There is no convincing evidence that combined antipsychotics are more effective than a single antipsychotic. Reference Centorrino, Goren, Hennen, Salvatore, Kelleher and Baldessarini1 Also, combining antipsychotics is a major cause of high-dose prescribing, Reference Harrington, Lelliott, Paton, Ochoka, Duffett and Sensky2 an increased side-effect burden, Reference Centorrino, Goren, Hennen, Salvatore, Kelleher and Baldessarini1 and possibly increased mortality. Reference Joukamaa, Heliovaara, Knekt, Aromaa, Raitasalo and Lehtinen3 Thus, evidence-based treatment guidelines for schizophrenia recommend antipsychotic monotherapy in standard dosage. 4,Reference Herz, Liberman, Lieberman, Marder, McGlashan, Wyatt and Wang5 Despite this, cross-sectional surveys consistently find that 40–50% of people receiving psychiatric in-patient treatment are prescribed combined antipsychotics. Reference Harrington, Lelliott, Paton, Ochoka, Duffett and Sensky2,Reference De Hert, Wampers and Peuskens6 We report on a quality improvement initiative to reduce the prevalence of high-dose and combination antipsychotic prescribing in acute adult and psychiatric intensive care wards in the UK.
Method
All National Health Service (NHS) trusts and private healthcare organisations in the UK that provide specialist mental health services were invited to participate in a quality improvement project by the UK Prescribing Observatory for Mental Health (POMH), focusing on high-dose and combination antipsychotic prescribing in acute adult psychiatric wards. Services that chose to participate were invited to send a project team to a workshop to discuss and review the aims, objectives and methodology of the project. The study consisted of three distinct phases: a baseline audit of prescribing practice with feedback of the benchmarked data to each participating service; the delivery of quality improvement interventions to trust teams; and then a further audit of prescribing practice 1 year after the baseline audit. The audit standards and three phases of the project are described.
Audit standards
Three standards were derived from treatment guidelines and consensus statements. First, the daily dose of an individual antipsychotic should be within licensed limits or, if a combination of antipsychotics is prescribed, the cumulative dose using the percentage method should not exceed 100%. 7 Second, a single antipsychotic should be prescribed; exceptions are patients who are switching from one antipsychotic to another and those who have derived insufficient benefit from clozapine monotherapy. 4 Finally, first-generation (typical) and second-generation (atypical) antipsychotic drugs should not be prescribed concurrently; exceptions are patients who are switching from one antipsychotic to another. 8
Phase 1: baseline audit of prescribing practice
Participating services were asked to submit data for all patients who, on a given census day during January 2006, occupied a bed on an acute adult or psychiatric intensive care ward and were being prescribed one or more antipsychotic drugs. The following data were collected for each patient: demographic variables (age, gender, ethnicity), clinical variables (ICD–10 diagnostic grouping, 9 Mental Heath Act status); names and doses of all regular and pro re nata (p.r.n., or ‘as required’) antipsychotic drugs prescribed. The maximum prescribed dose that could be administered over a 24-h period was recorded, irrespective of whether it was administered or not. For patients who were prescribed more than one antipsychotic, the primary reason for the combination (as determined by the clinical team) was recorded. Data were entered on a web-based form and submitted through a secure web system.
Phase 2: delivery of quality improvement interventions
Following the baseline audit, nine change interventions were developed and made available to participating services (further information is presented in a data supplement to the online version of this paper). All interventions were informed by the findings of the baseline audit. The interventions were of a type that research evidence has suggested has some effect on changing the behaviour of individuals working in healthcare settings. Reference Iles and Sutherland10 Participating trusts were encouraged to disseminate the audit report and interventions as widely as possible, and present the audit findings to local clinicians. The precise implementation strategy was determined by each trust's local POMH project team.
Phase 3: re-audit of prescribing practice
Data collection, as described for phase 1, was repeated 1 year after the baseline audit. The census day was in January 2007.
Data analysis
Data from the baseline audit were analysed at national level to allow the characteristics of the whole sample to be described; simple descriptive statistics were used. For patients with a diagnosis of schizophrenia or related disorder (ICD–10 categories F20–29), a binary logistic regression analysis was conducted with high dose as the dependent variable, and combined antipsychotics and variables that have previously been shown to be associated with high dose Reference Lelliott, Paton, Harrington, Konsolaki, Sensky and Okocha11 as the independent variables: age band, ward type, Mental Health Act status and gender. Data were further analysed at service level, to allow comparison of the findings against other services and the national data. Finally, data were analysed at ward level, allowing participating wards to compare themselves with their service as a whole and the national data. All analyses were repeated using the data from the second audit. Only the national data are described here. Data were analysed using SPSS version 14 for Windows.
Results
Thirty-two services, all of them NHS, participated in the baseline audit, submitting data for 3492 patients from 218 wards. All 32 services participated in the second audit, submitting data for 3271 patients from 209 wards. The demographic and clinical variables of patients occupying a bed at the point of the second audit did not differ from those of patients who occupied a bed during the baseline audit (Table 1).
Table 1 Demographic characteristics of the baseline and 1-year audit in-patient samples
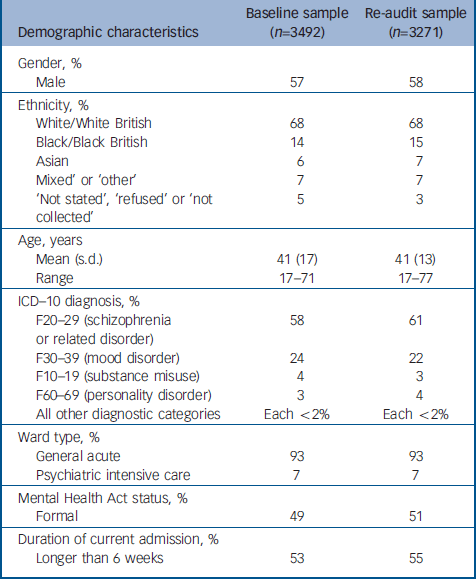
| Demographic characteristics | Baseline sample (n=3492) | Re-audit sample (n=3271) |
|---|---|---|
| Gender, % | ||
| Male | 57 | 58 |
| Ethnicity, % | ||
| White/White British | 68 | 68 |
| Black/Black British | 14 | 15 |
| Asian | 6 | 7 |
| Mixed' or ‘other’ | 7 | 7 |
| ‘Not stated’, ‘refused’ or ‘not collected’ | 5 | 3 |
| Age, years | ||
| Mean (s.d.) | 41 (17) | 41 (13) |
| Range | 17–71 | 17–77 |
| ICD–10 diagnosis, % | ||
| F20–29 (schizophrenia or related disorder) | 58 | 61 |
| F30–39 (mood disorder) | 24 | 22 |
| F10–19 (substance misuse) | 4 | 3 |
| F60–69 (personality disorder) | 3 | 4 |
| All other diagnostic categories | Each <2% | Each <2% |
| Ward type, % | ||
| General acute | 93 | 93 |
| Psychiatric intensive care | 7 | 7 |
| Mental Health Act status, % | ||
| Formal | 49 | 51 |
| Duration of current admission, % | ||
| Longer than 6 weeks | 53 | 55 |
Prescribing practice compared with audit standards
Table 2 summarises the extent to which prescribing practice deviated from the three audit standards at baseline and follow-up. For all three standards, and on both occasions, standards were not met for a substantial minority of patients. There was no meaningful change in prescribing practice between the two audits.
Table 2 Performance against the audit standards in the total national sample at baseline and re-audit
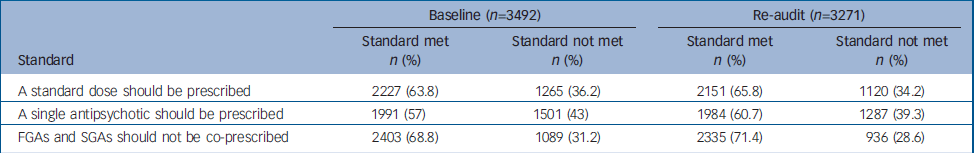
| Baseline (n=3492) | Re-audit (n=3271) | |||||
|---|---|---|---|---|---|---|
| Standard | Standard met n (%) | Standard not met n (%) | Standard met n (%) | Standard not met n (%) | ||
| A standard dose should be prescribed | 2227 (63.8) | 1265 (36.2) | 2151 (65.8) | 1120 (34.2) | ||
| A single antipsychotic should be prescribed | 1991 (57) | 1501 (43) | 1984 (60.7) | 1287 (39.3) | ||
| FGAs and SGAs should not be co-prescribed | 2403 (68.8) | 1089 (31.2) | 2335 (71.4) | 936 (28.6) | ||
FGA, first-generation antipsychotic; SGA, second-generation antipsychotic
Reasons for prescribing combined antipsychotics
Of the 1503 patients prescribed combined antipsychotics in the baseline audit, the clinical team reported that the reason for this was to control disturbed behaviour in 683 (45%) cases, because of a poor response to antipsychotic monotherapy in 259 (17%) cases, to cover a period of acute positive symptom exacerbation in 183 (12%) of cases and because the patient was switching from one antipsychotic to another in 125 (8%) cases. For 142 (9%) patients, the clinical team was unsure of the reason. In 56 (22%) of the 259 cases where the reason given was poor response to monotherapy, the combination included clozapine.
At the 1-year audit, 1287 patients were prescribed combined antipsychotics; 489 (38%) for the control of disturbed behaviour, 195 (15%) for a period of acute positive symptom exacerbation, 192 (15%) because of a poor response to antipsychotic monotherapy and 144 (11%) because they were switching from one antipsychotic to another. For 131 (10%) patients the clinical team was unsure of the reason or no reason was given. In 56 (29%) of the 192 cases where the reason given was poor response to monotherapy, the combination included clozapine.
Uptake of quality improvement interventions
All participating services received a copy of the audit report benchmarking their organisation as a whole and their individual wards against the total national sample. (Details of the number of services that ordered copies of the quality improvement interventions are presented in the data supplement to the online version of this paper.)
Predictors of high-dose and combination antipsychotic prescribing
Combined antipsychotics were the major cause of high-dose prescribing at baseline and re-audit; at baseline, 8.8% of prescriptions for a single antipsychotic were for a high dose and 72.6% of prescriptions for combined antipsychotics were for a high dose. At re-audit these figures were 9.4% and 72.5% respectively. On both occasions the majority of prescriptions for combined antipsychotics were due to p.r.n. prescribng. Figure 1 shows the contribution of p.r.n. to high-dose prescribing. At baseline, 78.5% of prescriptions for combined first- and second-generation antipsychotics included p.r.n. At re-audit, this proportion was 72.9%.
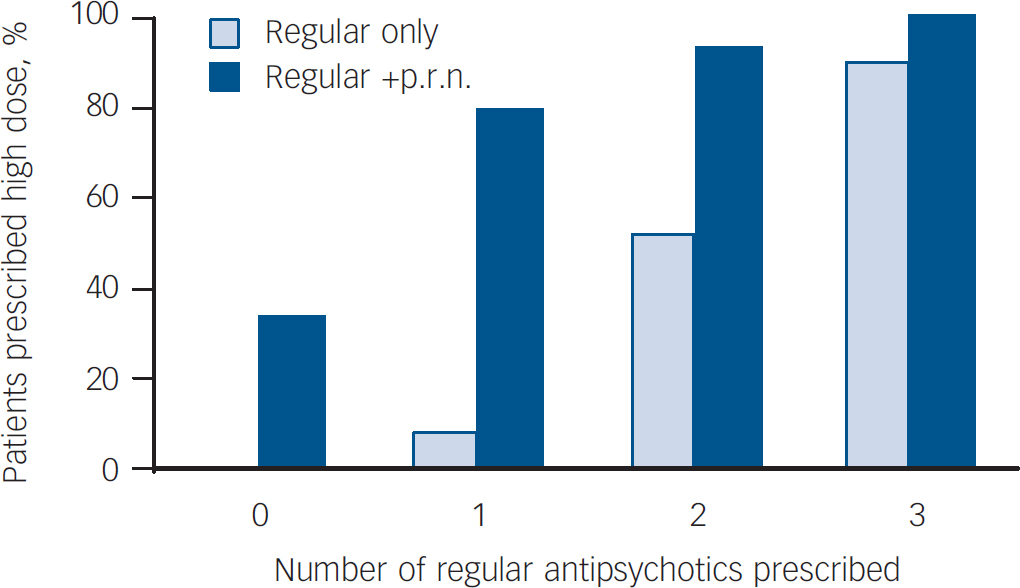
Fig. 1 Effect of pro re nata (p.r.n.) medication on high-dose prescribing: re-audit data.
The binary logistic regression analysis of the baseline audit data with ‘high dose’ as the dependent variable found that, together, combined antipsychotics, age band, ward type, Mental Health Act status and gender correctly predicted the prescription of high dose or not high dose in 82% of cases. The only variable in this model that was significant at the level of P<0.001 was ‘combined antipsychotics’. A patient who was prescribed combined antipsychotics was more than 20 times more likely to be prescribed a high dose as defined in our audit than a patient who was prescribed a single antipsychotic (odds ratio (OR)=23, 95% CI 18–29). The regression analysis was repeated after removing the combined antipsychotics variable, in order to examine further the possible influence of the other variables. This model was relatively poor at predicting high dose or not high dose, yielding only 60% correct classifications. Adding ethnicity to the model did not improve its predictive power. Similar results were obtained when the analysis was repeated using the data from the 1-year audit. On this occasion, combined antipsychotics, age band, ward type, Mental Health Act status and gender together predicted the prescription of high dose or not high dose in 81.5% of cases. The only variables in this model that were significant at the level of P<0.001 were combined antipsychotics and Mental Health Act status. Again, adding ethnicity to the model did not improve its predictive power. As at baseline, a patient who was prescribed combined antipsychotics was more than 20 times more likely to be prescribed a high dose as defined in our audit than a patient who was prescribed a single antipsychotic (OR=20.8, 95% CI 16.1–26.3).
Discussion
We found a high prevalence for the prescription of both high-dose and combined antipsychotics in adult in-patient settings in the UK. Only a small proportion of this was consistent with recommended treatment strategies such as cross-titration when switching from one antipsychotic to another, or augmentation of clozapine. Most was due to p.r.n. prescribing of antipsychotic drugs for the management of behavioural disturbance. Thus, prescribing practice fell well below the audit standards derived from the recommendations made in clinical guidelines. Although the use of combined antipsychotics significantly predicted high dosage, none of the other demographic and clinical variables collected in the audits did so. This included ethnicity, a finding that is consistent with Connolly et al. Reference Connolly, Rogers and Taylor12 Our quality improvement interventions, designed to encourage reflective practice and based on methods known to produce change, had little impact within the year to re-audit (see online Table DS1).
We propose three possible explanations for the failure of our quality improvement programme to change practice. First, the standards might not have been accepted by those to whose practice they relate. Second, the change interventions might not have reached – or, if they did reach, might not have influenced – those for whom they were intended; in allowing trusts to determine their own strategies for implementing interventions, it is likely that there was variation in the way the interventions were disseminated and used. Third, there might be cultural or organisation system factors that hindered change. We suspect that all three contributed and that the most important common element was p.r.n. prescribing.
We did not measure changes in the staff or patient group from baseline to re-audit. However, there is considerable consistency of practice across the UK regarding staffing and use of acute psychiatric wards. This allows some assumptions to be made with confidence. Although there would have been relatively little change in the senior medical or nursing staff, most wards would have had different junior doctors at baseline and re-audit, and the patient cohort would have changed greatly. Thus, the relative stability of the prevalence of p.r.n. prescribing over the period of our audit cycle suggests that such practice is not due to transient factors such as a particularly challenging group of patients at the time of the audit census or the prescribing practice of a particular cohort of junior doctors.
Pro re nata prescribing of antipsychotic drugs is neither supported by a robust evidence base demonstrating effectiveness and safety, Reference Whicher, Morrison and Douglas-Hall13 nor routinely covered by institutional policies, procedures or guidelines, Reference Szczesny and Miller14 yet is common in psychiatric in-patient settings. Reference Geffen, Sorensen, Stokes, Cameron, Roberts and Geffen15,Reference Paton, Lelliott, Harrington, Okocha, Sensky and Duffet16 Most patients who have a diagnosis of schizophrenia or mania have at least one dose of p.r.n. antipsychotic administered during their hospital stay. Reference Geffen, Sorensen, Stokes, Cameron, Roberts and Geffen15 The risk of a patient being given a high dose may not be recognised by either the prescribing doctor or the administering nurse. In addition, several studies have found that neither the therapeutic outcome nor the side-effect burden after p.r.n. antipsychotics is adequately documented in medical records, Reference Geffen, Sorensen, Stokes, Cameron, Roberts and Geffen15,Reference Paton, Lelliott, Harrington, Okocha, Sensky and Duffet16 which means that clinical teams may continue with this approach without the ability to systematically evaluate its risks and benefits.
The ‘custom and practice’ of p.r.n. prescribing within clinical teams leads to a loss of clarity regarding responsibility. Reference Baker, Lovell and Harris17 Although p.r.n. medication is usually prescribed by junior doctors, it is strongly influenced by the requests of ward-based nurses for access to additional medication. Reference Ito, Koyama and Higuchi18 Previous studies suggest that a large proportion of p.r.n. prescriptions do not contain clear indications for use or frequency of administration. Reference Craven, Voore and Voineskos19 The same dose may be prescribed by the oral or intramuscular route, and the use of dose ranges is common. Reference Bowden20 Thus, the choice of p.r.n. dose, route, frequency and indication may be left to the nursing staff. Some data indicate that the reasons for administration may differ from those intended. For example, where both benzodiazepines and antipsychotics are prescribed p.r.n., nursing staff preferentially administer the antipsychotic, Reference Usher and Luck21,Reference Geffen, Cameron, Sorensen, Stokes, Roberts and Geffen22 whereas doctors prefer the use of benzodiazepines for most indications. Reference Geffen, Cameron, Sorensen, Stokes, Roberts and Geffen22 Nurses identify more indications for the use of p.r.n. antipsychotics than do doctors, notably for the acute management of hallucinations, delusions and thought disorder, and their preferred antipsychotics are haloperidol and chlorpromazine. Reference Geffen, Cameron, Sorensen, Stokes, Roberts and Geffen22 Concerns have been raised that nurses do not have enough knowledge of psychopharmacology to make appropriate choices from a range of p.r.n. options and to monitor for side-effects, Reference Brooker, Falloon, Butterworth, Goldberg, Graham-Hole and Hillier23 and research in this area has been considered a priority. Reference Usher, Holmes, Lindsay and Lack24
There is some evidence that patients who receive p.r.n. antipsychotics have an increased burden of side-effects, particularly sedation, confusion, extrapyramidal side-effects and postural hypotension, Reference Geffen, Sorensen, Stokes, Cameron, Roberts and Geffen15 and are at greater risk of clinically significant drug interactions. Reference Davies, Lennard, Ghahramani, Pratt, Robertson and Potokar25 In addition, we found that p.r.n. prescribing commonly led to co-prescription of first- and second-generation antipsychotics, which is likely to negate the main advantage of the newer drugs, that being their lower liability to cause extrapyramidal side-effects. This is supported by the findings of a large survey of prescribing practice: patients prescribed combined first- and second-generation antipsychotics were just as likely to be prescribed anticholinergic agents as patients prescribed first-generation drugs alone. Reference Paton, Lelliott, Harrington, Okocha, Sensky and Duffet16 Further, the majority of antipsychotics have some potential to prolong the cardiac QTc interval, an effect that may rarely result in serious cardiac arrhythmias. In 2006, the Medicines and Healthcare Regulatory Authority conducted a review of the cardiac safety of all antipsychotic drugs available in the UK. 26 This led to the recommendation that the wording ‘avoid concomitant neuroleptics’ should be added to the ‘special warnings and precautions for use’ section of the summary of product characteristics (product licence) of every antipsychotic; the routine prescription of antipsychotics on a p.r.n. basis is in contrast to the caution that is recommended.
Strategies to reduce the use of p.r.n. prescribing
Our audit findings support the notion that p.r.n. antipsychotic medication is an embedded practice in many in-patient clinical teams, which leads to high-dose and combined antipsychotic prescribing and seems to be resistant to educational change interventions. However, small studies have demonstrated that interventions that integrate the use of p.r.n. medication into a care pathway can significantly reduce prescribing and administration. Examples include standard setting Reference Bowden20 and the use of stat (immediate) doses in place of p.r.n. Reference Thapa, Palmer, Owen, Huntley, Clardy and Miller27 Further, studies have demonstrated that senior nursing staff are able to identify more alternatives to p.r.n. than junior or part-time staff, Reference Geffen, Cameron, Sorensen, Stokes, Roberts and Geffen22 and that mandatory review of patients receiving p.r.n. by staff with specialist skills in behavioural management and psychopharmacology reduced the subsequent use of p.r.n. medication. Reference Donat28 Simple strategies such as the provision of structured daytime activities can also be effective. Reference Donat28
Acknowledgements
Acknowledgements are due to the Health Foundation which funded the UK Prescribing Observatory for Mental Health (POMH–UK) with a tapering grant from 2005, the local POMH–UK project teams of the participating services and the National Health Service clinicians and administrators who collected the audit data, and Dr Andy Thompson and Simon Strange who acted as advisors. In addition to the authors, the members of the Topic 1 project group were Janey Antoniou, Elizabeth Hancock and Mo Hutchison.






eLetters
No eLetters have been published for this article.