In the past decade a series of studies has shown that coronary heart disease, hypercholesterolaemia, hypertension, stroke and non-insulin-dependent diabetes mellitus (NIDDM) have their origins in foetal life (Reference Barker, Osmond and WinterBarker et al, 1989; Reference BarkerBarker, 1998). Low birth weight in singletons born at term and a low growth rate in early infancy leading to low weight at 1 year are both implicated in these conditions. These studies have been replicated in many parts of the world (Reference Forsen, Eriksson and TuomilehtoForsen et al, 1997) and show that the associations are independent of social class or adult lifestyle. A few studies have suggested that in monozygotic twins discordant for hypertension (Reference Poulter, Chang and MacGregorPoulter et al, 1999) and diabetes (Reference Poulsen, Vaag and KyvikPoulsenet al, 1997) the lighter baby is most likely to develop the illness, demonstrating that these effects may operate independently of genetic vulnerability.
The growth of a foetus is determined mainly by the intra-uterine environment and is limited by the nutrients and oxygen it receives (Reference MortonMorton, 1955; Reference McCance and WiddowsonMcCance & Widdowson, 1974; Reference BarkerBarker, 1998). Individual organs differ in the time of maximum growth rates and therefore have different critical times in their development. Male foetuses have more rapid growth trajectories than females and therefore are more vulnerable to impaired nutrition (Reference BarkerBarker, 1998).
Hormonal programming
The relationship between undernutrition and later disease is mediated by persisting changes to the internal environment of the individual. In particular there are long-lasting effects known as ‘hormonal programming’ in which the plasma levels of hormones or the set points of neuroendocrine systems are altered permanently. These have been shown to occur in the hypothalamic—pituitary—adrenal (HPA), growth-hormone, thyroxine and insulin axes. There is a clear association between depressive disorder and cardiovascular disease in community samples (Reference VaillantVaillant, 1998) and this relationship is still present after controlling for smoking (Reference Anda, Williamson and JonesAnda et al, 1993). The association is stronger in men than in women (Reference Hippisley-Cox, Fielding and PringleHippisley-Cox et al, 1998). The cause of the association is unknown and in some cases it may be simply a reaction to the physical disability. Other commentators have suggested that depression itself may induce physical disease, possibly by increasing levels of stress hormones (Reference DinanDinan, 1998). Another possibility that has not been discussed previously is that both depression and cardiovascular disease may share a common vulnerability factor in foetal life.
Relevance to depression
The causes of depression are incompletely understood. Adverse life events often are the proximal cause of a depressive illness (Reference MurphyMurphy, 1982) but act selectively on vulnerable individuals (Reference Kendler, Karkowski and PrescottKendler et al, 1999). Although a genetic predisposition usually is postulated, particularly in more severe forms, no individual genes have been identified reliably as yet. Some estimates place the heritability at only 36-44% in women and rather less in men (Reference Bierut, Heath and BucholzBierut et al, 1999). Thus, there are reasons to doubt an overwhelming effect of genetic influences on the predisposition to depression and to anticipate other constitutional factors, particularly in men, for whom the heritability appears to be lower.
One such constitutional factor may be foetal undernutrition, leading to low birth weight. This possibility is indicated by two studies of the Dutch Hunger Winter (Brown et al, Reference Brown, Susser and Lin1995, Reference Brown, van Os and Driessens2000) in which a second-trimester exposure to famine conditions was associated with an increased risk of admission for affective psychosis in later life. These studies had the limitation of relying on hospital admissions for case identification, which may have introduced a nosocomial artefact.
In a previous study, we have used death certificates to identify cases of suicide in a cohort born during 1911-1930 in Hertfordshire, England (Reference Barker, Osmond and RodinBarker et al, 1995). The standardised mortality ratio for suicide was higher in babies who were lighter at 1 year. Although this study supported an association between low growth rates and depression, it only did so circumstantially, because the relationship between depressive disorder and suicide is not invariable. We have therefore tested the hypothesis further by determining the rates of depressive disorder among surviving members of the Hertfordshire birth cohort.
METHOD
Subjects
The study population has been described previously (Reference Osmond, Barker and WinterOsmond et al, 1993). All births in the English county of Hertfordshire from 1911 onwards were notified to the county medical officer of health by the attending midwife. Health visitors saw the babies periodically through infancy and recorded the method of feeding, date of birth, birth weight, name, address and paternal occupation, and wrote general comments on the babies' health and well-being. At 1 year the babies were weighed again. In the early 1990s the National Health Service Central Register (NHSCR) was used to trace all singleton births that had survived until 5 years of age.
We traced 10 141 of the boys and 5585 of the girls. Previously we had studied growth in infancy in relation to cardiovascular disease and toHelicobacter pylori infection in subsamples of 631 men and 389 women born during 1920-1930 and still living in the east or north-west districts of the county, and these formed the population for the current study. They comprised 5% of all men and women born in east and north-west Hertfordshire during 1920-1930 and 42% of all such births known to be still living in the county (Reference Fall, Goggin and HawtinFall et al, 1997). As part of the current study we wrote to these 1020 subjects, asking them to take part in a study looking at the effects of early development on ‘how stress affects people’.
Procedures
The subjects were interviewed in their home by a research nurse who had met them already in the course of previous studies. The nurses were unaware of the data on the subjects' birth records. They also asked about known risk factors for depression in the elderly, including social isolation (Reference Green, Copeland and DeweyGreen et al, 1992), bereavement (Reference Beekman, Deeg and GeerlingsBeekman et al, 2001) and illness (Reference Prince, Harwood and ThomasPrinceet al, 1998).
Two standard measures were used to assess current depression. The first was the 15-item version of the self-administered Geriatric Depression Scale (GDS), a score of 5 or more indicating a probable case of depression (Reference Almeida and AlmeidaAlmeida & Almeida, 1999). This threshold has a sensitivity of 92.7% and a specificity of 62.5% using the ICD-10 (World Health Organization, 1992) depressive episode as a gold standard. The second was the Geriatric Mental State B version (GMS), a semi-structured interview adapted from the Present State Examination (Reference McWilliam, Copeland and DeweyMcWilliam et al, 1988). A score of 3 or more indicates depressive disorder and the assessment distinguishes between psychotic depression, neurotic depression and generalised anxiety disorder.
The research nurses attended a 5-day GMS training course and were supervised regularly throughout the study. Interrater reliability for the GMS was assessed by rating of videos before and during the study. As an additional measure of quality control, the GMS was repeated within 1 week by I. R. on 42 (4.8%) subjects selected at random.
Because our hypothesis was formed from the recognition of the comorbidity of depression and coronary heart disease, it was important to show that any association between low birth weight and depression was not mediated solely by this effect. For each subject the results of the Rose questionnaire (a measure of disability due to ischaemic heart disease; Reference Rose and BlackburnRose & Blackburn, 1968) and electrocardiogram evidence of cardiac ischaemia were available from previous studies and were used to adjust for coronary heart disease in the analyses.
Statistical analysis
Three definitions of depression were used in the analyses: depression according to either the GMS or GDS definition; GDS score above threshold; and a GDS diagnosis of depression (any category). The first definition was the primary analysis of the study because there was no a priori reason to expect an association to be restricted to any given diagnostic sub-category of depression. Secondary analyses examined the relationships between birth weight, weight at 1 year and individual definitions of depression.
Logistic regression models were used to analyse the association between depression and birth weight and weight at 1 year while adjusting for known risk factors of depression. Analyses were carried out for men and women separately. Summary tables of these associations used the same divisions into weight categories as in previous analyses (Reference Fall, Goggin and HawtinFall et al, 1997). Continuous measures of birth weight and weight at 1 year were entered in the logistic regression models. All analyses were carried out using STATA, release 5 (StataCorp, 1997). The effect of birth weight was adjusted for weight at 1 year, as has been the case in previous analyses of the effects in other diseases. Weight at 1 year is influenced less by the intra-uterine environment and so corrects to some degree for catch-up growth to the adult size.
RESULTS
Of the 1020 subjects 882 agreed to participate. Others refused to participate, had died, moved away or were lost to contact. There were 542 men (mean age 68 years) and 340 women (mean age 68 years).
All subjects completed the GDS and 74 (42 men and 32 women) thereby were classified as having depression. A total of 867 subjects completed the GMS. Twelve subjects (five men and seven women) were classified as having psychotic depression; 54 (25 men and 29 women) had neurotic depression; and 14 had generalised anxiety disorder, of whom 12 also met the criteria for a depression diagnosis and therefore were included as ‘depressed’ in the analysis. The two with pure anxiety were classified as ‘not depressed’.
Altogether, 113 subjects (57 men and 56 women) had depression according to either of the assessments and were treated as cases in the primary analysis. The prevalence of depression, so defined, was 11% in men and 16% in women. Twenty-four subjects (12 men and 12 women) had depression according to both assessments.
Known risk factors for depression
Twenty-four per cent of the men were in social classes I and II; 10% were in III non-manual, 45% were in III manual and 22% were in IV and V. The corresponding figures for women were 25%, 20%, 31% and 25%.
Table 1 shows that by using social class as a trend variable the prevalence of any depression (GMS or GDS) was, as expected, higher in the lower social classes. There was a similar trend with social class at birth. Both current social class and social class at birth made independent contributions to the risk of depression. In both males and females the risk of depression was increased also by bereavement in the previous year, living alone, having an illness causing pain or having an illness preventing activity. In women, but not men, depression was associated also with social isolation. The adjusted odds ratios in Table 1 show that all of these known risk factors for depression exerted an independent effect, although only the odds ratios for social class and illness were statistically significant. All of the risk factors in Table 1 were adjusted for in subsequent analyses, whether or not they were statistically significant.
Table 1 Odds ratios for depression according to known risk factors in late life
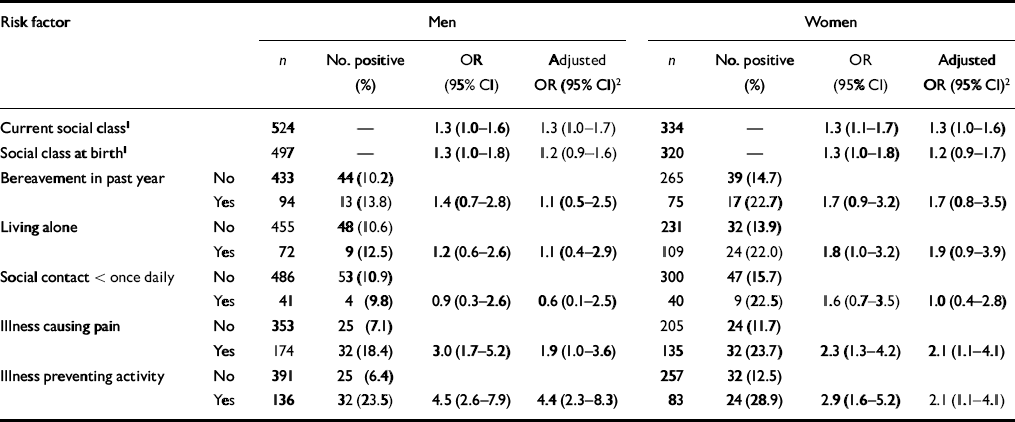
| Risk factor | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | No. positive (%) | OR (95% Cl) | Adjusted OR (95% Cl)2 | n | No. positive (%) | OR (95% Cl) | Adjusted OR (95% Cl)2 | ||
| Current social class1 | 524 | — | 1.3 (1.0-1.6) | 1.3 (1.0-1.7) | 334 | — | 1.3 (1.1-1.7) | 1.3 (1.0-1.6) | |
| Social class at birth1 | 497 | — | 1.3 (1.0-1.8) | 1.2 (0.9-1.6) | 320 | — | 1.3 (1.0-1.8) | 1.2 (0.9-1.7) | |
| Bereavement in past year | No | 433 | 44 (10.2) | 265 | 39 (14.7) | ||||
| Yes | 94 | 13 (13.8) | 1.4 (0.7-2.8) | 1.1 (0.5-2.5) | 75 | 17 (22.7) | 1.7 (0.9-3.2) | 1.7 (0.8-3.5) | |
| Living alone | No | 455 | 48 (10.6) | 231 | 32 (13.9) | ||||
| Yes | 72 | 9 (12.5) | 1.2 (0.6-2.6) | 1.1 (0.4-2.9) | 109 | 24 (22.0) | 1.8 (1.0-3.2) | 1.9 (0.9-3.9) | |
| Social contact < once daily | No | 486 | 53 (10.9) | 300 | 47 (15.7) | ||||
| Yes | 41 | 4 (9.8) | 0.9 (0.3-2.6) | 0.6 (0.1-2.5) | 40 | 9 (22.5) | 1.6 (0.7-3.5) | 1.0 (0.4-2.8) | |
| Illness causing pain | No | 353 | 25 (7.1) | 205 | 24 (11.7) | ||||
| Yes | 174 | 32 (18.4) | 3.0 (1.7-5.2) | 1.9 (1.0-3.6) | 135 | 32 (23.7) | 2.3 (1.3-4.2) | 2.1 (1.1-4.1) | |
| Illness preventing activity | No | 391 | 25 (6.4) | 257 | 32 (12.5) | ||||
| Yes | 136 | 32 (23.5) | 4.5 (2.6-7.9) | 4.4 (2.3-8.3) | 83 | 24 (28.9) | 2.9 (1.6-5.2) | 2.1 (1.1-4.1) | |
Associations with birth weight, weight at 1 year and infant feeding
Table 2 shows that among men but not women the odds ratios for depression fell with increasing birth weight. Adjusting for weight at 1 year increased this effect. Relative to those born over 8.5 pounds, the odds ratios rose from 2.8 (95% CI 0.9-8.9) for those weighing 7.5-8.5 lb, to 3.2 (95% CI 1.0-10.5) for those weighing 6.5-7.5 lb, to 3.5 (95% CI 1.0-12.8) for those weighing up to 6.5 lb (for the trendP=0.007). For the small group of men who weighed up to 5.5 lb at birth compared with those who weighed more than 9.5 lb the odds ratio was 18.5 (95% CI 4.0-86.1; P<0.0001). In a simultaneous analysis the trend in the risk of depression with birth weight (P=0.01) was independent of the trend with social class at birth (P=0.07).
Table 2 Odds ratios for depression according to birthweight and weight at 1 year
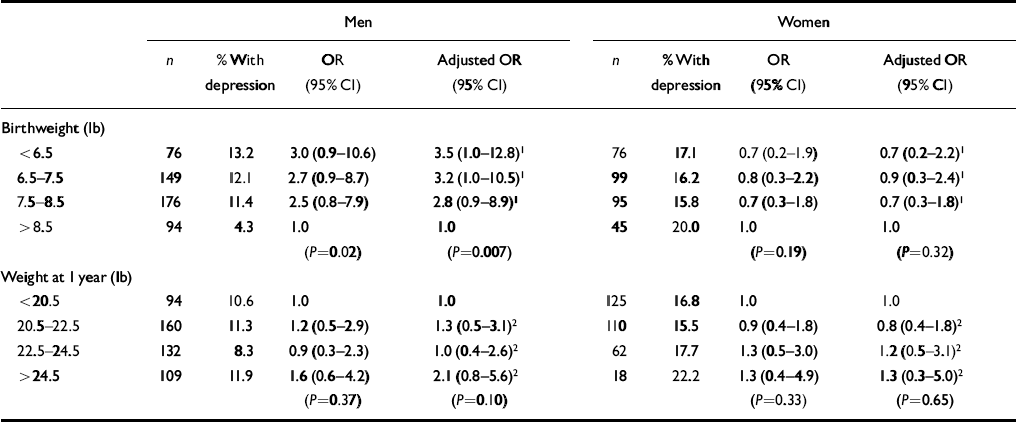
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| n | % With depression | OR (95% Cl) | Adjusted OR (95% Cl) | n | % With depression | OR (95% Cl) | Adjusted OR (95% Cl) | |
| Birthweight (lb) | ||||||||
| < 6.5 | 76 | 13.2 | 3.0 (0.9-10.6) | 3.5 (1.0-12.8)1 | 76 | 17.1 | 0.7 (0.2-1.9) | 0.7 (0.2-2.2)1 |
| 6.5-7.5 | 149 | 12.1 | 2.7 (0.9-8.7) | 3.2 (1.0-10.5)1 | 99 | 16.2 | 0.8 (0.3-2.2) | 0.9 (0.3-2.4)1 |
| 7.5-8.5 | 176 | 11.4 | 2.5 (0.8-7.9) | 2.8 (0.9-8.9)1 | 95 | 15.8 | 0.7 (0.3-1.8) | 0.7 (0.3-1.8)1 |
| > 8.5 | 94 | 4.3 | 1.0 | 1.0 | 45 | 20.0 | 1.0 | 1.0 |
| (P=0.02) | (P=0.007) | (P=0.19) | (P=0.32) | |||||
| Weight at 1 year (lb) | ||||||||
| < 20.5 | 94 | 10.6 | 1.0 | 1.0 | 125 | 16.8 | 1.0 | 1.0 |
| 20.5-22.5 | 160 | 11.3 | 1.2 (0.5-2.9) | 1.3 (0.5-3.1)2 | 110 | 15.5 | 0.9 (0.4-1.8) | 0.8 (0.4-1.8)2 |
| 22.5-24.5 | 132 | 8.3 | 0.9 (0.3-2.3) | 1.0 (0.4-2.6)2 | 62 | 17.7 | 1.3 (0.5-3.0) | 1.2 (0.5-3.1)2 |
| > 24.5 | 109 | 11.9 | 1.6 (0.6-4.2) | 2.1 (0.8-5.6)2 | 18 | 22.2 | 1.3 (0.4-4.9) | 1.3 (0.3-5.0)2 |
| (P=0.37) | (P=0.10) | (P=0.33) | (P=0.65) | |||||
In view of the hybrid definition of depression used in the primary analysis the two definitions were entered separately into the same analysis described above. None of these analyses gave significant results in the females. The GMS definition yielded an odds ratio between birth weight (adjusted for weight at 1 year) and depression of 0.72 (P=0.05), lighter male babies being at higher risk. There was also a significant association between greater weight at 1 year (adjusted for birth weight) and increased risk of depression (OR=1.18, P=0.05). The GDS gave similar trends but these were not significant (adjusted OR for birth weight=0.79, P=0.17; adjusted OR for weight at 1 year=1.08, P=0.36).
There were 65 men and 37 women with definite coronary heart disease in the primary analysis. After adjusting for coronary heart disease the associations between birth weight and depression in men remained and became more significant. Relative to those born over 8.5 lb the odds ratios for birth weight, adjusted for weight at 1 year, were 3.0 (95% CI 0.9-9.8) for those 7.5-8.5 lb, 3.1 (95% CI 0.9-10.4), for those 6.5-7.5 lb and 4.1 (95% CI 1.1-15.2) for those under 6.5 lb (for the trend P=0.005). The association with weight at 1 year, adjusted for coronary heart disease as well as birth weight, was not significant, and the associations in females remained non-significant.
We then removed the subjects with definite coronary heart disease and repeated the primary analysis on the remaining 428 men and 274 women. Once again the odds ratios increased in value as the birth weight declined (overallP<0.004).
In contrast to the trend with birth weight, odds ratios rose with increasing weight at 1 year, although this trend was statistically significant only in the GMS data adjusted for birth weight and including those with coronary heart disease. The highest risk was found among men who had low birth weight but higher weight at 1 year, whereas the lowest risk was among men who had high birth weight, but lower weight at 1 year. We also examined the method of infant feeding because such data were available from the contemporary records. Of the subjects 605 were exclusively breast-fed, 221 were breast— and bottle-fed before weaning and 41 were exclusively bottlefed. There was no relationship to the risk of depression.
DISCUSSION
What does this study add to previous findings?
These results place late-life depression in the same category of illness as cardiovascular disease, NIDDM and hypertension, the vulnerability to which begins in foetal life. The findings explain some of the comorbidity between depression and cardiovascular disease and diabetes (Reference Popkin, Callies and LentzPopkin et al, 1988) because they appear to be related to a common vulnerability factor. They add to our previous study of suicide in the same cohort (Reference Barker, Osmond and RodinBarker et al, 1995) and to the studies of affective psychosis after the Dutch Hunger Winter (Brownet al, Reference Brown, Susser and Lin1995, Reference Brown, van Os and Driessens2000). Sacker et al (Reference Sacker, Done and Crow1995) also found lower birth weight in patients admitted with affective psychosis who were part of the British Perinatal Mortality Survey of 1958. Unlike these previous samples, the definition of depression in our study was not dependent upon admission to hospital or on the accuracy of hospital records, but was made on the basis of a self-rating scale or a semi-structured interview of the survivors of a birth cohort. In addition, unlike the Dutch sample, the cohort was born in peace time and had not been subjected to unusual or extreme conditions.
The findings demonstrate that, among men, low birth weight increases the risk of depression in late life, almost 70 years later, and does so independently of social class at birth, current social class and the other known risk factors of social isolation, recent bereavement and illness. Considering the high levels of comorbidity between depression and coronary heart disease, it is particularly important that the associations remained after both adjusting for and excluding those with coronary heart disease.
In addition to affective disorders, low birth weight has been associated with schizophrenia in adults (Reference Rifkin, Lewis and JonesRifkinet al, 1994; Reference Sacker, Done and CrowSackeret al, 1995; Reference Susser, Neugebauer and HoekSusseret al, 1996; Reference Jones, Rantakallio and HartikainenJoneset al, 1998). Among those with schizophrenia and bipolar disorder, patients with poorer premorbid functioning had been born at the lower weight (Reference Foerster, Lewis and OwenFoerster et al, 1991; Reference Cannon, Jones and GilvarryCannon et al, 1997).
It is important to differentiate between very-low-birth-weight premature babies, who often need special baby care, and the effect of birth weight at term expressed as a continuous variable, as in our results. However, such very-low-birth-weight groups also appear to show a range of neurodevelopmental abnormalities (Reference BreslauBreslau, 1995), including attention-deficit hyper-activity disorder and intellectual deficits.
What are the limitations of our findings?
Our study used a hybrid definition of depression in the primary analysis, consisting of a high score on a rating scale and/or an interview-based diagnosis. Although not as ideal as using a single definition, it is unlikely that this hybrid definition affected the results, for two reasons: they depend on trends within the sample; and the association was found also in the secondary analysis of the interview-based diagnostic variable (GMS) alone. Using the primary outcome variable, 113 subjects (21%) were rated as having depression, reflecting the inclusiveness of the GMS system. The use of this broad definition in our study suggests that the association between birth weight and depression was not confined to severe or psychotic cases such as those that have been studied previously (Brown et al, Reference Brown, Susser and Lin1995, Reference Brown, van Os and Driessens2000).
Our subjects were interviewed only once at a late stage in life and the absence of life-time histories of depression would have introduced a conservative bias into the current study. Depression at younger ages, although different in some respects from late-life depression (National Institutes of Health, 1992), also is known to be a risk factor for depression in the elderly (Reference Beekman, Deeg and GeerlingsBeekman et al, 2001) and our findings therefore might be expected to apply to depression in younger adults. However, more information is needed for life-time histories, derived for example from medical records and by studying a younger cohort, before this can be confirmed. Such a study currently is under way.
Interpretation of the findings
It has been known for many years that foetal growth is determined by the intra-uterine environment (Reference McCance and WiddowsonMcCance & Widdowson, 1974), specifically the ability of the mother to deliver nutrients. We postulate that male foetuses that are nutritionally stressed and grow slowly in utero are more likely to become depressed in adulthood. Female foetuses grow more slowly than males and therefore are less vulnerable at critical periods of development (Reference Forsen, Eriksson and TuomilehtoForsen et al, 1997).
Possible confounding variables
Genetic influences could confound our interpretation only if mothers with depression have low-birth-weight babies and (separately) confer a genetic vulnerability to depression on their offspring. An association between postnatal depression and low birth weight has recently been reported (Reference Bergant, Heim and UlmerBergant et al, 1999) but this was accounted for by obstetric complications. A predominantly genetic explanation would have been expected to produce a larger effect in female than in male offspring (Reference Bierut, Heath and BucholzBierut et al, 1999). It is noteworthy that not only our study but also the studies of the Dutch Hunger Winter found the effect to be greater in males than in females (Brown et al, Reference Brown, Susser and Lin1995, Reference Brown, van Os and Driessens2000).
It is equally unlikely that social factors mediate the association because by the age of 1 year the affected individuals had caught up in their growth. Nevertheless, even though we controlled for social class at birth and at interview, it remains possible that the effects of socio-economic deprivation are not accounted for entirely by the employment descriptions that we used. If the risk of living in deprivation continued throughout life, and such deprivation caused low birth weight and independently caused depression in old age, then it is possible that the results were an artefact of socio-economic deprivation. However, it is possible also that the biological effects of socio-economic deprivation on foetal growth and on other prenatal biological variables partially mediate its effect on depression. Some of the mechanisms by which this may arise will be discussed now.
Candidate mechanisms
There are several mechanisms by which undernutrition of the foetus might cause permanent changes that increase later vulnerability to depression. Foremost among these is programming of the HPA axis. Small male babies have increased urinary adrenal androgen and glucocorticoid metabolite excretion at age 9 years (Reference Clark, Hindmarsh and ShiellClark et al, 1996) and higher fasting cortisol concentrations as adults (Reference Philips, Barker and FallPhilips et al, 1998). Raised plasma cortisol level is the most consistently demonstrated biological abnormality in primary depressive disorder (Reference Nemeroff, Widerlov and BisetteNemeroff et al, 1984; Reference MurphyMurphy, 1991; Reference Lopez, Chalmers and LittleLopez et al, 1998). The growth hormone axis is another candidate. Median 24-h plasma growth hormone concentrations are related to weight at 1 year (Reference Fall, Hindmarsh and DennisonFall et al, 1998), and in depression the control of growth hormone secretion is known to be disturbed. For example, growth hormone secretion in response to both clonidine (Reference Checkley, Glass and ThompsonCheckley et al, 1984) and slow wave sleep (Reference Sakkas, Soldatos and BergiannakiSakkas et al, 1998) is reduced in patients with depression. Thyroid function also may be set during foetal growth and infant feeding (Reference Philips, Barker and OsmondPhilips et al, 1993) and reduced plasma thyrotropin levels coupled with impaired response to thyrotropin-releasing hormone (Reference Schule, Laakman and BaghaiSchuleet al, 1997) are associated with depression (Reference Oomen, Schippering and DrexhageOomen et al, 1996).
Although the brain is relatively protected during intra-uterine life, foetal undernutrition at critical periods can have neurodevelopmental effects — reducing cellular growth (Reference Winick, Rosso and BraselWinicket al, 1972), later IQ (Reference Davies and StewartDavies & Stewart, 1975) and learning performance (Reference KatzKatz, 1980). The effect on the serotonergic system has not been studied in any detail.
Implications
Future research should attempt to replicate the findings using a lifetime definition of depression in an elderly cohort, and repeating the study in a younger cohort. If they are replicated successfully our findings have a number of implications. First, they provide a second inter-generational cause of depression to complement the genetic contribution and thus they increase the proportion of the variance in vulnerability that can be explained by known risk factors. Second, they introduce a new explanation of the comorbidity between depression and coronary heart disease in adulthood, which otherwise cannot be explained fully by smoking or other lifestyle factors. Third, they suggest an integrative hypothesis of the associations between neuroendocrine abnormalities and depression that has been lacking previously from the psycho-neuroendocrinology literature, because many of the identified abnormalities may be programmed during foetal life.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• A non-genetic inter-generational risk factor has been implicated in depression.
-
• Depression and cardiovascular disease may share a common origin in foetal life.
-
• The study has shown a gender difference in risk factors for depression.
LIMITATIONS
-
• Late-life depression may differ from depression in earlier life and these findings may or may not extrapolate to life-time risk.
-
• A hybrid of self-rating scale and interview-based diagnosis was used for case definition of current depression.
-
• Certain social or illness variables, such as dementia, may have remained uncontrolled.





eLetters
No eLetters have been published for this article.