Although obsessive—compulsive disorder (OCD) is still the subject of aetiological controversy, a number of studies over the last 20 years have provided strong evidence that abnormalities in frontal—subcortical circuitry are involved in the pathophysiology of OCD (Reference Saxena, Brody and SchwartzSaxena et al, 1998). Most of the functional imaging studies using positron emission tomography (PET) consistently have revealed increased metabolic rates in the orbitofrontal cortex and subcortical areas such as the caudate nucleus or the putamen, both in a resting state (Reference Baxter, Schwartz and MazziottaBaxter et al, 1988) and during periods of provoked OCD symptoms (Reference Rauch, Jenike and AlpertRauch et al, 1994). In contrast to these functional studies, the findings of volumetric studies using magnetic resonance imaging (MRI) have been contradictory. Some studies have reported that patients with OCD had significantly smaller volumes of the orbitofrontal cortex (Reference Szeszko, Robinson and AlvirSzeszko et al, 1999) and the caudate nucleus (Reference Robinson, Wu and MunneRobinson et al, 1995; Reference Rosenberg, Keshavan and O'HearnRosenberg et al, 1997) than those of normal controls, suggesting that a degenerative process might be implicated in the pathophysiology of OCD. Such results are not consonant, however, with those of other studies reporting no differences (Reference Jenike, Breiter and BaerJenike et al, 1996; Reference Bartha, Stein and WilliamsonBartha et al, 1998) or even increases in volume (Reference Scarone, Colombo and LivianScarone et al, 1992). Moreover, such volume reduction findings run counter to the regional cerebral metabolic elevation found in those areas of patients with OCD. These inconsistent findings in volumetric studies may originate from methodological differences, particularly in the arbitrary delineation of the region of interest (ROI). In contrast to the previous studies, our current study employs a novel voxel-by-voxel analysis of magnetic resonance images to compare the grey matter distribution between patients with OCD and their matched normal controls. On the basis of previous reports of increased metabolic activities in orbitofrontal and subcortical areas, we hypothesised that patients with OCD also would show increased grey matter density in those regions.
METHOD
Subjects
Twenty-five patients with OCD (17 men and 8 women) were recruited from an out-patient OCD clinic at Seoul National University Hospital. They fulfilled the DSM—IV criteria (American Psychiatric Association, 1994) for OCD as diagnosed using the Structured Clinical Interview for DSM—IV (SCID—IV). Exclusion criteria were any lifetime history of neurological or significant medical illnesses and any past history of substance misuse. Three of the 25 patients had major depressive disorder, 1 had major depressive disorder and bulimia nervosa, and 21 had OCD as their sole diagnosis. The normal control group consisted of 25 age— and gender-matched healthy volunteers (17 men and 8 women) who were recruited from the community through news-paper advertisements. Exclusion criteria for the controls were any current or lifetime history of DSM—IV Axis I disorder, which was screened using the SCID—IV. This study was carried out under guidelines for the use of human subjects established by the institutional review board. After a complete description of the scope of study to all subjects, written informed consent was obtained.
The mean ages for the OCD and control groups were 27.4 (s.d.=7.0) and 27.0 (s.d.=6.2) years (t=0.21, d.f.=4.8, P=0.83), respectively, and the mean periods of education were 14.2 (s.d.=2.2) and 15.3 (s.d.=1.8) years (t=1.97, d.f.=48, P=0.06), respectively. Composition of handedness was identical in both groups; 24 were right-handed and 1 was left-handed. At the time of the study, the patients had a mean duration of illness of 8.4 (s.d.=6.7) years, ranging from 1 to 23 years. Eight patients were drug-naïve and 17 had a history of anti-obsessional medication (four included a history of combined therapy with neuro-leptics), but they all remained psychotropic-free for at least 4 weeks. Clinical assessment included the Yale—Brown Obsessive Compulsive Scale (Y—BOCS; Reference Goodman, Price and RasmussenGoodman et al, 1989) for measuring OCD symptom severity (mean score for obsessive symptoms=13.0, s.d.=3.3; mean score for compulsive symptoms=11.2, s.d.=5.0; mean total score=24.2, s.d.=8.0).
Magnetic resonance imaging acquisition and image processing
Three-dimensional T1-weighted spoiled gradient echo magnetic resonance images were acquired on a 1.5-T GE SIGNA scanner (GE Medical Systems, Milwaukee, USA). Imaging parameters were as follows: 1.5-mm sagittal slices, echo time=5.5 ms, repetition time=14.4 ms, number of excitations=1, rotation angle=20°, field of view=21 × 21 cm and a matrix of 256 × 256.
Magnetic resonance images were processed using an image-processing software package, ANALYZE (version 3.0, Mayo Foundation, USA). Images were resampled to 1.0-mm3 voxels, reoriented to the conventional position and spatially normalised so that the anterior—posterior axis of the brain was aligned parallel to the inter-commissural line and the other two axes were aligned along the inter-hemispheric fissure. The data sets then were filtered using anisotropic diffusion methods to improve the signal-to-noise ratio. Images of tissues exterior to the brain were removed by the semi-automated region growing method. The extracted brain images were segmented into grey matter, white matter and cerebrospinal fluid, employing the fuzzy C-means algorithm (Reference Cannon, Dave and BezdekCannon et al, 1986). This method was chosen because it did not require a priori probability. Grey matter data were flipped to reverse right and left (to comply with neurological convention) and then reconstructed into binary grey matter images; grey matter voxels were set to a uniform intensity value of unity and others were set to a value of zero.
Processing of grey matter images for the regional analysis was performed using Statistical Parametric Mapping (SPM) 99 (Institute of Neurology, University College of London, UK) implemented in Matlab (Mathworks Inc., USA) (Reference Friston, Holmes and WorsleyFriston et al, 1995). Binary grey matter images were smoothed using an 8-mm full width at half-maximum (FWHM) Gaussian kernel to assign a weighted sum of grey matter values to each voxel for voxel-based statistical analysis of grey matter differences. These images were spatially normalised to remove variability due to differences in head size and to facilitate a voxel-based analysis. This process consisted of the following two steps. First, to determine the transformation parameters, filtered T1-weighted imaged were spatially normalised into the MNI (Montreal Neurological Institute, McGill University, CA) standard T1 template at the Standard Talairach spaces — (Reference Talairach and TournouxTalairach & Tournoux, 1988), and affine transformation was performed to determine the 12 optimal parameters to register the brain on the template. Second, by applying the parameters produced in the first step, the smoothed grey matter images were spatially transformed. Spatially normalised grey matter again were smoothed by convolution with an isotropic Gaussian kernel with 12-mm FWHM. Parameters for these two separate smoothing process steps were applied following those of Wright et al (Reference Wright, McGuire and Poline1995).
Statistical analysis
Total intracranial volume and global grey matter volume prior to the processing for regional analyses were calculated and compared between the patients and the controls using Student's t-test. P values less than 0.05 were considered significant.
Voxel-based regional analyses of the processed grey matter images were performed using SPM. The effects of the global grey matter intensity were removed by proportional scaling in which the count of each voxel was normalised relative to the total count of the brain. Any significant changes of regional grey matter density in the patients then were estimated by comparing their pre-processed grey matter images with those of the controls using t-statistics at every voxel. For ease of interpretation, t values were transformed to Z scores in the standard Gaussian distribution. The clusters consisting of a minimum of 50 contiguous voxels with a threshold of uncorrected P<0.001 (Z=3.09) were considered to have significant differences and were displayed on three orthogonal planes.
RESULTS
Global volume measurements
Mean total intracranial volume was 1410.8 cc (s.d.=131.8) for the patient group and 1357.5 cc (s.d.=106.3) for the control group. Mean global grey matter volumes were 849.8 cc (s.d.=83.3) and 834.4 cc (s.d.=71.1), respectively. Significant group differences for total intracranial volume and global grey matter volume were not observed (t=1.57, d.f.=48, P=0.12 and t=0.70, d.f.=48, P=0.48, respectively).
Regional analysis
The SPM results for the grey matter differences between the two groups are summarised in Table 1. As shown in Fig. 1a, regions where the patient group had significantly increased grey matter were observed to be distributed over multiple areas: left side of the orbitofrontal cortex, superior temporal gyrus, inferior parietal lobule and thalamus, right side of the insula, middle temporal gyrus and inferior occipital cortex and both sides of the hypothalamus.
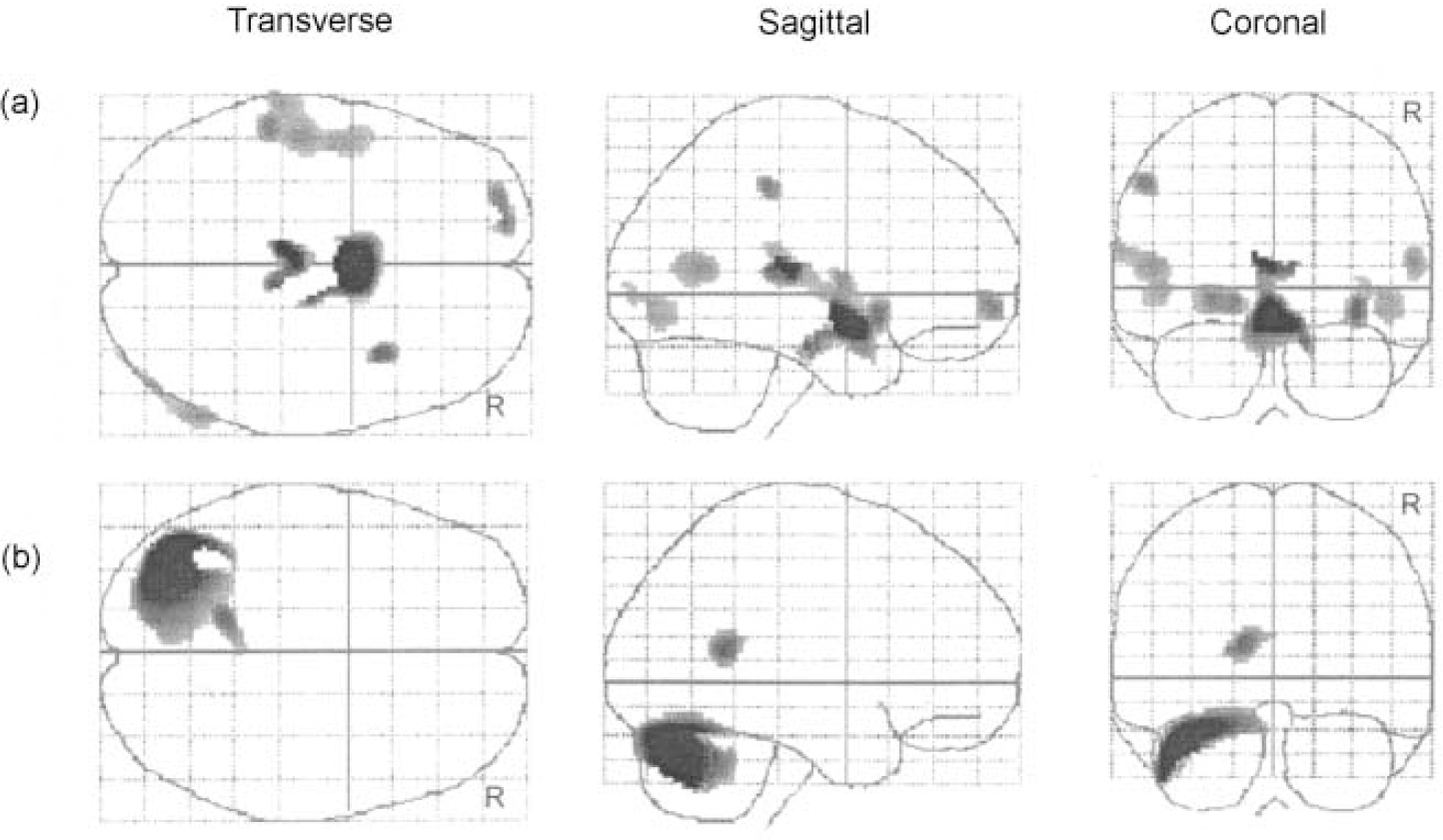
Fig. 1 Statistical parametric maps displaying abnormalities in grey matter density in patients with obsessive—compulsive disorder: (a) increased areas; (b) decreased areas.
Table 1 Regional grey matter changes in patients with obsessive—compulsive disorder
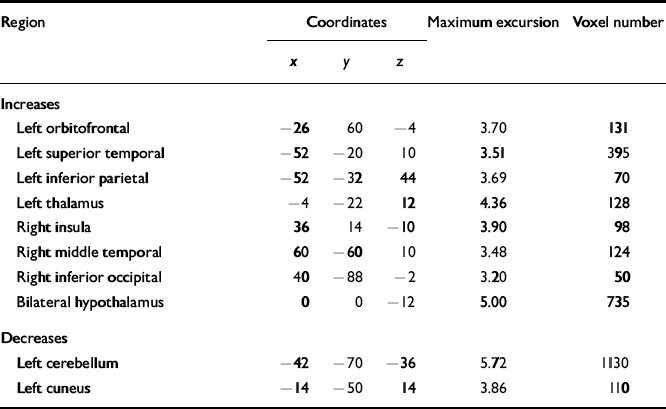
| Region | Coordinates | Maximum excursion | Voxel number | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Increases | |||||
| Left orbitofrontal | -26 | 60 | -4 | 3.70 | 131 |
| Left superior temporal | -52 | -20 | 10 | 3.51 | 395 |
| Left inferior parietal | -52 | -32 | 44 | 3.69 | 70 |
| Left thalamus | -4 | -22 | 12 | 4.36 | 128 |
| Right insula | 36 | 14 | -10 | 3.90 | 98 |
| Right middle temporal | 60 | -60 | 10 | 3.48 | 124 |
| Right inferior occipital | 40 | -88 | -2 | 3.20 | 50 |
| Bilateral hypothalamus | 0 | 0 | -12 | 5.00 | 735 |
| Decreases | |||||
| Left cerebellum | -42 | -70 | -36 | 5.72 | 1130 |
| Left cuneus | -14 | -50 | 14 | 3.86 | 110 |
On the other hand, as shown in Fig. 1b, regions where the patient group had significantly reduced grey matter appeared to be confined to the posterior part of the brain. Areas of reduction were distributed in a large circumscribed region in the left cerebellum and in a small region in the left cuneus.
DISCUSSION
Methodological considerations
Although a number of functional studies of OCD converge to suggest orbitofrontal and subcortical hyperfunction, structural findings using MRI have been contradictory. Different study methods and heterogeneous samples among the studies could, in part, account for the conflicting findings. Most of the earlier volumetric studies have employed the ROI approach in which there have been significant differences in the definition of the studied regions. Consequently, results have varied depending on how regions are demarcated and which external landmarks are used in circumstances without clear sulcal or structural boundaries (Reference Kim, Crespo-Facorro and AndreasenKim et al, 2000a ). For instance, one of the structural studies reported greater opercular volume in a sample of 10 females with OCD (Reference Jenike, Breiter and BaerJenike et al, 1996), but such an increase was not found in another study that applied a different ROI system to the same sample (Reference Grachev, Breiter and RauchGrachev et al, 1998). In addition, the delineated ROIs could be too large to detect subtle changes and thus a significant change in some neuronal field is likely to be obscured if surrounding areas within the ROI exhibit no changes. Such features are analogous to the inconsistent findings of functional imaging studies using the ROI approach that could be accounted for by the simultaneous existence of both hyper-and hypofunctioning regions in some areas (Reference Kim, Mohamed and AndreasenKim et al, 2000b ).
On the other hand, a novel voxel-based approach using SPM seems to have promise in the detection of subtle changes. Without artificial delineation, changes are searched for in the whole brain rather than in preselected regions. This approach has been verified as being useful to find subtle structural abnormalities in schizophrenia (Reference Sowell, Levitt and ThompsonSowell et al, 2000), as well as maturational changes in childhood and adolescence (Reference Paus, Zijdenbos and WorsleyPaus et al, 1999).
It should be noted that the regional grey matter differences that we detected may have arisen by chance (type I error) following multiple comparisons. We attempted, therefore, to apply a strict criterion: clusters of more than 50 contiguous voxels with a threshold of P<0.001. On the contrary, consideration should be given to the fact that changes in variably located area or areas with highly variable volume cannot be detected. Because of the possibility of these type II errors, the regional differences detected in the current study need to be viewed as representing foci of maximal change rather than regions that are exclusively affected.
Increased grey matter density in the orbitofrontal cortex
The voxel-by-voxel analyses of grey matter density in patients with OCD revealed regional brain abnormalities that were generally consistent with our a priori hypothesis based on previous results from functional imaging studies. Abnormality in the left orbitofrontal cortex is particularly impressive. It is noteworthy that the orbitofrontal cortex might play a potential role in inhibitory motor control (Reference Rubia, Russell and OvermeyerRubia et al, 2001). This may be of special importance given the compulsions presented in OCD and the patients' attempts to resist performing these actions.
Increased regional grey matter density in the orbitofrontal cortex, together with those in the thalamus and hypothalamus, is in agreement with the hyperfunctioning of the frontal—subcortical circuits in OCD, although the mechanism of the relationship between them is still unclear. One study also identified increased volume of the anterior cingulate gyrus in paediatric patients with OCD (Reference Rosenberg and KeshavanRosenberg & Keshavan, 1998). This concurs with the surgical interruption of frontal, cingulate or related fibres that has been found effective in the treatment of severe OCD (Reference Jenike, Baer and BallantineJenike et al, 1991), probably by interfering with these inappropriately overactive circuits.
Hyperfunctional frontal—subcortical circuits
Hyperfunctioning in these circuits may lead to increased grey matter density, in common with results regarding the exposure to neuroleptics among patients with schizophrenia. Neuroleptic treatment has been shown repeatedly to be related to enlarged basal ganglia structures, possibly resulting from the compensatory overactivities secondary to dopamine blockade (Reference Gur, Maany and MozleyGur et al, 1998). Likewise, long-standing metabolic and perfusional increases in frontal—subcortical circuits that have been provoked by OCD symptoms also might result in increased grey matter density.
It is possible also that increased grey matter density in frontal—subcortical circuits is a result of neurodevelopmental abnormality. Some failure in normally programmed cell death during brain development may contribute to greater amounts of cerebral cortex in patients with OCD. Frontal—subcortical circuits consisting of transient juvenile projections that would have been extinguished eventually may be retained abnormally and evoke obsessional symptoms. Alternatively, these circuits may stem from impaired neuronal pruning during development.
Parietal and cerebellar involvements in OCD
In contrast to the orbitofrontal cortex, the left cuneus in the current study was observed to be of a reduced area. This finding is consistent with a previous one where the retrocallosal region was reduced in patients with OCD (Reference Breiter, Filipek and KenneyBreiter et al, 1994). Several functional imaging studies also have suggested that similar areas have reduced metabolic activity, although such reductions have not been important in their own consideration (Reference Nordahl, Benkelfat and SempleNordahl et al, 1989; Reference Lucey, Costa and BlanesLucey et al, 1995). Given that these extrastriate areas have been activated with cognitive tasks related to visual imagery (Reference Mellet, Tzourio and CrivelloMellet et al, 1996), the defective features of these areas found in both functional and structural imaging studies may be related to visuospatial processing and visual memory deficits in some patients with OCD (Reference Purcell, Maruff and KyriosPurcell et al, 1998).
It is noteworthy that cerebellar grey matter is characteristically reduced in the left upper part. In fact, the cerebellum has not been an area of interest in psychiatric imaging studies, so it has not been investigated frequently as an ROI in previous studies. Cerebellar involvement in the symptomatology of OCD might be negligible because the binding site for serotonergic drugs such as paroxetine is nearly absent from the cerebellum (Reference Gumming and GjeddeCumming & Gjedde, 1993). However, consideration should be given to the important role that the cerebellum plays in coordinating complex mental and non-motor higher cognitive functions (Reference Kim, Andreasen and O'LearyKim et al, 1999), as well as in controlling motor functions through abundant cortical—subcortical—cerebellar connections (Reference Andreasen, Paradiso and O'LearyAndreasen et al, 1998). The cerebellar reduction identified in the current study, therefore, might be related to some cognitive deficits in OCD, including executive and visual memory dysfunction, although this is somewhat speculative. Previous researchers have interpreted abnormal neuropsychological performances in OCD as reflecting dysfunction of frontal—subcortical circuits (Reference Galderisi, Mucci and CatapanoGalderisi et al, 1995). Our finding indicates that such an interpretation needs to be extended to the abnormal circuits, including the cerebellum. Further research is necessary to verify cerebellar involvement in the pathophysiology of OCD.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• This study suggests that patients with obsessive—compulsive disorder (OCD) may have increased grey matter density as well as metabolic elevation in the frontal—subcortical circuits.
-
• Our finding of a marked reduction of cerebellar grey matter suggests that further research is necessary to verify cerebellar involvement in the pathophysiology of OCD.
-
• The overall morphometric changes observed suggest that neurodevelopmental abnormalities might be an underlying cause of OCD.
LIMITATIONS
-
• Despite the application of a strict statistical criterion, the regional grey matter differences detected may have arisen by chance following multiple comparisons.
-
• Grey matter changes in variably located areas or areas with highly variable volume could not be detected.
-
• It is still unclear whether or not grey matter changes are related to the severity of clinical symptoms and cognitive deficits.





eLetters
No eLetters have been published for this article.