spatio-temporal kymograph of the curvature. (c) Plot of
![]() ${r}_{\mathrm{e}}$
and
${r}_{\mathrm{e}}$
and
![]() ${r}_{\mathrm{a}}$
with the diagonal
${r}_{\mathrm{a}}$
with the diagonal
![]() ${r}_{\mathrm{e}} = {r}_{\mathrm{a}}$
(dotted line) based on the dataset in Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023. (d) Spatial distribution of cell wall extensibility. Greater magnitude of cell wall extensibility indicates a high strain rate at the extension zone. (e) Three different profiles of cell wall extensibility were considered: case (1)
${r}_{\mathrm{e}} = {r}_{\mathrm{a}}$
(dotted line) based on the dataset in Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023. (d) Spatial distribution of cell wall extensibility. Greater magnitude of cell wall extensibility indicates a high strain rate at the extension zone. (e) Three different profiles of cell wall extensibility were considered: case (1)
![]() $\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
, case (2)
$\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
, case (2)
![]() $\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
, and case (3)
$\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
, and case (3)
![]() $\Phi (S) = 1-S/{l}_{\mathrm{g}}$
. (f) Ellipse fitting revealed that the aspect ratio of tip shape becomes close to 1.00 in the case of a cosine-type profile. The values for the three examples are
$\Phi (S) = 1-S/{l}_{\mathrm{g}}$
. (f) Ellipse fitting revealed that the aspect ratio of tip shape becomes close to 1.00 in the case of a cosine-type profile. The values for the three examples are
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.01$
for cosine,
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.01$
for cosine,
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.11$
for square of cosine, and
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.11$
for square of cosine, and
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.23$
for linear function.
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.23$
for linear function.
The time-averaged velocities of the three example zygotes reported by Kang et al. (Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023) are presented in supplementary Figure S4, where the tip has a maximum growth rate that gradually decreases as the meridional distance increases. We also confirmed that the curvature away from the tip did not change significantly over time for all the samples shown in supplementary Figure S5. This indicates that the zygote cells grow in the manner of tip growth with a hemisphere-like shape. Acknowledging that early studies (Green & King Reference Green and King1966; Green Reference Green1969; Dumais et al., Reference Dumais, Long and Shaw2004) demonstrated that the hemispherical shape is sufficient for cosine-type wall extensibility in our model, the hemispherical shape is ensured by the cosine-type wall extensibility only if the growth is transversely isotropic (
![]() $\dot{\varepsilon}_{\mathrm{s}} = \dot{\varepsilon}_{\unicode{x3b8}}$
, see Methods). Therefore, we sought different types of
$\dot{\varepsilon}_{\mathrm{s}} = \dot{\varepsilon}_{\unicode{x3b8}}$
, see Methods). Therefore, we sought different types of
![]() $\Phi (S)$
without assuming transversely isotropic growth. We employed three different formulations: case (1)
$\Phi (S)$
without assuming transversely isotropic growth. We employed three different formulations: case (1)
![]() $\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
, case (2)
$\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
, case (2)
![]() $\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
and case (3)
$\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
and case (3)
![]() $\Phi (S) = 1-S/{l}_{\mathrm{g}}$
, as shown in Figure 3E. We then quantified the ellipse-fitting parameters
$\Phi (S) = 1-S/{l}_{\mathrm{g}}$
, as shown in Figure 3E. We then quantified the ellipse-fitting parameters
![]() $\left({r}_{\mathrm{a}},{r}_{\mathrm{e}}\right)$
using the radial half axis
$\left({r}_{\mathrm{a}},{r}_{\mathrm{e}}\right)$
using the radial half axis
![]() ${r}_{\mathrm{a}}$
and the circumferential half axis
${r}_{\mathrm{a}}$
and the circumferential half axis
![]() ${r}_{\mathrm{e}}$
for our model (Figure 3F). By definition, the aspect ratio
${r}_{\mathrm{e}}$
for our model (Figure 3F). By definition, the aspect ratio
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}>1$
corresponds to a tapered shape,
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}>1$
corresponds to a tapered shape,
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}} = 1$
corresponds to a hemispherical shape and
${r}_{\mathrm{a}}/{r}_{\mathrm{e}} = 1$
corresponds to a hemispherical shape and
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}<1$
corresponds to a flattened shape. As shown in Figure 3D, the aspect ratios obtained were close to the value
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}<1$
corresponds to a flattened shape. As shown in Figure 3D, the aspect ratios obtained were close to the value
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.01$
for (1),
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.01$
for (1),
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.11$
for (2) and
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.11$
for (2) and
![]() ${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.23$
for (3). The actual data for the aspect ratio of cell shape were approximately equal to 1.00, corresponding to a hemisphere-like shape, indicating that the cosine-type wall extensibility profile is well fitted to tip-growing cells in plant zygotes.
${r}_{\mathrm{a}}/{r}_{\mathrm{e}}\approx 1.23$
for (3). The actual data for the aspect ratio of cell shape were approximately equal to 1.00, corresponding to a hemisphere-like shape, indicating that the cosine-type wall extensibility profile is well fitted to tip-growing cells in plant zygotes.
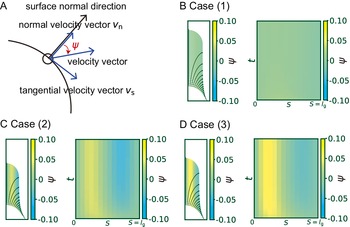
The hemisphere-like cell growth model exhibits a normal growth direction around the cell tip
To clarify what happens to the growth direction in the model with a hemisphere-like shape, we investigated the directional angle
![]() $\psi$
, which measures the deviated angle from the normal surface direction to the direction of surface point velocity (Figure 4A). The colour diagrams of
$\psi$
, which measures the deviated angle from the normal surface direction to the direction of surface point velocity (Figure 4A). The colour diagrams of
![]() $\psi$
as a function of
$\psi$
as a function of
![]() $S$
show that
$S$
show that
![]() $\psi$
is approximately equal to 0 regardless of
$\psi$
is approximately equal to 0 regardless of
![]() $S$
, which indicates a normal growth direction around the cell tip (Figure 4B). By contrast, the angle
$S$
, which indicates a normal growth direction around the cell tip (Figure 4B). By contrast, the angle
![]() $\psi$
for cases (2) and (3) shows positive values in the subapical regions (Figure 4C and Figure 4D), indicating that the growth direction is not normal. Therefore, the resulting shape in cases (2) and (3) becomes more tapered. Therefore, we concluded that the cosine-type wall extensibility leads to the normal growth direction.
$\psi$
for cases (2) and (3) shows positive values in the subapical regions (Figure 4C and Figure 4D), indicating that the growth direction is not normal. Therefore, the resulting shape in cases (2) and (3) becomes more tapered. Therefore, we concluded that the cosine-type wall extensibility leads to the normal growth direction.

Figure 4. Hemispherical shape results from the normal growth direction during cell elongation. (a) Definition of the growth angle
![]() $\psi$
. (b–d) Growth trajectories of selected points (black lines) with colour code
$\psi$
. (b–d) Growth trajectories of selected points (black lines) with colour code
![]() $\psi$
are shown in the left panel, and spatio-temporal plots of the corresponding color code
$\psi$
are shown in the left panel, and spatio-temporal plots of the corresponding color code
![]() $\psi$
are shown in the right panel for case (1)
$\psi$
are shown in the right panel for case (1)
![]() $\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
(B), case (2)
$\Phi (S) = \cos \left(\pi S/2{l}_{\mathrm{g}}\right)$
(B), case (2)
![]() $\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
(C), and case (3)
$\Phi (S) = \mathrm{co}{\mathrm{s}}^2\left(\pi S/2{l}_{\mathrm{g}}\right)$
(C), and case (3)
![]() $\Phi (S) = 1-S/{l}_{\mathrm{g}}$
(D).
$\Phi (S) = 1-S/{l}_{\mathrm{g}}$
(D).
An elongating zygote can be reconstructed computationally from cell contour data alone
As described above, we obtained a mathematically supported logical connection between cosine-type wall extensibility and the normal growth direction for hemisphere-like zygote shapes. The relationship points to the possibility of inferring model parameters from only cell contour data using such a constraint for wall extensibility. In our previously reported live-imaging time sequence of zygote plasma membrane markers, we obtained cell contours using the cell contour–based coordinate normalisation (CCN) method (Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023). Using the cell contour, we quantified the so-called morphospace
![]() $\left({r}_{\mathrm{e}},\frac{\mathrm{d}y}{\mathrm{d}t}\right),$
where
$\left({r}_{\mathrm{e}},\frac{\mathrm{d}y}{\mathrm{d}t}\right),$
where
![]() $\mathrm{d}y/\mathrm{d}t$
is the growth rate in the y-axis (Figure 5A). Among the previously reported samples (Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023), parameters were distributed in the range
$\mathrm{d}y/\mathrm{d}t$
is the growth rate in the y-axis (Figure 5A). Among the previously reported samples (Kang et al., Reference Kang, Matsumoto, Nonoyama, Nakagawa, Ishimoto, Tsugawa and Ueda2023), parameters were distributed in the range
![]() ${r}_{\mathrm{e}}\in \left[3,5\right]$
and
${r}_{\mathrm{e}}\in \left[3,5\right]$
and
![]() $\mathrm{d}y/\mathrm{d}t\in \left[1,5\right]$
. For the sake of simplicity, we considered the time-averaged values for each sample (Figure 5B). Based on perturbation analysis of
$\mathrm{d}y/\mathrm{d}t\in \left[1,5\right]$
. For the sake of simplicity, we considered the time-averaged values for each sample (Figure 5B). Based on perturbation analysis of
![]() $P$
and
$P$
and
![]() ${l}_{\mathrm{g}}$
in the simulations (Figure 5C), we noticed that
${l}_{\mathrm{g}}$
in the simulations (Figure 5C), we noticed that
![]() $P$
only affects the growth rate, while
$P$
only affects the growth rate, while
![]() ${l}_{\mathrm{g}}$
predominantly changes the radius. Therefore, we classified all the samples into group 1 or group 2 and fitted the parameter set
${l}_{\mathrm{g}}$
predominantly changes the radius. Therefore, we classified all the samples into group 1 or group 2 and fitted the parameter set
![]() $\left(P,{l}_{\mathrm{g}}\right)$
for the sample-averaged values of
$\left(P,{l}_{\mathrm{g}}\right)$
for the sample-averaged values of
![]() $\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
denoted by Examples 1 and 2, respectively. To further confirm what happens for intermediate parameters, we included Example 3, with almost the same growth rate as Example 1 and almost the same radius as Example 2. Using typical values for each quantity
$\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
denoted by Examples 1 and 2, respectively. To further confirm what happens for intermediate parameters, we included Example 3, with almost the same growth rate as Example 1 and almost the same radius as Example 2. Using typical values for each quantity
![]() $\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
with the previously estimated range of
$\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
with the previously estimated range of
![]() $P\;\left(0.3\hbox{--} 1.0\;\mathrm{MPa}\right)$
(Cosgrove, Reference Cosgrove1993; Lintilhac et al., Reference Lintilhac, Wei, Tanguay and Outwater2000; Radotić et al., Reference Radotić, Roduit, Simonović, Hornitschek, Fankhauser, Mutavdžić, Steinbach, Dietler and Kasas2012) (Figure 5C), we applied an exhaustive search of
$P\;\left(0.3\hbox{--} 1.0\;\mathrm{MPa}\right)$
(Cosgrove, Reference Cosgrove1993; Lintilhac et al., Reference Lintilhac, Wei, Tanguay and Outwater2000; Radotić et al., Reference Radotić, Roduit, Simonović, Hornitschek, Fankhauser, Mutavdžić, Steinbach, Dietler and Kasas2012) (Figure 5C), we applied an exhaustive search of
![]() ${l}_{\mathrm{g}}$
parameters that matched the data and obtained the fitted parameter
${l}_{\mathrm{g}}$
parameters that matched the data and obtained the fitted parameter
![]() ${l}_{\mathrm{g}}$
. With these parameters, we reconstructed the elongating zygote computationally (Figure 5D). Note that we assumed a constant cell wall thickness
${l}_{\mathrm{g}}$
. With these parameters, we reconstructed the elongating zygote computationally (Figure 5D). Note that we assumed a constant cell wall thickness
![]() $\left(\delta = 0.5\;\unicode{x3bc} \mathrm{m}\right)$
in this study based on the acknowledgement that the parameter
$\left(\delta = 0.5\;\unicode{x3bc} \mathrm{m}\right)$
in this study based on the acknowledgement that the parameter
![]() ${\Phi}_0$
was affected by
${\Phi}_0$
was affected by
![]() $\delta$
.
$\delta$
.

Figure 5. Reconstruction of model parameters using only cell contour data. (a) Morphospace analysis using the circumferential half-axis
![]() ${r}_{\mathrm{e}}$
and the growth rate in the y-axis
${r}_{\mathrm{e}}$
and the growth rate in the y-axis
![]() $\mathrm{d}y/\mathrm{d}t$
. (b) The time average of the growth rate and the radius for each sample. (c) Perturbation analysis of
$\mathrm{d}y/\mathrm{d}t$
. (b) The time average of the growth rate and the radius for each sample. (c) Perturbation analysis of
![]() $P$
and
$P$
and
![]() ${l}_{\mathrm{g}}$
in the mechanical simulations. (d) The samples are classified into group 1 or group 2, with the sample-averaged values of
${l}_{\mathrm{g}}$
in the mechanical simulations. (d) The samples are classified into group 1 or group 2, with the sample-averaged values of
![]() $\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
denoted by Examples 1 and 2, respectively. The left panel shows the reconstructed model for Example 1 and one data point from the contour data for group 1. The right panel shows the reconstructed model for Example 2 and one data point from the contour data for group 2.
$\left({r}_{\mathrm{e}},\mathrm{d}y/\mathrm{d}t\right)$
denoted by Examples 1 and 2, respectively. The left panel shows the reconstructed model for Example 1 and one data point from the contour data for group 1. The right panel shows the reconstructed model for Example 2 and one data point from the contour data for group 2.

Figure 6. Tip-growing behavior is controlled by non-dimensional parameters
![]() $\left(\alpha, \beta \right)$
. (a) Schematic illustration of the non-dimensional parameters
$\left(\alpha, \beta \right)$
. (a) Schematic illustration of the non-dimensional parameters
![]() $\alpha$
and
$\alpha$
and
![]() $\beta$
. (b) Morphospace for the elongating zygote where the stable elongating cell shape depends only on the non-dimensional parameters
$\beta$
. (b) Morphospace for the elongating zygote where the stable elongating cell shape depends only on the non-dimensional parameters
![]() $\left(\alpha, \beta \right)$
.
$\left(\alpha, \beta \right)$
.
To summarise, we were able to reconstruct the elongating zygote computationally only from the cell contour data, with the parameter ranges inherited from the data range.
Non-dimensional control parameters
 $\left(\boldsymbol{\alpha}, \boldsymbol{\beta} \right)$
characterise tip-growing behaviour
$\left(\boldsymbol{\alpha}, \boldsymbol{\beta} \right)$
characterise tip-growing behaviour
Through the above model reconstruction, we determined effective parameters as follows. First, as the growth rate is affected by
![]() $P,{\Phi}_0,\mathrm{and}\;{l}_{\mathrm{g}}$
, we defined the cell growth sensitivity as
$P,{\Phi}_0,\mathrm{and}\;{l}_{\mathrm{g}}$
, we defined the cell growth sensitivity as
This is a non-dimensional parameter that reveals the vertical growth length (Figure 6A). As the parameter
![]() ${r}_{\mathrm{e}}$
is another characteristic of the zygote cell, we can also define the constant
${r}_{\mathrm{e}}$
is another characteristic of the zygote cell, we can also define the constant
This is the other non-dimensional parameter and is the ratio between meridional growth length and hemispherical half axis length (Figure 6A). Under the hypothetical condition of the cosine-type wall extensibility profile,
![]() $\beta = \pi /2$
. By definition, the parameters
$\beta = \pi /2$
. By definition, the parameters
![]() $\alpha\;\mathrm{and}\;\beta$
are independent of cell size; therefore, these can be used to sufficiently characterise tip-growing behaviour, as shown in Figure 6B.
$\alpha\;\mathrm{and}\;\beta$
are independent of cell size; therefore, these can be used to sufficiently characterise tip-growing behaviour, as shown in Figure 6B.
Discussion
The viscoelastic–plastic deformation model developed in this study explores three new aspects of cell elongation analysis. (1) Simultaneous evaluation of shape, mechanics and deformation, which will be a powerful tool for understanding multiple factors during cell elongation. (2) Quantitative verification of normal growth direction to produce a hemisphere-like shape. (3) Model reconstruction using only the cell contours derived from live-imaging data. Some possible future directions are summarised below.
In the field of plant mechanobiology, mechanical measurement is critical, such as in atomic force microscopy (Beauzamy et al., Reference Beauzamy, Derr and Boudaoud2015; Tsugawa et al., Reference Tsugawa, Yamasaki, Horiguchi, Zhang, Muto, Nakaso, Ito, Takebayashi, Okano, Akita, Yasukuni, Demura, Mimura, Kawaguchi and Hosokawa2022) and in mechanical inference from stem shape (Nakata et al., Reference Nakata, Takahara, Sakamoto, Yoshida and Mitsuda2018). However, these methods are only applicable to the surface cells and external shapes of organs, whereas the mechanics of developmentally important cells, such as the zygote cells within the seed and lateral root primordia or vascular cells inside the root, have not yet been fully examined. Using our data–model combined method, we defined the model parameters using only the quantification of cell contours. Therefore, our method can precisely infer cellular mechanics, which cannot be determined solely by current mechanical measurement techniques, opening a new avenue for studying cellular mechanics not only in plant developmental biology but also in other multicellular systems including animal cells.
Considering the Lockhart equations (Lockhart, Reference Lockhart1965a, Reference Lockhart1965b), which guide the reconstruction of the plastic deformation of plant cells, the cosine-type distribution
![]() $\Phi (S)$
and its effect on morphology can be thought of as a spatial example of plastic deformation relating to hemisphere-like tip shape. Biological events regulating cosine-type distribution should include the heterogeneous distribution of microtubule ring structure (Kimata et al., Reference Kimata, Higaki, Kawashima, Kurihara, Sato, Yamada, Hasezawa, Berger, Higashiyama and Ueda2016), where the microtubule-associated cell wall deposition acts as a mechanical hoop to regulate hemisphere-like tip shape. Furthermore, since our model includes viscoelastic deformation, it also considers the later-proposed viscoelastic–plastic deformation (Ortega, Reference Ortega1985). As revisited by Green et al. (Reference Green, Erickson and Buggy1971), a possible first approach for reconstructing plastic deformation including viscoelasticity is to use models that take into account a threshold level of viscoelasticity. The present model precisely reflects the understanding that cell wall loosening occurs before water uptake (Cosgrove, Reference Cosgrove1985, Reference Cosgrove1987), implying that the parameter
$\Phi (S)$
and its effect on morphology can be thought of as a spatial example of plastic deformation relating to hemisphere-like tip shape. Biological events regulating cosine-type distribution should include the heterogeneous distribution of microtubule ring structure (Kimata et al., Reference Kimata, Higaki, Kawashima, Kurihara, Sato, Yamada, Hasezawa, Berger, Higashiyama and Ueda2016), where the microtubule-associated cell wall deposition acts as a mechanical hoop to regulate hemisphere-like tip shape. Furthermore, since our model includes viscoelastic deformation, it also considers the later-proposed viscoelastic–plastic deformation (Ortega, Reference Ortega1985). As revisited by Green et al. (Reference Green, Erickson and Buggy1971), a possible first approach for reconstructing plastic deformation including viscoelasticity is to use models that take into account a threshold level of viscoelasticity. The present model precisely reflects the understanding that cell wall loosening occurs before water uptake (Cosgrove, Reference Cosgrove1985, Reference Cosgrove1987), implying that the parameter
![]() $\Phi (S)$
is the most important wall parameter involved in morphology, stress and strain. In addition, we identified the non-dimensional indices
$\Phi (S)$
is the most important wall parameter involved in morphology, stress and strain. In addition, we identified the non-dimensional indices
![]() $\alpha$
and
$\alpha$
and
![]() $\beta$
that fully characterise the dynamics of our tip-growing cells.
$\beta$
that fully characterise the dynamics of our tip-growing cells.
In summary, we developed a data-driven reconstruction method of mechanical model for studying cell elongation. This method represents a promising tool for studying the growth mechanisms and biological connections between morphology, mechanics and deformation, paving the way for understanding tip-growing cells.
Acknowledgements
The authors thank Koichi Fujimoto and his lab members (Hiroshima University) and Takumi Higaki and his lab members (Kumamoto University) and Yusuke Kimata (Tohoku University) for helpful discussions.
Financial statement
This work was supported by the Japan Society for the Promotion of Science [JSPS KAKENHI (JP20K15832 to S.T.), a Grant-in-Aid for Early-Career Scientists (JP22K15135 to H.M.), a Grant-in-Aid for Scientific Research on Innovative Areas (JP19H05670 and JP19H05676 to M.U.), a Grant-in-Aid for Scientific Research (B) (JP23H02494 to M.U.), International Leading Research (JP22K21352 to M.U.)], the Japan Science and Technology Agency [CREST (JPMJCR2121)], the Suntory Rising Stars Encouragement Program in Life Sciences (SunRiSE; to M.U.) and the Toray Science Foundation (20-6102 to M.U.).
Competing interest
The authors have no competing interests.
Author contributions
Z.K. and S.T. conceived and designed the study. Z.K. and S.T. developed and implemented models and algorithms. H.M., S.N. and M.U. carried out data acquisition from live cell imaging. Z.K. and S.T. wrote the manuscript. Z.K., T.N., S.T., M.U. and Y.I. edited and reviewed the manuscript. S.T., H.M. and M.U. provided research funding. All authors have read and approved the manuscript.
Data availability statement
The data supporting the findings of this study are available from the author, Zichen Kang, upon a reasonable request. The code used in this study is available on GitHub (https://github.com/blues0910/A-viscoelastic-plastic-deformation-model-of-hemisphere-like-tip-growth).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/qpb.2024.13.










































Comments
Dear Editor:
We would like to submit our manuscript “An elasto-plastic deformation model of hemispherical tip growth in Arabidopsis zygotes” for publication in Quantitative Plant Biology as an original research article.
In a single cell term in Arabidopsis zygote, a unidirectional anisotropic elongation is crucially important because it determines the axial and internal/external axes of the individual. In our previous study (Kang et al., Plant Cell Physiology, 2023), we found that the type of the elongation was tip growth and the tip shape has a characteristic hemispherical shape. In this study, an elasto-plastic deformation model was employed to elucidate the mechanism underlying the formation of the hemisphere-shaped tip of Arabidopsis zygote. As a main result, we obtained growth parameters in the model that matched the hemispherical shape in the data and reconstructed the computational elongating zygote using model parameters associated with turgor pressure and cell wall expansion derived from the actual zygote cell contour data. Based on this finding, we further identified that two non-dimensional parameters are sufficient to characterize the size and shape of an elongating zygote.
We believe that our study makes a significant contribution to the literature of the studies of zygote because of our novel approach in terms of model-data correspondence based on the so-called morphospace analysis to combine live-imaging data with the mathematical model. Furthermore, our finding that zygote morphology can be evaluated only by the two non-dimensional parameters would allow us to analyze the other types of cells or organs in the same manner. This approach enables us to predict cellular mechanics, which cannot be determined solely by current mechanical measurement techniques, opening a new avenue for studying cellular mechanics not only in plant developmental biology but also in other multicellular systems including animal cells.
We believe that the findings of this study are nicely fit to the scope of the journal Plant Quantitative biology and will be of interest to its broad readership.
Sincerely,
Satoru Tsugawa
Satoru Tsugawa
Faculty of Systems Science and Technology,
Akita Prefectural University,
Akita, 015-0055, Japan
Tel: + 81-18-427-2191;
Email: tsugawa@akita-pu.ac.jp