Lithium salts have been extensively used in the past decades for the treatment of bipolar disorder and major depression. Glycogen synthase kinase 3 beta (GSK3B) activity has a central role in the pathophysiology of Alzheimer's disease. Reference Hooper, Killick and Lovestone1 Glycogen synthase kinase 3 beta is the predominant tau-kinase in the brain and modulates the cleavage of amyloid precursor protein. Reference Ishiguro, Shiratsuchi, Sato, Omori, Arioka and Mobayashi2,Reference Muyllaert, Kremer, Jaworski, Borghgraef, Devijver and Croes3 There is substantial support from experimental models that lithium, via inhibition of GSK3B, may modify Alzheimer's disease-specific pathological processes Reference Chuang and Manji4 such as the hyperphosphorylation of tau, Reference Hong, Chen, Klein and Lee5,Reference Lovestone, Davis, Webster, Kaech, Brion and Matus6 the over-production of the amyloid-β (Aβ42) peptide Reference Phiel, Wilson, Lee and Klein7,Reference Rockenstein, Torrance, Adame, Mante, Bar-on and Rose8 and Aβ-induced neurotoxicity. Reference Koh, Noh and Kim9 Thus, lithium, via the inhibition of GSK3B, may hamper mechanisms that lead to the formation of amyloid plaques and neurofibrillary tangles, and, consequently, having neuroprotective effects against Alzheimer's disease. Indeed, in a previous study we reported that chronic lithium intake was associated with lower prevalence of dementia in older adults with bipolar disorder. Reference Nunes, Forlenza and Gattaz10 Our findings were confirmed by two additional studies in Denmark with different methodological approaches. Reference Kessing, S⊘ndergård, Forman and Andersen11,Reference Kessing, Forman and Andersen12 Thus, chronic lithium intake may protect against the development of Alzheimer's disease and GSK3B inhibition may be a target for the development of interventions with disease-modifying properties in Alzheimer's disease.
Two clinical trials with lithium were recently conducted in participants with mild and moderate dementia. In an open-label study, lithium carbonate (serum levels 0.3–0.8 mmol/l) was administered to 22 people with Alzheimer's disease for up to 1 year. In spite of a high discontinuation rate, side-effects were mild and reversible leading to the conclusion that the prescription of lithium salts for individuals with Alzheimer's disease is relatively safe. Nevertheless, no cognitive benefits were observed among participants who completed the study. Reference Macdonald, Briggs, Poppe, Higgins, Velayudhan and Lovestone13 In a multicentre, single-blind study, participants with mild Alzheimer's disease were treated with lithium sulfate (0.6–0.8 mmol/l) or placebo for 10 weeks. Reference Hampel, Ewers, Bürger, Annas, Mörtberg and Bogstedt14 Lithium treatment did not have significant effects on cognitive performance and on the cerebrospinal fluid (CSF) concentrations of Alzheimer's disease-related biomarkers.
We hypothesised that these negative results were because of the investigation of lithium in people with clinically manifest Alzheimer's disease, whereas the protective effects of lithium would be better tested during a longer follow-up of individuals at risk of but without dementia, such as people with amnestic mild cognitive impairment (aMCI). Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen15 Therefore, the primary objective of this study was to assess the effect of long-term lithium treatment on the progression of cognitive deficits in individuals with aMCI. Secondary objectives were to assess the modification to CSF concentrations of Alzheimer's disease biomarkers (total tau (T-tau), phosphorylated tau (P-tau) and Aβ42), along with safety and tolerability issues.
Method
Participant recruitment and study design
This was a single-centre, randomised, double-blind, placebo-controlled study to assess the potential neuroprotective effects of chronic low-dose lithium treatment in people with aMCI. Participants were community-dwelling out-patients recruited from a cohort dedicated to the study of cognitive ageing at the Institute of Psychiatry, Faculty of Medicine, University of São Paulo. Reference Diniz, Nunes, Yassuda, Pereira, Flaks and Viola16 This study was approved by the local ethical committee and was conducted in adherence with the Helsinki Declaration and Good Clinical Practice recommendations; the trial is registered with clinicaltrials.gov: NCT01055392.
Participants were enrolled to this study after signing informed consent. Inclusion criteria were: age 60 years or older; diagnosis of aMCI according to Mayo Clinic criteria Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen15 and no evidence of ongoing psychiatric disorders. For individuals with prevalent medical comorbidities requiring continuous pharmacological treatment, enrolment was reliant on the approval of the general practitioner.Figure 1 displays the flow chart for the first 12 months of follow-up. The Appendix outlines the clinical assessment methods and other procedures pertaining to this protocol. All participants were recruited, enrolled, monitored and prescribed by one single physician (O.V.F.) who did not take part in the assessment of baseline or outcome variables. No significant differences in total Cambridge Cognitive Test (CAMCOG) Reference Roth, Tym, Mountjoy, Huppert, Hendrie and Verina17 scores were observed between randomised and non-randomised patients (88.4 (s.d. = 5.9) and 90.3 (s.d. = 3.6) respectively, P = 0.3). The CAMCOG is part of the Cambridge Examination for Mental Disorders in the Elderly (CAMDEX). Reference Roth, Tym, Mountjoy, Huppert, Hendrie and Verina17 It is a brief neuropsychological battery designed to assess global cognitive function and ascertain the impairments that are required for the diagnosis of dementia. Its scores range from 0 to 107 points through 60 items, and it is composed by 8 domains: orientation, memory, language, attention, praxis, perception, abstraction, and calculation.
Lithium dose titration
Identical tablets containing 150 mg, 300 mg, 450 mg or 600 mg of lithium carbonate or placebo were produced at the Central Pharmacy of Hospital das Clínicas, University of São Paulo, and packaged into identical coded blisters. After randomisation, participants started daily doses of 150 mg of lithium, which was titrated to target serum levels of 0.25–0.5 mmol/l through weekly visits, controlling for tolerability. This range, which is lower than that commonly used for the treatment of affective disorders, was chosen to reduce the risk of discontinuation because of adverse events. Preliminary data from our laboratory showed that treatment with this concentration range for 2 weeks in healthy volunteers caused a 50% reduction in GSK3B activity in leukocytes (data available from the authors on request). Dispensing was carried out by three pharmacists who did not take part in any other intervention. All participants were instructed to take their prescriptions (lithium or placebo) twice a day at 08.00 h and 20.00 h, preferentially with meals. If there was any relevant adverse event during the titration phase, the lithium dose was adjusted back to the highest tolerable dose within the treatment range. Serum lithium levels were determined weekly in the titration phase, 12 h after the last dose. Once stable target levels were achieved, the prescription was maintained until the next visit, which was scheduled at 3-monthly intervals. Any adverse events or modifications in other ongoing prescriptions were to be noted and, if necessary, the lithium treatment re-evaluated.
Outcome variables
The primary outcomes of this study were the modification of cognitive or functional status and concentrations of amyloid-β42 peptide (Aβ42), total tau (T-tau) and phosphorylated tau (P-tau) in the CSF. The Clinical Dementia Rating (CDR) scale, Reference Morris18 including the Sum of Boxes (SoB) score, and the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-Cog) Reference Rosen, Mohs and Davis19 were administered to assess global functional and cognitive state. Memory, attention and executive function were further evaluated with the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Reference Morris, Mohs, Rogers, Fillenbaum and Heyman20 delayed recall test, Sequence of Letters and Numbers (SLN), Reference Wechsler21 and the Trail Making Test (TMT). Reference Reitan22 Secondary outcomes were the conversion from aMCI to Alzheimer's disease, as well as safety and tolerability analysis according to longitudinal clinical and laboratory data.
Cerebrospinal fluid biomarkers
Cerebrospinal fluid samples were obtained by lumbar puncture (L3/L4 or L4/L5 inter-vertebral spaces) between 10.00 h and 12.00 h, with a 23-gauge needle and using polypropylene tubes. Samples were immediately centrifuged at 3200 g for 10 min at 4 °C, and then stored in 500 μl aliquots at – 80 °C until analysis. The analysis of the CSF concentrations of T-tau, P-tau and Aβ42 were done in duplicate with the INNo-Bia AlzBio3 assay (Innogenetics, Belgium), a multiplexed, microsphere-based method for xMAP-Luminex platform allowing the simultaneous analysis of all biomarkers in the same aliquot.
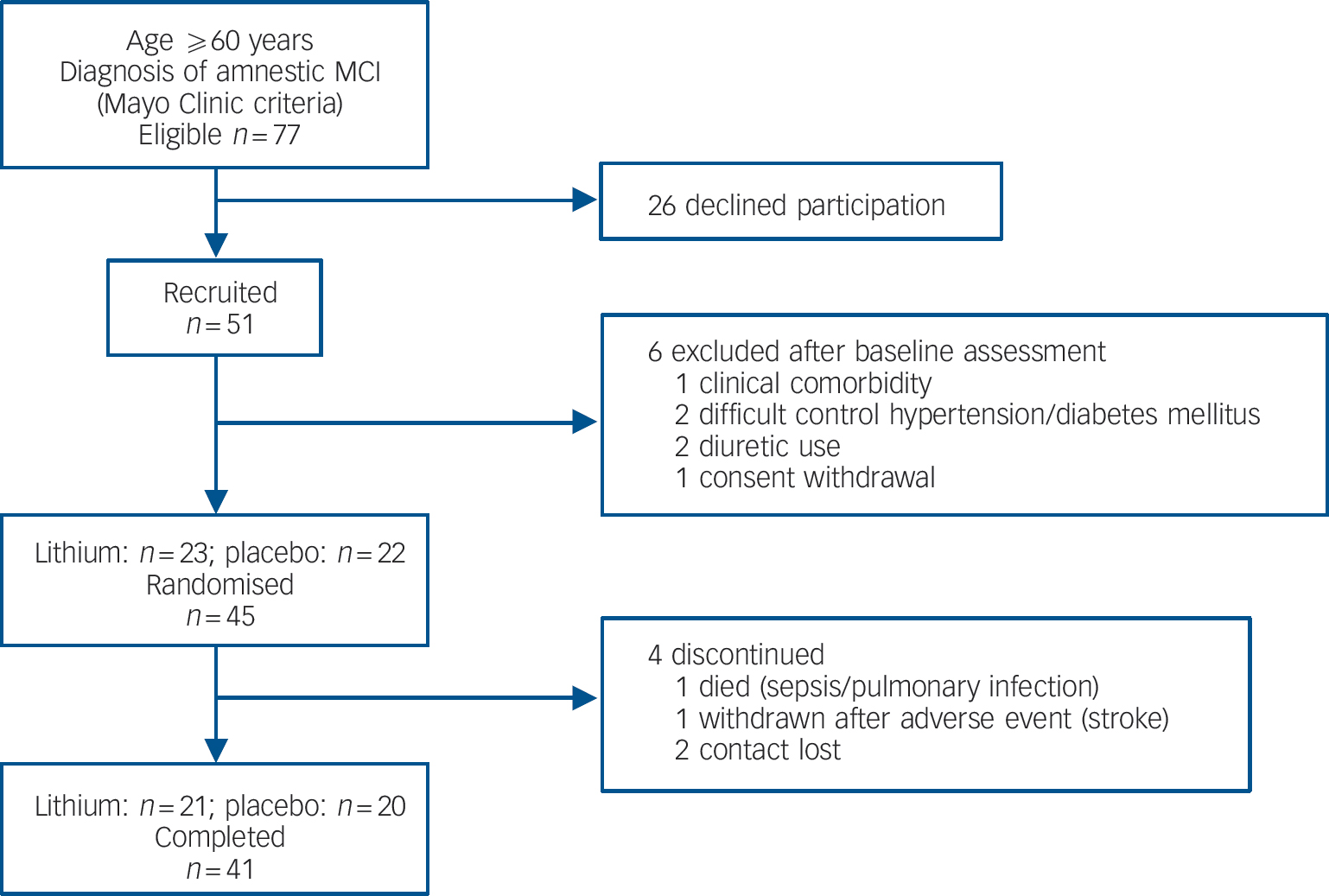
Fig. 1 Study flow chart from recruitment until 12-month follow-up. MCI, mild cognitive impairment.
After pre-wetting of the filter plate with a wash buffer, a suspension of microspheres carrying the corresponding capturing antibodies (AT120 for T-tau, AT270 for P-tau phosphorylated at Threonine181, and 4D7A3 for Aβ42) was added to the plate. A mixture of biotinylated detection monoclonal antibodies, designed to specifically recognise one of the capturing antibodies (HT7 for T-tau and P-tau, and 3D6 for Aβ42), and 75 μl of CSF aliquots or standards were added to the plate and incubated overnight, protected from light. The following morning, the plate was washed and a detection conjugate (phycoerythrin-labelled streptavidin) was added and incubated for 1 h at room temperature. A reading solution (phosphate buffer saline) was added to the plate after a final wash and the assay was analysed on a Luminex 100 IS platform (Luminex, Austin, USA). Standard curves were constructed for each biomarker using a sigmoid curve fitting method, and mean fluorescence values for duplicate samples were used to yield CSF concentrations of T-tau, P-tau and Aβ42 in pg/ml of CSF.
Statistical analysis
Chi-squared and Fisher's exact tests were carried out to assess differences in categorical data (gender distribution and conversion status) between lithium and placebo groups. Student's t-tests (independent samples) were carried out to assess mean differences in baseline clinical, cognitive and biological variables between treatment groups and between the subsets of participants who progressed to Alzheimer's disease as opposed to those who remained stable. Paired sample t-tests were carried out to address differences in the cognitive and biological variables between baseline and the 1-year follow-up within-treatment groups. Non-parametric tests (Mann–Whitney U-test and Wilcoxon rank test) were further carried out for analysis of biological data in subanalyses with limiting sample sizes.
Results
Baseline assessment
No differences were found between those participants treated with lithium and those treated with placebo regarding sociodemographic, cognitive and biological variables prior to treatment (Table 1).
Table 1 Sociodemographic variables, cognitive performance and cerebrospinal fluid biomarkers at baseline according to treatment groups
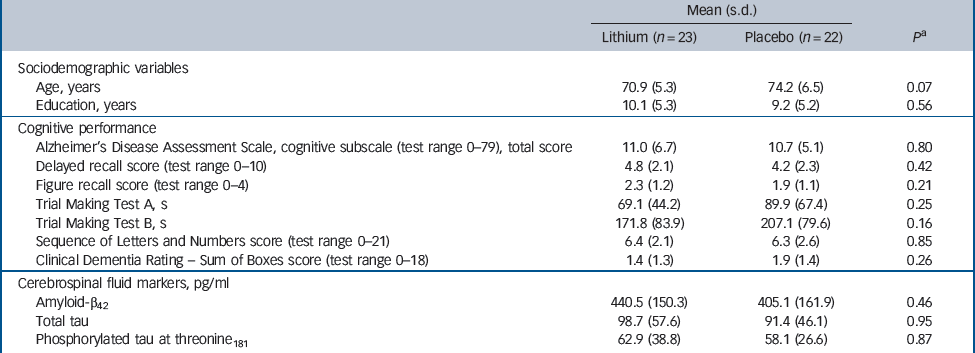
| Mean (s.d.) | |||
|---|---|---|---|
| Lithium (n = 23) | Placebo (n = 22) | P a | |
| Sociodemographic variables | |||
| Age, years | 70.9 (5.3) | 74.2 (6.5) | 0.07 |
| Education, years | 10.1 (5.3) | 9.2 (5.2) | 0.56 |
| Cognitive performance | |||
| Alzheimer's Disease Assessment Scale, cognitive subscale (test range 0–79), total score | 11.0 (6.7) | 10.7 (5.1) | 0.80 |
| Delayed recall score (test range 0–10) | 4.8 (2.1) | 4.2 (2.3) | 0.42 |
| Figure recall score (test range 0–4) | 2.3 (1.2) | 1.9 (1.1) | 0.21 |
| Trial Making Test A, s | 69.1 (44.2) | 89.9 (67.4) | 0.25 |
| Trial Making Test B, s | 171.8 (83.9) | 207.1 (79.6) | 0.16 |
| Sequence of Letters and Numbers score (test range 0–21) | 6.4 (2.1) | 6.3 (2.6) | 0.85 |
| Clinical Dementia Rating – Sum of Boxes score (test range 0–18) | 1.4 (1.3) | 1.9 (1.4) | 0.26 |
| Cerebrospinal fluid markers, pg/ml | |||
| Amyloid-β42 | 440.5 (150.3) | 405.1 (161.9) | 0.46 |
| Total tau | 98.7 (57.6) | 91.4 (46.1) | 0.95 |
| Phosphorylated tau at threonine181 | 62.9 (38.8) | 58.1 (26.6) | 0.87 |
Outcome (12-month) assessment
Forty-one participants (91% of the total sample) completed 1 year of follow-up, with similar representation in the lithium (n = 21) and placebo (n = 20) groups (χ2 = 0.178, P = 0.52). Four participants discontinued treatment within 12 months (Fig. 1). There were no significant differences in sociodemographic data, cognitive variables and CSF biomarkers between completers and non-completers (data not shown). Eleven participants (24% of the original sample) progressed to Alzheimer's disease after 12 months of follow-up. As expected, these individuals displayed at baseline the typical ‘Alzheimer's disease signature’ in the CSF, i.e. higher concentrations of T-tau and P-tau and lower concentrations of Aβ42, as compared with non-converters (data not shown). The number of conversions was higher in the placebo group (7/20) compared with the lithium group (4/21), but this difference was not significant (Fisher's, P = 0.2). All participants irrespective of treatment with lithium or placebo, had a slight but significant worsening of global functional state, as indicated by mean CDR–SoB scores (P<0.04). However, the magnitude of cognitive decline was smaller in the participants treated with lithium than in the placebo group, as shown by the modification of the ADAS–Cog and in the SLN test scores (Table 2).
Table 2 Baseline and follow-up scores on cognitive tests and concentrations of cerebrospinal fluid biomarkers according to treatment group
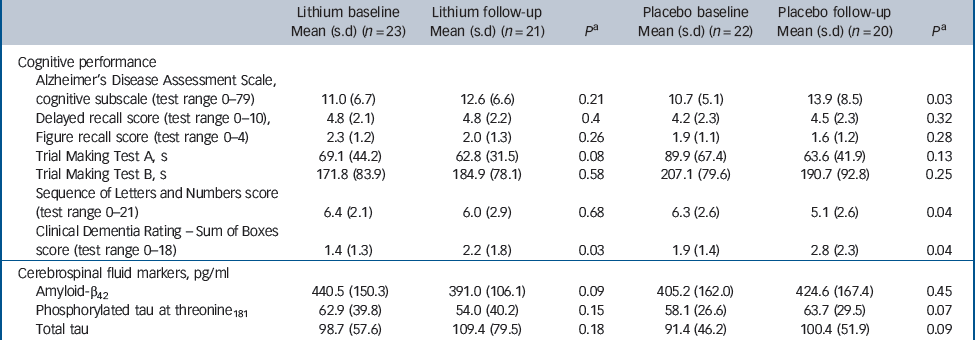
| Lithium baseline Mean (s.d) (n = 23) | Lithium follow-up Mean (s.d) (n = 21) | P a | Placebo baseline Mean (s.d) (n = 22) | Placebo follow-up Mean (s.d) (n = 20) | P a | |
|---|---|---|---|---|---|---|
| Cognitive performance | ||||||
| Alzheimer's Disease Assessment Scale, cognitive subscale (test range 0–79) | 11.0 (6.7) | 12.6 (6.6) | 0.21 | 10.7 (5.1) | 13.9 (8.5) | 0.03 |
| Delayed recall score (test range 0–10), | 4.8 (2.1) | 4.8 (2.2) | 0.4 | 4.2 (2.3) | 4.5 (2.3) | 0.32 |
| Figure recall score (test range 0–4) | 2.3 (1.2) | 2.0 (1.3) | 0.26 | 1.9 (1.1) | 1.6 (1.2) | 0.28 |
| Trial Making Test A, s | 69.1 (44.2) | 62.8 (31.5) | 0.08 | 89.9 (67.4) | 63.6 (41.9) | 0.13 |
| Trial Making Test B, s | 171.8 (83.9) | 184.9 (78.1) | 0.58 | 207.1 (79.6) | 190.7 (92.8) | 0.25 |
| Sequence of Letters and Numbers score (test range 0–21) | 6.4 (2.1) | 6.0 (2.9) | 0.68 | 6.3 (2.6) | 5.1 (2.6) | 0.04 |
| Clinical Dementia Rating – Sum of Boxes score (test range 0–18) | 1.4 (1.3) | 2.2 (1.8) | 0.03 | 1.9 (1.4) | 2.8 (2.3) | 0.04 |
| Cerebrospinal fluid markers, pg/ml | ||||||
| Amyloid-β42 | 440.5 (150.3) | 391.0 (106.1) | 0.09 | 405.2 (162.0) | 424.6 (167.4) | 0.45 |
| Phosphorylated tau at threonine181 | 62.9 (39.8) | 54.0 (40.2) | 0.15 | 58.1 (26.6) | 63.7 (29.5) | 0.07 |
| Total tau | 98.7 (57.6) | 109.4 (79.5) | 0.18 | 91.4 (46.2) | 100.4 (51.9) | 0.09 |
The participants treated with lithium had a decrease in the concentrations of P-tau (end-point minus baseline values: –8.9 pg/ml, s.d. = 24.3), whereas a slight increase was observed in participants who received placebo (5.6 pg/ml, s.d. = 11.4); this difference was significant (P = 0.02). No such effect was observed for the concentrations of T-tau (Fig. 2,Table 2). Per definition, the participants who converted to dementia in both treatment groups had an increase in the CDR–SoB scores, as opposed to those who remained stable. However, this cognitive and functional worsening in those who progressed to Alzheimer's disease was attenuated by lithium (baseline: 3.3 (s.d. = 1.3), end-point: 4.4 (s.d. = 1.5), P = 0.3) as compared with placebo (baseline: 3.4 (s.d. = 1.4), end-point: 5.6 (s.d. = 1.5), P = 0.03).Table 3 displays the modifications in CSF biomarkers in four subsets of participants: those who remained stable and were receiving lithium; those who progressed to Alzheimer's disease receiving lithium; those who remained stable and were receiving placebo; and those who progressed to Alzheimer's disease receiving placebo. The reduction of CSF P-tau in those treated with lithium occurred only in the participants who remained stable over time (P = 0.006), whereas no significant reduction was found in those treated with lithium who progressed to Alzheimer's disease (P = 0.90). In the placebo group both those who were stable and those who progressed to Alzheimer's disease had a slight increase in P-tau. Irrespective of treatment groups and conversion status, no significant changes were noted in the concentrations of T-tau and Aβ42.
Table 3 Concentrations of cerebrospinal fluid biomarkers at baseline and after 12 months of treatment with lithium or placebo and according to conversion status
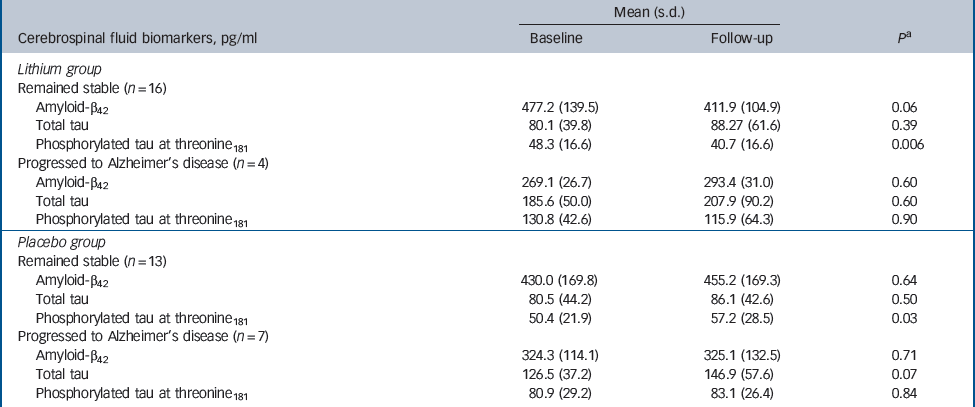
| Mean (s.d.) | |||
|---|---|---|---|
| Cerebrospinal fluid biomarkers, pg/ml | Baseline | Follow-up | P a |
| Lithium group | |||
| Remained stable (n = 16) | |||
| Amyloid-β42 | 477.2 (139.5) | 411.9 (104.9) | 0.06 |
| Total tau | 80.1 (39.8) | 88.27 (61.6) | 0.39 |
| Phosphorylated tau at threonine181 | 48.3 (16.6) | 40.7 (16.6) | 0.006 |
| Progressed to Alzheimer's disease (n = 4) | |||
| Amyloid-β42 | 269.1 (26.7) | 293.4 (31.0) | 0.60 |
| Total tau | 185.6 (50.0) | 207.9 (90.2) | 0.60 |
| Phosphorylated tau at threonine181 | 130.8 (42.6) | 115.9 (64.3) | 0.90 |
| Placebo group | |||
| Remained stable (n = 13) | |||
| Amyloid-β42 | 430.0 (169.8) | 455.2 (169.3) | 0.64 |
| Total tau | 80.5 (44.2) | 86.1 (42.6) | 0.50 |
| Phosphorylated tau at threonine181 | 50.4 (21.9) | 57.2 (28.5) | 0.03 |
| Progressed to Alzheimer's disease (n = 7) | |||
| Amyloid-β42 | 324.3 (114.1) | 325.1 (132.5) | 0.71 |
| Total tau | 126.5 (37.2) | 146.9 (57.6) | 0.07 |
| Phosphorylated tau at threonine181 | 80.9 (29.2) | 83.1 (26.4) | 0.84 |
Safety analysis
Forty-three individuals were included in the safety analysis. General tolerability was good given the high adherence rate (91%) at 1 year (Fig. 1). The occurrence of side-effects was similar in the lithium-(58%) and placebo-treated (42%) groups (χ2 = 2.17, P = 0.13). Most of the adverse events were mild and transient, and involved the gastrointestinal system, and no action was required. Three participants in the placebo group had side-effects of moderate intensity (nausea and hand tremor) and dose reduction was sufficient to relieve complaints. One person in the lithium group presented with a major cerebro-vascular event (ischemic stroke) during treatment and was withdrawn from the study. One participant in the placebo group died during the study due to sepsis secondary to pneumonia. These serious adverse events were considered unrelated to the studied drug.
Discussion
Main findings
This is the first double-blind, placebo-controlled study addressing the protective properties of lithium in people with aMCI. We found that lithium treatment for a year reduced the cognitive decline, as compared with placebo. Moreover, long-term lithium treatment was associated with a significant reduction in CSF concentration of P-tau. It should be stressed that lithium treatment was safe and well-tolerated at serum concentrations of 0.25–0.5 mmol/l.
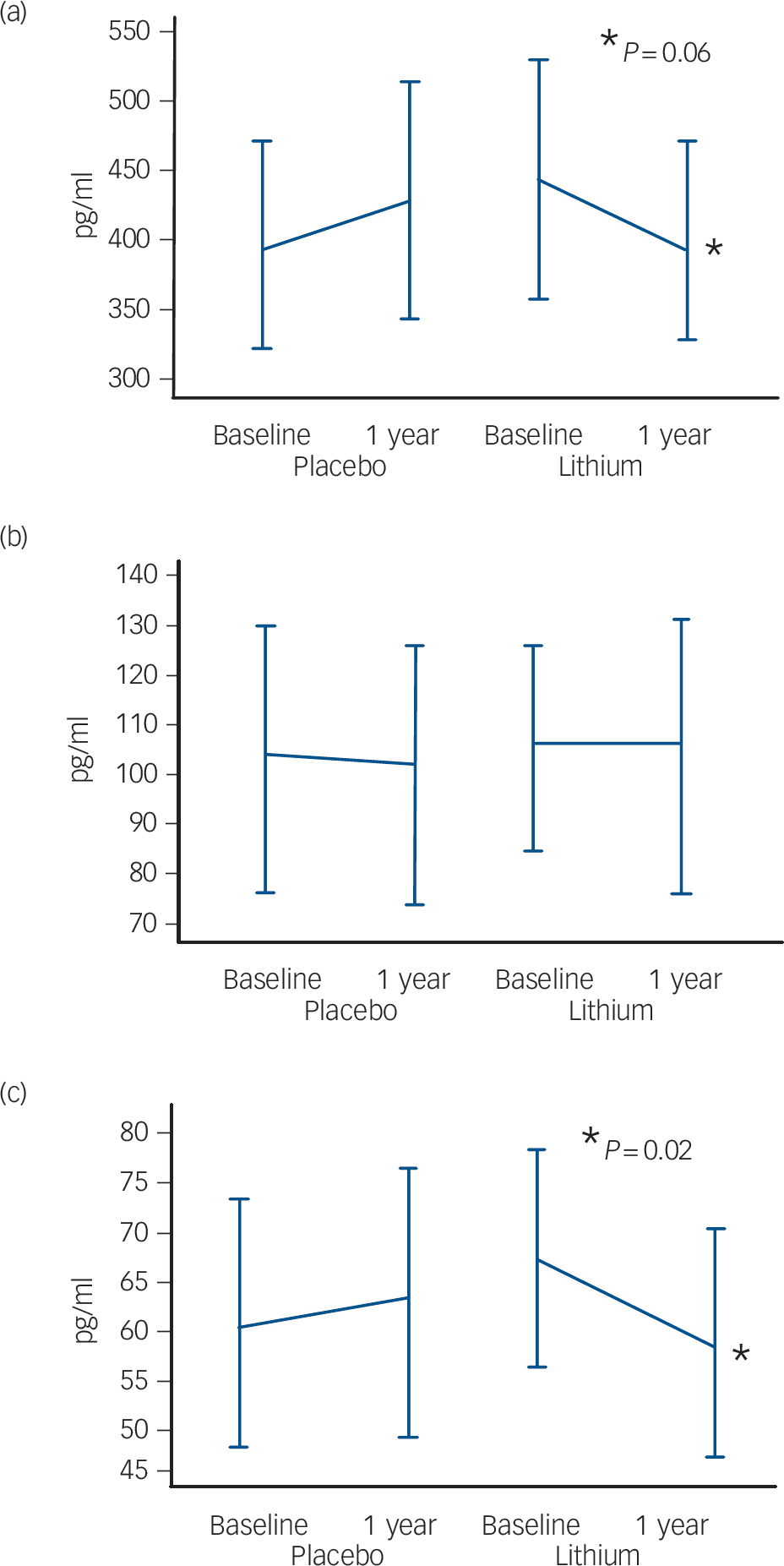
Fig. 2 Modification of cerebrospinal fluid biomarkers at follow-up according to treatment groups. (a) Amyloid-β42, (b) total tau, (c) phosphorylated tau.
Other studies
Two previous studies failed to find a significant effect of lithium either on cognition or in Alzheimer's disease-related biomarkers. Reference Macdonald, Briggs, Poppe, Higgins, Velayudhan and Lovestone13,Reference Hampel, Ewers, Bürger, Annas, Mörtberg and Bogstedt14 Possible reasons were the higher side-effects and drop-out rates associated with higher lithium levels; the shorter follow-up periods; and the inclusion of people in more advanced stages of cognitive deterioration, i.e. mild and moderate Alzheimer's disease. In contrast, the present study was designed in concordance with two recent consensus papers addressing methodological aspects of trials on disease-modification in Alzheimer's disease. Reference Vellas, Andrieu, Sampaio and Wilcock23,Reference Vellas, Andrieu, Sampaio, Coley and Wilcock24 Accordingly, such trials should start as early as possible in the disease continuum to warrant clinically relevant benefits, and should preferentially include biological information for the definition of cases and/or outcomes. From that viewpoint, people with incipient or prodromal Alzheimer's disease will more likely benefit from disease modification than those with clinically manifest dementia. Thus, the strengths of the present study are: the inclusion of individuals without dementia but with aMCI; the determination of baseline and end-point biomarkers; the long duration of follow-up; and the low-dose lithium regimen, yielding a low drop-out rate.
Implications
In the present study, lithium treatment reduced CSF P-tau in participants with aMCI who did not convert to Alzheimer's disease. Since increased P-tau is a specific marker of the pathological process in Alzheimer's disease, it is tempting to speculate that the reduction of the concentrations of P-tau in the CSF may be a useful parameter to predict the preventive effects of lithium regarding the conversion from mild cognitive impairment to Alzheimer's disease. Reference Diniz, Pinto Júnior and Forlenza25
We further hypothesise that the disease-modifying effect of lithium in Alzheimer's disease may be stage-dependent. Among converters, baseline P-tau and Aβ42 levels were similar to those found in clinically manifest Alzheimer's disease, suggesting that the more severe homeostatic imbalance is less responsive to lithium. In contrast, individuals with less severe deficits (i.e. individuals with mild cognitive impairments who remained stable) may benefit most from the neurobiological effects of lithium as they are at earlier stages in the disease continuum. If replicated in future studies, we believe that the understanding of this dissociated effect of lithium according to the magnitude of intracerebral pathology may have important clinical implications, particularly referring to the question of when to start treatment with antidementia drugs. Presumably, maximum benefits from treatment are subject to the early diagnosis of Alzheimer's disease, preferentially at mild cognitive impairment stages or earlier, when the pathological changes are still incipient and the restoration of normal physiological status is more likely to occur. Our data, together with evidence from the literature discussed, suggest that long-term lithium treatment at relatively lower serum levels may be a safe and inexpensive strategy to prevent, or at least to delay the progression from pre-dementia stages to clinical Alzheimer's disease.
In conclusion, the present findings reinforce the notion that in an individual at risk for Alzheimer's disease, lithium may have a protective effect on the progression of cognitive impairment to dementia. This is probably a consequence of the effect of lithium on GSK3B Reference Bhat, Budd Haeberlein and Avila26 and possibly on other pivotal cascades involved in the pathophysiology of Alzheimer's disease. We acknowledge that the relatively small sample size of this single-centre study is a limitation to the generalisation of the current findings. Therefore, we think that the present results warrant replication in multicentric trials with a larger sample.
Appendix Clinical examination, cognitive assessment and laboratory procedures
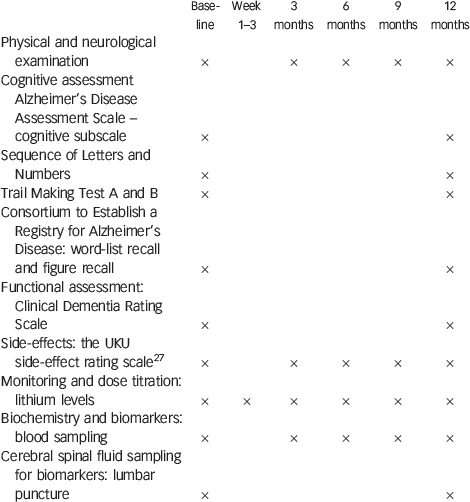
| Baseline | Week 1–3 | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| Physical and neurological examination | × | × | × | × | × | |
| Cognitive assessment | ||||||
| Alzheimer's Disease | ||||||
| Assessment Scale – cognitive subscale | × | × | ||||
| Sequence of Letters and Numbers | × | × | ||||
| Trail Making Test A and B | × | × | ||||
| Consortium to Establish a Registry for Alzheimer's | ||||||
| Disease: word-list recall and figure recall | × | × | ||||
| Functional assessment: | ||||||
| Clinical Dementia Rating Scale | × | × | ||||
| Side-effects: the UKU side-effect rating scale Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen27 | × | × | × | × | × | |
| Monitoring and dose titration: lithium levels | × | × | × | × | × | × |
| Biochemistry and biomarkers: blood sampling | × | × | × | × | × | |
| Cerebral spinal fluid sampling for biomarkers: lumbar puncture | × | × |
Funding
The present work was supported by Conselho Nacional de Pesquisa Científica (CNPq, Project 554535/2005-0), Alzheimer's Association () and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Project 02/13633-7). The Laboratory of Neuroscience (LIM-27) receives financial support from Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Acknowledgements
We are indebted to the pharmacists Aaron Barbosa, Maria F. Castanheira and Severiano Tolentino de Freitas Neto for their contribution to this study dispensing trial drugs.









eLetters
No eLetters have been published for this article.