Rapid-cycling bipolar disorder is associated with high morbidity and poor treatment outcome. Reference Oppenheim1-Reference Leverich, Altshuler, Frye, Suppes, McElroy and Keck7 Some studies suggest an association between rapid cycling and antidepressant use, Reference Wehr and Goodwin5,Reference Leverich, Altshuler, Frye, Suppes, McElroy and Keck7-Reference Goldberg and Ghaemi11 although this has not been universally observed. Reference Altshuler, Kiriakos, Calcagno, Goodman, Gitlin and Frye12-Reference Yildiz and Sachs18 Controlled trials have reported good efficacy and low mood conversion rates during antidepressant use in bipolar II disorder. Reference Altshuler, Post, Leverich, Mikalauskas, Rosoff and Ackerman13,Reference Kupfer, Chengappa, Gelenberg, Hirschfeld, Goldberg and Sachs15,Reference Parker, Tully, Olley and Hadzi-Pavlovic16,Reference Amsterdam, Shults, Brunswick and Hundert19-Reference Amsterdam and Shults21 We performed an exploratory analysis of a randomised, double-blind, placebo-controlled trial to examine the efficacy and mood conversion rate of long-term fluoxetine v. lithium monotherapy in patients with rapid- v. non-rapid-cycling bipolar II disorder who recovered from a major depressive episode during initial fluoxetine monotherapy (trial registration NCT00044616). We hypothesised that lithium monotherapy would provide greater relapse prevention with fewer treatment-emergent mood conversion episodes in patients with rapid- v. non-rapid-cycling bipolar II disorder.
Method
Participants
The participants were out-patients ≥18 years old with a DSM-IV 22 Axis I diagnosis of bipolar II disorder who recovered from a major depressive episode with a 17-item Hamilton Rating Scale for Depression (HRSD) Reference Williams23 score ≤8. A description of inclusion and exclusion criteria has been previously published. Reference Amsterdam and Shults21 Patients provided informed consent in accordance with the ethical standards of the Institutional Review Board of the University of Pennsylvania. The study was conducted using the Good Clinical Practice guidelines with oversight by the local Office of Human Research and independent Data & Safety Monitoring Board.
Procedures
Psychiatric diagnosis was verified using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders format. Reference First, Spitzer, Gibbon and Williams24 Estimates of the number of prior syndromal depressive and hypomanic episodes (defined by DSM-IV criteria) and subsyndromal hypomanic episodes, were obtained. The participants' condition was classified as rapid cycling if they had an average of ≥4 affective episodes per year during the course of their illness:

Structured HRSD, Young Mania Rating Scale (YMRS) Reference Young, Biggs, Ziegler and Meyer25 and mood conversion measures were obtained as previously described. Reference Amsterdam, Shults, Brunswick and Hundert19-Reference Amsterdam and Shults21 Treatment-emergent hypomania was defined as: (a) syndromal hypomania meeting DSM-IV criteria of ≥4 symptoms lasting ≥4 days; (b) type I subsyndromal hypomania of ≥4 symptoms lasting ≤3 days; (c) type II subsyndromal hypomania of ≤3 symptoms lasting ≥4 days; and (d) type III subsyndromal hypomania of ≤3 symptoms lasting ≤3 days. Treatment-emergent depression was defined as: (a) major depressive episode meeting DSM-IV criteria plus a HRSD score 14; (b) type I minor depressive episode of ≥5 symptoms lasting <14 days or ≤4 symptoms lasting ≥14 days; (c) type II minor depressive episode of ≥4 symptoms lasting ≥7 days; and (d) type III minor depressive episode of ≥4 symptoms lasting <7 days. Patients experiencing a major depressive episode were discontinued from the study. Patients experiencing syndromal or subsyndromal hypomania, or subsyndromal depression underwent double-blind rescue therapy. Reference Amsterdam and Shults21
Treatment
Initial fluoxetine monotherapy 80 mg daily was administered up to 12 weeks. Patients with a final HRSD score ≤8 were randomised to long-term monotherapy with either fluoxetine 10–40 mg daily, lithium 300–1200 mg daily (with a serum level of 0.5–1.5 mmol/l) or placebo for 50 weeks.
Outcome
The HRSD, YMRS and mood conversion measures were obtained at baseline (i.e. randomisation) and during double-blind therapy, as previously described. Reference Amsterdam and Shults21 The primary outcome measure was the proportion of participants with rapid- v. non-rapid-cycling bipolar disorder with relapse or recurrence of a major depressive episode. Secondary outcomes included the hazard for depressive relapse, change over time in YMRS scores (in patients experiencing change in YMRS scores) and frequency of syndromal and subsyndromal mood conversion episodes.
Sample size justification
The power estimate for the primary analysis has been previously described. Reference Amsterdam and Shults21 The current exploratory study was not powered to detect small to moderate differences in efficacy or mood conversion rates between rapid v. non-rapid-cycling groups.
Statistical procedures
Exploratory analyses were conducted using Stata 11 on Windows, with two-sided tests of hypotheses and a P-value <0.05 for statistical significance. Proportions of participants with rapid- v. non-rapid-cycling bipolar disorder who discontinued treatment or had an increase in YMRS scores were compared using χ2 and t-tests. Confidence intervals for proportions were based on the exact Binomial distribution.
Log rank tests were used to compare survival distributions to relapse for each treatment group by cycling status. Mean time to relapse was estimated. Logistic regression was used to estimate the odds of relapse. Cox regression was used to estimate the hazard ratio of relapse. Quasi-least squares (QLS) analysis was used to compare change in YMRS score using covariates of rapid cycling, time, and rapid cycling time. The largest intraparticipant change in YMRS scores (in patients experiencing a change in YMRS scores) was compared in participants with rapid- v. non-rapid-cycling bipolar disorder using a t-test.
Results
Enrolment
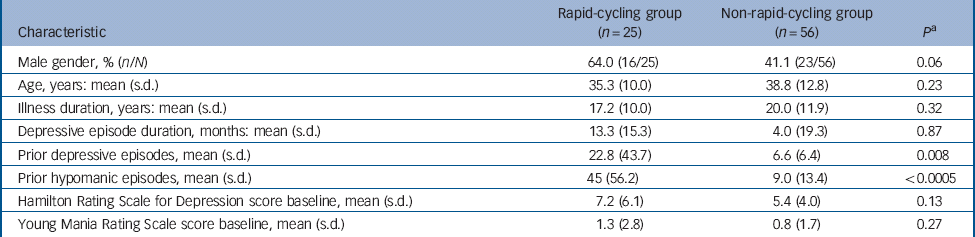
In total 167 people enrolled: 89 women with a mean age of 36.9 years (s.d. = 12.7) and 78 men with a mean age of 37.9 years (s.d. = 12.9). Cycling status was available for 166 patients (99.4%): 42 with rapid (25.3%) and 124 with non-rapid (74.7%). Overall, 37 participants with rapid- and 111 with non-rapid-cycling bipolar disorder received initial fluoxetine. Of these, 12 (32.4%, 95% CI 18.0–49.8) with rapid- v. 53 (47.7%, 95% CI 38.2–57.4) with non-rapid-cycling bipolar disorder discontinued treatment (P = 0.10); whereas, 25 (67.6%, 95% CI 50.2–82.0) with rapid- v. 58 (52.3%, 95% 42.6–61.8) with non-rapid-cycling bipolar disorder recovered (P = 0.10) (Fig. 1). Table 1 provides details of participant characteristics at the start of double-blind therapy.
Depressive relapse
Relapse occurred in 9 (36.0%, 95% CI 18.0–57.5) in the rapid- v. 29 (51.8%, 95% CI 38.0–65.3) in the non-rapid-cycling group (P = 0.20). The proportion of those with rapid-cycling bipolar disorder who relapsed was similar for fluoxetine (28.6%, 95% CI 13.2–48.7), lithium (34.6%, 95% CI 17.2–55.7) and placebo (29.6%, 95% CI 13.8–50.2) (P = 0.88). There was no significant difference between those in the rapid- v. non-rapid-cycling groups for the odds of relapse (odds ratio (OR) = 0.6, 95% CI 0.2–1.8) (P = 0.36) or for the hazard of relapse (hazard ratio 0.87, 95% CI 0.40–1.91).
YMRS scores
The QLS analysis identified no significant difference in change over time in YMRS scores among treatment conditions, and no significant difference in change in YMRS scores among those in the rapid- v. non-rapid-cycling groups. The estimated regression coefficient for the rapid-cycling treatment duration was 0.0004 (P = 0.86). The largest mean increase in YMRS score (for patients who experienced an increase) was 4.28 (s.d. = 6.2) for those in the rapid- v. 3.3 (s.d. = 4.2) for those in the non-rapid-cycling group (P = 0.40).
Mood conversion episodes
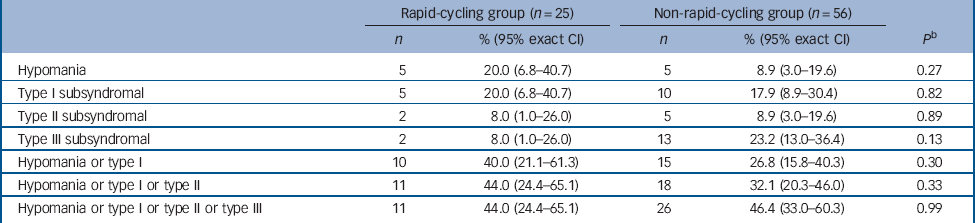
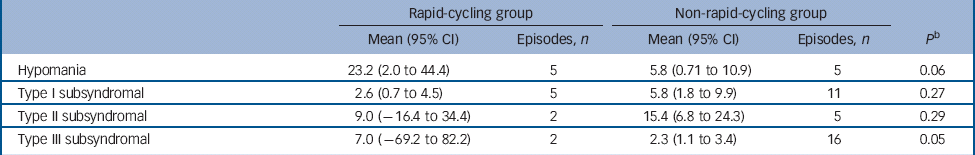
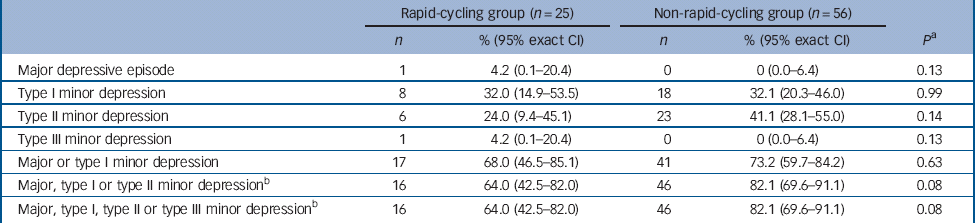
There was no significant difference in the proportion of those in the rapid- v. non-rapid-cycling groups with syndromal and/or subsyndromal hypomania (Table 2). There was a non-significant trend for a longer duration of hypomania in individuals in the rapid-cycling group (P = 0.06), although this observation was based on only five episodes in two individuals in this group. There was also a significantly longer duration of type III subsyndromal hypomania in those in the rapid-cycling group (P = 0.05), which was based on only two episodes (Table 3). There was no significant difference in the proportion of participants in the rapid- v. non-rapid-cycling groups with major or minor depressive episodes (Table 4). The duration of minor depressive episodes did not differ significantly between the two groups of patients (Table 5).
Treatment discontinuation
One participant (4.0%, 95% CI 0.1–20.4) in the rapid- v. 4 (5.4%, 95% CI 1.1–14.9) in the non-rapid-cycling group prematurely discontinued double-blind treatment because of an adverse event (P = 0.60).
Discussion
Findings from other studies
Few double-blind, placebo-controlled trials have examined efficacy and safety of antidepressant v. mood stabiliser monotherapy in bipolar disorder. Leverich et al Reference Leverich, Altshuler, Frye, Suppes, McElroy and Keck7 found only a 23% sustained response rate without mood conversion episodes in patients with bipolar I and II disorder maintained on antidepressants (plus mood stabilisers). Schneck et al Reference Schneck, Miklowitz, Miyahara, Araga, Wisniewski and Gyulai26 followed 1191 individuals with bipolar disorder (356 rapid cycling) for 1 year and found a 34% recovery rate, with a 61% mood conversion rate during antidepressant therapy. A double-blind, placebo-controlled comparison of quetiapine v. paroxetine found no advantage for paroxetine v. placebo in bipolar depression, Reference McElroy, Weisler, Chang, Olausson, Paulsson and Brecher27 whereas a recent meta-analysis of controlled studies found no advantage for antidepressants per se in treating bipolar depression. Reference Sidor and McQueen's28
In contrast, a 5-year naturalistic study of 54 people with bipolar disorder found that antidepressants plus lithium maintained a response for an average of 17.2 months longer than if taking lithium alone, with a mood conversion rate of 14%. Reference Peselow, Fieve, Difiglia and Sanfilipo29 A 1-year study of individuals with bipolar disorder who had recovered on antidepressants plus mood stabilisers (n = 19) v. mood stabilisers alone (n = 25) found a 32% and 68% depressive relapse rate, respectively (P = 0.0065). Antidepressants produced a threefold lower risk of relapse. Reference Altshuler, Kiriakos, Calcagno, Goodman, Gitlin and Frye30 A similar benefit from long-term

Fig. 1 Flow chart of participants.
Table 1 Characteristics of participants with rapid- v. non-rapid-cycling bipolar disorder at the start of double-blind therapy
| Characteristic | Rapid-cycling group (n = 25) | Non-rapid-cycling group (n = 56) | P a |
|---|---|---|---|
| Male gender, % (n/N) | 64.0 (16/25) | 41.1 (23/56) | 0.06 |
| Age, years: mean (s.d.) | 35.3 (10.0) | 38.8 (12.8) | 0.23 |
| Illness duration, years: mean (s.d.) | 17.2 (10.0) | 20.0 (11.9) | 0.32 |
| Depressive episode duration, months: mean (s.d.) | 13.3 (15.3) | 4.0 (19.3) | 0.87 |
| Prior depressive episodes, mean (s.d.) | 22.8 (43.7) | 6.6 (6.4) | 0.008 |
| Prior hypomanic episodes, mean (s.d.) | 45 (56.2) | 9.0 (13.4) | <0.0005 |
| Hamilton Rating Scale for Depression score baseline, mean (s.d.) | 7.2(6.1) | 5.4 (4.0) | 0.13 |
| Young Mania Rating Scale score baseline, mean (s.d.) | 1.3(2.8) | 0.8 (1.7) | 0.27 |
a P-values are from Student's t-test except for gender, which is from χ2.
Table 2 Proportion of participants in the rapid- v. non-rapid-cycling group with treatment-emergent hypomania a
| Rapid-cycling group (n = 25) | Non-rapid-cycling group (n = 56) | ||||
|---|---|---|---|---|---|
| n | % (95% exact CI) | n | % (95% exact CI) | P b | |
| Hypomania | 5 | 20.0 (6.8–40.7) | 5 | 8.9 (3.0–19.6) | 0.27 |
| Type I subsyndromal | 5 | 20.0 (6.8–40.7) | 10 | 17.9 (8.9–30.4) | 0.82 |
| Type II subsyndromal | 2 | 8.0 (1.0–26.0) | 5 | 8.9 (3.0–19.6) | 0.89 |
| Type III subsyndromal | 2 | 8.0 (1.0–26.0) | 13 | 23.2 (13.0–36.4) | 0.13 |
| Hypomania or type I | 10 | 40.0 (21.1–61.3) | 15 | 26.8 (15.8–40.3) | 0.30 |
| Hypomania or type I or type II | 11 | 44.0 (24.4–65.1) | 18 | 32.1 (20.3–46.0) | 0.33 |
| Hypomania or type I or type II or type III | 11 | 44.0 (24.4–65.1) | 26 | 46.4 (33.0–60.3) | 0.99 |
a Some patients had more than one subsyndromal episode. Thus, the number of patients experiencing an episode shown in Table 2 may be smaller than the total number of episodes shown in Table 3.
b Fisher's exact test for comparison of rapid- v. non-rapid-cycling groups.
Table 3 Duration of treatment-emergent hypomanic episodes (in days) in the rapid- v. non-rapid-cycling group a
| Rapid-cycling group | Non-rapid-cycling group | ||||
|---|---|---|---|---|---|
| Mean (95% CI) | Episodes, n | Mean (95% CI) | Episodes, n | P b | |
| Hypomania | 23.2 (2.0 to 44.4) | 5 | 5.8 (0.71 to 10.9) | 5 | 0.06 |
| Type I subsyndromal | 2.6 (0.7 to 4.5) | 5 | 5.8 (1.8 to 9.9) | 11 | 0.27 |
| Type II subsyndromal | 9.0 (–16.4 to 34.4) | 2 | 15.4 (6.8 to 24.3) | 5 | 0.29 |
| Type III subsyndromal | 7.0 (–69.2 to 82.2) | 2 | 2.3 (1.1 to 3.4) | 16 | 0.05 |
a Some patients had more than one subsyndromal episode. Thus, the number of patients experiencing an episode shown in Table 2 may be smaller than the total number of episodes shown in Table 3.
b P-value is for comparison of mean duration between groups.
Table 4 Proportion of patients (with 95% exact CI) with a major or minor depressive episode
| Rapid-cycling group (n = 25) | Non-rapid-cycling group (n = 56) | ||||
|---|---|---|---|---|---|
| n | % (95% exact CI) | n | % (95% exact CI) | P a | |
| Major depressive episode | 1 | 4.2 (0.1–20.4) | 0 | 0 (0.0–6.4) | 0.13 |
| Type I minor depression | 8 | 32.0 (14.9–53.5) | 18 | 32.1 (20.3–46.0) | 0.99 |
| Type II minor depression | 6 | 24.0 (9.4–45.1) | 23 | 41.1 (28.1–55.0) | 0.14 |
| Type III minor depression | 1 | 4.2 (0.1–20.4) | 0 | 0 (0.0–6.4) | 0.13 |
| Major or type I minor depression | 17 | 68.0 (46.5–85.1) | 41 | 73.2 (59.7–84.2) | 0.63 |
| Major, type I or type II minor depression b | 16 | 64.0 (42.5–82.0) | 46 | 82.1 (69.6–91.1) | 0.08 |
| Major, type I, type II or type III minor depression b | 16 | 64.0 (42.5–82.0) | 46 | 82.1 (69.6–91.1) | 0.08 |
a Fisher's exact test for comparison of rapid- v. non-rapid-cycling groups.
b The same patients who experienced major, type I, type II or type III minor depressive episodes also experienced a type III minor depressive episode, resulting in similar values for the last two rows in Table 4.
Table 5 Duration of treatment-emergent depressive episodes (in days) in the rapid- v. non-rapid-cycling group

| Rapid-cycling group | Non-rapid-cycling group | ||||
|---|---|---|---|---|---|
| Minor depression | Mean (95% CI) | Episodes, n | Mean (95% CI) | Episodes, n | P a |
| Type I | 10.4 (3.9 to 17.0) | 9 | 14.6 (4.3 to 24.9) | 18 | 0.47 |
| Type II | 97.7 (–27.8 to 223.0) | 12 | 14.7 (8.8 to 20.6) | 27 | 0.10 |
| Type III | 3.0 | 1 | 0.0 | 0 | – |
a P-value is for comparison of mean duration between groups.
antidepressant therapy was reported by Kupfer et al, Reference Kupfer, Chengappa, Gelenberg, Hirschfeld, Goldberg and Sachs15 whereas a double-blind, placebo-controlled study of maintenance fluoxetine in individuals with bipolar II disorder who had recovered showed a clinically meaningful trend for fewer depressive relapses (P = 0.08). Reference Amsterdam, Shults, Brunswick and Hundert19,Reference Amsterdam and Shults20 Finally, the primary analysis from the current study showed the estimated hazard for depressive relapse as 2.5 times greater during lithium v. fluoxetine monotherapy (P = 0.04). Reference Amsterdam and Shults21
Limitations
Several caveats should be considered when interpreting the current findings. Results of this exploratory analysis are not definitive. The study was not powered to detect significant differences in efficacy or mania ratings between the rapid- v. non-rapid-cycling groups. The failure to identify significant differences in efficacy and safety in the current analysis does not mean that differences do not exist. We note the limited sample size of the rapid- v. non-rapid-cycling group within each treatment condition. Larger samples would be needed to detect small differences in mood conversion rates between groups.
In the current study, patients were stabilised on fluoxetine prior to randomisation. This design methodology may have influenced the long-term efficacy and safety ratings in favour of fluoxetine, whereby patients randomised to fluoxetine were more likely to stay well and less likely to experience mood conversion episodes.
Our definition of rapid cycling differed from the DSM-IV definition and was based on an average of ≥4 affective episodes per year over the course of the illness (rather than ≥4 affective episodes in the preceding year). This difference may have resulted in a rapid-cycling cohort with fewer affective episodes. Reference Schneck, Miklowitz, Miyahara, Araga, Wisniewski and Gyulai26 Analysis of rapid cycling by DSM-IV criteria may have produced different results.
Finally, we limited our YMRS analysis to participants who experienced a change in YMRS scores over baseline in order to avoid averaging zero values from individuals with no change in YMRS scores. The frequency and severity of mood conversion episodes may have differed between groups had we used different threshold criteria for subsyndromal episodes, or if we employed a longer treatment duration. For example, Schneck et al Reference Schneck, Miklowitz, Miyahara, Araga, Wisniewski and Gyulai26 found a 61% mood conversion rate in patients with stabilised bipolar disorder during 1 year of antidepressant therapy, whereas Koukopoulos et al Reference Koukopoulos, Sani, Koukopoulos, Minnai, Girardi and Pani3 reported rapid cycling in 88% of patients taking antidepressants in a 36-year naturalistic study. In contrast, the current study found no difference in the proportion of the patients in the rapid- v. non-rapid-cycling group in any treatment condition with increases in YMRS scores. Although it is possible that this low mood conversion rate resulted from the inclusion of more patients who were mildly ill with bipolar II disorder with a lower propensity for developing manic symptoms, the illness severity of the current patient cohort was similar to that of prior cohorts with bipolar II disorder in studies by us and others. Reference Leverich, Altshuler, Frye, Suppes, McElroy and Keck7,Reference Ghaemi, Klara, Ko, Baldassano, Kontos and Baldessarini10,Reference Goldberg and Ghaemi11,Reference Kupfer, Chengappa, Gelenberg, Hirschfeld, Goldberg and Sachs15-Reference Baldassano, Datto, Littman and Lipari17
Implications
Although not definitive, these findings suggest that maintenance lithium or fluoxetine monotherapy are similar to placebo in preventing depressive relapse and treatment-emergent mood conversion episodes during long-term relapse-prevention therapy of rapid- and non-rapid-cycling bipolar II disorder. The findings call into question practice guideline recommendations to avoid maintenance antidepressants in patients with rapid- and non-rapid-cycling bipolar II disorder. Reference Bauer, Callahan, Jampala, Petty, Sajatovic and Schaefer31-Reference Sachs, Printz, Kahn, Carpenter and Docherty34









eLetters
No eLetters have been published for this article.