The neurodevelopmental conceptualisation of attention-deficit hyperactivity disorder (ADHD) is based on the fact that the disorder has its onset before puberty, has a steady course rather than a remitting and relapsing pattern, and affects males predominantly.Reference Thapar, Cooper and Rutter1 However, clinical and population-based studies do not support all these assumptions. Despite the absolute predominance of boys in clinical settings, there is a more balanced gender ratio in the child population,Reference Kooij, Bijlenga, Salerno, Jaeschke, Bitter and Balázs2 or even a preponderance of females in community samples of adults.Reference Kooij, Buitelaar, van den Oord, Furer, Rijnders and Hodiamont3,Reference Vitola, Bau, Salum, Horta, Quevedo and Barros4 Still, the persistent course is not observed in all affected individuals, with remission occurring in at least a third of patients, both in children and adults.Reference Faraone, Biederman and Mick5,Reference Karam, Breda, Picon, Rovaris, Victor and Salgado6 Additionally, one study raised the possibility of a remitting and relapsing symptom course in children.Reference Lahey, Pelham, Loney, Lee and Willcutt7 However, the most intriguing findings were those regarding the possibility of the disorder beginning after puberty. Epidemiological cohorts suggested that a subset of individuals with the disorder may have its onset late in adolescenceReference Lecendreux, Konofal, Cortese and Faraone8 or even in adulthood, with three different cohorts showing that around 80% of adults with ADHD did not present with the disorder in childhood.Reference Moffitt, Houts, Asherson, Belsky, Corcoran and Hammerle9–Reference Caye, Rocha, Anselmi, Murray, Menezes and Barros11 These results challenged both the common notion that adults with ADHD are persistent cases with onset in childhood, as well as the classic neurodevelopmental nature of the disorder itself.
Empirical data on late-onset ADHD
Several factors contribute to the plausibility of the existence of late-onset ADHD cases. As opposed to clinical cohorts, where individuals are followed up from childhood until adulthood, a birth cohort design is more appropriate for detecting tardive incident cases.Reference Lecendreux, Konofal, Cortese and Faraone8 Three birth cohorts that assessed ADHD in >8000 individuals from New Zealand, Brazil and the UK showed that around 80% of adults with current ADHD syndrome did not have the disorder in childhood.Reference Moffitt, Houts, Asherson, Belsky, Corcoran and Hammerle9–Reference Caye, Rocha, Anselmi, Murray, Menezes and Barros11 Furthermore, those adults with late-onset ADHD did not differ from their full-criteria counterparts in terms of clinical profile, severity, comorbidities and impairment.Reference Agnew-Blais, Polanczyk, Danese, Wertz, Moffitt and Arseneault10–Reference Chandra, Biederman and Faraone12 Two additional population studies that considered subtle ADHD symptoms in childhood as evidence of a neurodevelopmental trajectory found that 25% of adults with ADHD were late-onset cases.Reference Manfro, Santoro, Polanczyk, Gadelha, Pan and Bressan13,Reference Cooper, Hammerton, Collishaw, Langley, Thapar and Dalsgaard14 The follow-up of individuals from control groups of case-control studies could also provide an estimation of the occurrence of late-onset ADHD, with the advantage of using more accurate methods to diagnose ADHD in both childhood and adulthood. In this sense, 18.8% of the individuals from the normative control group of the Multimodal Treatment Study of Children with Attention Deficit and Hyperactivity Disorder (MTA) had a formal diagnosis of late-onset ADHD. After a rigorous stepped procedure to rule out ‘false’ late-onset cases, 2% of the sample still had ADHD syndrome that started late in adolescence or adulthood.Reference Sibley, Rohde, Swanson, Hechtman, Molina and Mitchell15
Critics on birth cohort findings
Critics on the findings of these birth cohorts pointed out several methodological limitations of the studies to question the validity of their results.Reference Asherson and Agnew-Blais16 One of them is the ‘false positive paradox’, which occurs when the actual prevalence of the disorder is lower than the false-positive rate of the diagnostic test. Since the ADHD diagnosis is not fully validated in population samples, the false-positive paradox is an important aspect to be considered in those cohort studies. Another possible source of false-positive, late-onset diagnosis is the nonobservance of heterotypic neurodevelopmental trajectories, in which other psychiatric disorders precede ADHD. In this sense, a cohort study with enriched risk for psychopathology found that the majority of adolescent-onset ADHD cases had a heterotypic neurodevelopmental trajectory.Reference Manfro, Santoro, Polanczyk, Gadelha, Pan and Bressan13 Also, some of the population-based studies did not consider childhood subthreshold symptoms to rule out late-onset diagnosis.Reference Lecendreux, Konofal, Cortese and Faraone8 Furthermore, other psychiatric conditions preceding or co-occurring with ADHD might determine phenocopies.Reference Sibley, Rohde, Swanson, Hechtman, Molina and Mitchell15 The change in information source from childhood to adulthood (parent-, teacher- or self-report) is also quoted as a cause of possible false-positive and false-negative diagnoses.Reference Asherson and Agnew-Blais16 Finally, delayed ADHD diagnosis occurring in individuals with high IQ or highly supportive families was hypothesised as contributing to false-positive, late-onset ADHD.Reference Agnew-Blais, Polanczyk, Danese, Wertz, Moffitt and Arseneault17
The interpretation of those cohorts’ findings mentioned above was based on arbitrary categorical definitions (early- versus late-onset, persistent versus remitted). This aprioristic approach may have limited the detection of more subtle behaviors and ADHD symptomatology throughout time. There are few studies on hypothesis-free, empirically derived trajectories from childhood to adulthood.Reference Asherson and Agnew-Blais16 Riglin et alReference Riglin, Collishaw, Thapar, Dalsgaard, Langley and Smith18 showed the existence of low, intermediate, childhood-limited and persistent ADHD trajectories between 4 and 17 years of age. In that study, although a latent late-onset trajectory was not found, 2.5% of the cohort's individuals had late-onset ADHD. The only study using adulthood data also revealed low-, intermediate- and high-symptom trajectories, with around 3% of the individuals belonging to the high-persistent trajectory. The authors also found that hyperactivity/impulsivity symptoms have an overall declining pattern, and inattention tends to be more stable over time, but the study did not mention the specific trajectory of ADHD cases.Reference Döpfner, Hautmann, Görtz-Dorten, Klasen and Ravens-Sieberer19 Until now, no single study has followed children up to adulthood with a sufficiently long follow-up to permit identifying possible ascending trajectories.
We tested both polythetic definitions and latent growth modelling analyses to differentiate between neurodevelopmental and late-onset ADHD cases. Analyses were carried out with data from four waves of evaluation of ADHD up to adulthood (11, 15, 18 and 22 years of age) in individuals from the 1993 Pelotas birth cohort. We also assessed demographic and clinical factors associated with different latent ADHD trajectories.
Method
Sample and design
This study was carried out with data from 5249 individuals born in 1993, representing 99.1% of all live-born children in the southern Brazilian city of Pelotas (340 000 inhabitants), and followed up to 22 years of age. The participants underwent five evaluation waves at the perinatal period and at 11, 15, 18 and 22 years of age. In 2015, 4003 individuals were traced (including 193 deaths), with a final retention rate of 76.3%.Reference Gonçalves, Wehrmeister, Assunção, Tovo-Rodrigues, de Oliveira and Murray20
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the institutional review board (approval number 1.250.366) of the Universidade Federal de Pelotas. All individuals in the cohort provided written informed consent.
Clinical assessment
Interviewers
Trained psychologists performed face-to-face interviews to collect demographic and clinical information with parents and participants in the four waves.
Perinatal data
Data on prenatal tobacco exposure, newborn gender, birth weight and ethnicity were collected from the mothers in maternity.
Assessments at 11 and 15 years of age
Mental health status was assessed through the self- and parent-report Brazilian Portuguese version of the Strengths and Difficulties Questionnaire (SDQ). The SDQ is a diagnostic screening tool used to detect ADHD and other psychopathologies. The instrument was tested in different cultures and languages and had good psychometric properties.Reference Woerner, Fleitlich-Bilyk, Martinussen, Fletcher, Cucchiaro and Dalgalarrondo21 The hyperactive behaviour subset (SDQ-H) presents five affirmatives regarding ADHD symptoms: restlessness, fidgeting, poor concentration, impulsivity and finishing tasks. Each item has three possible answers: ‘not true’, ‘somewhat true’ or ‘certainly true’. To be considered present, the specific symptom had to be scored in the highest possible option (‘certainly true’ or ‘not true’, according to the direction that the affirmative pointed as a positive answer). Thus, participants could have a total score ranging from 0 to 10 points, or categorical symptoms ranging from 0 to 5 symptoms. The SDQ also has an impact supplement assessing impairment caused by symptoms at home, with friends, at school and during leisure time, with possible scores defined as ‘not at all’, ‘only a little’, ‘a medium amount’ or ‘a great deal’. Both self- and parent-reported SDQ information were obtained at 11 years of age, but only the parent-reported SDQ was available at 15 years of age. Individuals were considered as having ADHD at 11 or 15 years of age if they had a score of ≥8 in the SDQ-H by parent-report. This cut-off predicted the diagnosis with an area under the curve of 0.81 (95% CI, 0.74–0.88), with 85.7% sensitivity and 67.4% specificity. To improve specificity, we used at least one point of impairment in the impact SDQ supplement.Reference Caye, Rocha, Anselmi, Murray, Menezes and Barros11 We defined individuals with subthreshold ADHD as those with one standard deviation above the mean in the parent-report (≥6 points) or self-report (≥5 points) SDQ-H scores, regardless of impairment.
Internalising and externalising disorders were considered present when the participant scored ‘very high’ in the SDQ according to the four-band categorisation of the SDQ scores for 4- to 17-year-olds for emotional, peer and conduct problems (http://www.sdqinfo.org).
A confidential questionnaire regarding substance use and child maltreatment was applied at 11 and 15 years of age.
Assessments at 18 and 22 years of age
Between 2011 and 2012, when cohort individuals were 18 years old, all DSM-5 ADHD criteria were assessed in individuals presenting with at least two symptoms in the World Health Organization's Adult ADHD Self-Report Scale (ASRS)Reference Kessler, Adler, Ames, Demler, Faraone and Hiripi22 six-item screening tool. The screening tool assesses four symptoms of inattention (does not follow through, difficulty organising tasks, forgetful, reluctant to engage in tasks) and two symptoms of hyperactivity (fidgets, always on the go). ADHD data was collected through face-to-face interviews by clinical psychologists, using the DSM-5 ADHD criteria draft proposed by the DSM Externalizing Disorders Working Group, at that time freely available at www.dsm5.org.
At 22 years of age, the complete DSM-5 ADHD criteria were applied in the whole cohort and individuals were considered as having ADHD when they had all DSM-5 criteria but the age-at-onset criterion (i.e. at least five symptoms of inattention and/or hyperactivity/impulsivity, presence of symptoms in more than one setting and at least moderate impairment).
Other psychiatric disorders status was obtained through the Brazilian Portuguese version of the Mini-International Neuropsychiatric Interview,Reference Amorin23 using the major depressive disorder, bipolar disorder, social anxiety disorder, generalised anxiety disorder, post-traumatic stress disorder and antisocial personality disorder sections. Information on general health, income, substance use, years of schooling and behaviour was obtained with proper cohort protocol.Reference Gonçalves, Wehrmeister, Assunção, Tovo-Rodrigues, de Oliveira and Murray20 IQ was tested at 18 years of age, with a short version of the Wechsler Adult Intelligence Scale, Third Edition.Reference do Nascimento and de Figueiredo24
Statistical analyses
Polythetic analysis to test neurodevelopmental and late-onset trajectories in those with ADHD at 22 years of age
We took into account the possible causes for questionable late-onset diagnosis, as debated by Asherson and Agnew-Blais,Reference Asherson and Agnew-Blais16 to create hierarchical polythetic criteria to differentiate between true and false late-onset cases in the ADHD group at 22 years of age. Thus, we characterised false late-onset ADHD (doubtful cases) as those presenting with one or more of the following: (a) an SDQ-H score of ≥5 from self-report, or of ≥6 from parent-report at 11 years of age (defined as subthreshold ADHD); (b) an SDQ-H score of ≥6 in the parent-report at 15 years of age; (c) a heterotypic neurodevelopmental trajectory, defined as those without ADHD at 11 and 15 years of age but presenting with internalising or externalising problems at those ages; (d) high (>116) or low (<78) IQ (±1.5 s.d. in our sample); and (e) internalising (depression, anxiety disorders) or externalising disorders (antisocial personality disorder, substance use disorder) at 18 or 22 years of age that could potentially better explain ADHD symptoms. The same categorical method was applied in the non-ADHD individuals to illustrate how this approach would determine a group of supernormal controls not representative of the whole group of individuals without ADHD in the population (Fig. 1).
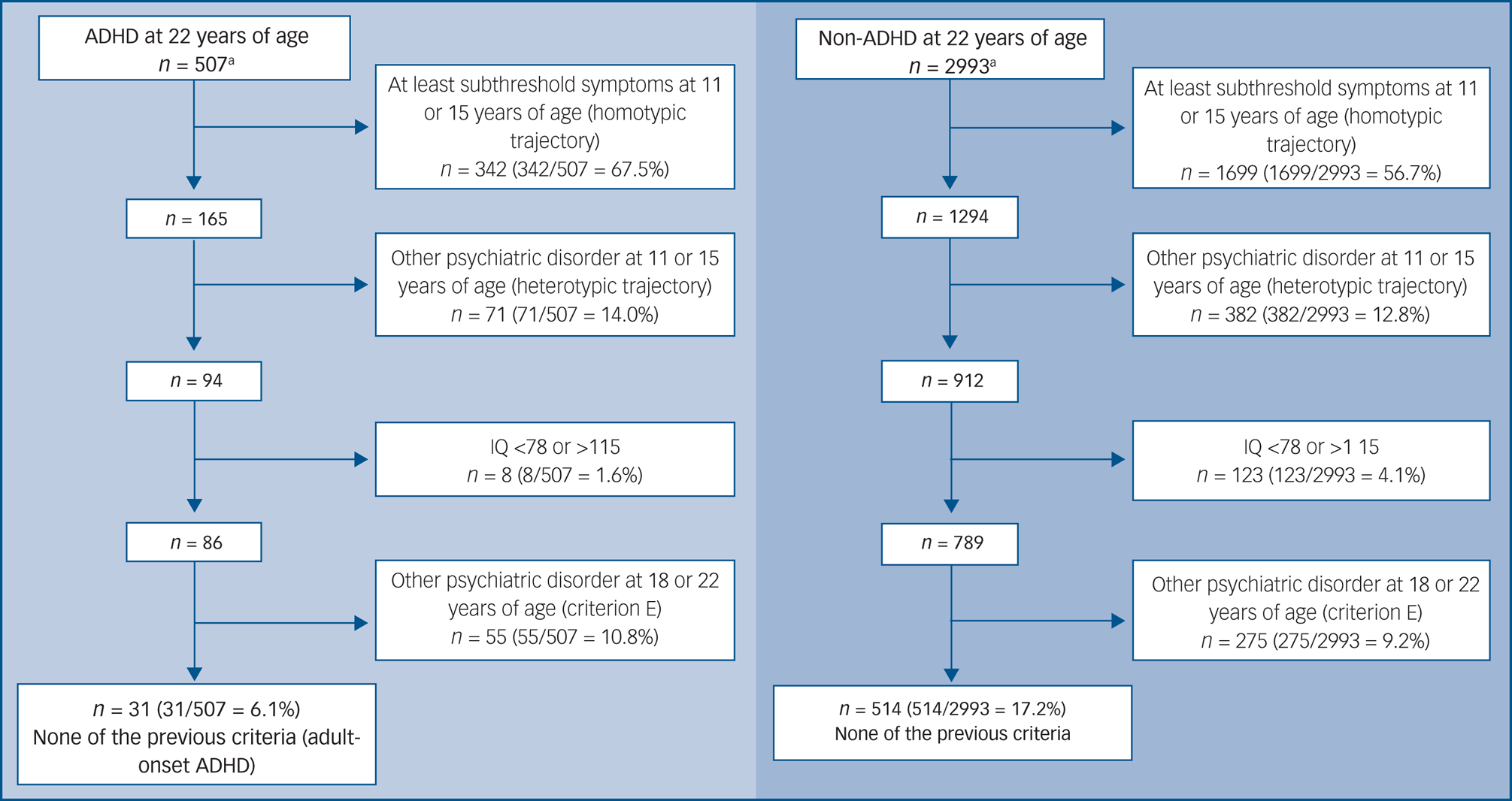
Fig. 1 Polythetic analysis of individuals with versus without ADHD at 22 years of age. ADHD, attention-deficit hyperactivity disorder.
a. Individuals with missing data were excluded.
Latent class mixed model to detect ADHD trajectories
We used a latent class mixed model (LCMM) with data on ADHD symptoms collected at 11, 15, 18 and 22 years of age to ascertain different trajectories of ADHD symptoms in the 4676 individuals of the cohort with at least one evaluation for ADHD symptoms, as well as in the 540 individuals with ADHD at 22 years of age. Z-scores derived from five symptoms of the SDQ-H subscale for 11 and 15 years of age, and the six-item ASRS screener at 18 and 22 years of age, were included in the latent analysis of trajectories.
LCMM was designed to differentiate groups of individuals following different developmental trajectories, providing specific trajectories, number of individuals belonging to each trajectory and the individual's probability of belonging to specific trajectories. Further, this methodology allows us to analyse ADHD measures from different instruments used to assess the disorder at different ages (e.g. the use of SDQ in adolescence and ASRS and DSM-5 in adulthood).Reference Proust-Lima, Philipps and Liquet25 The best-fitting solution between one class and four classes was defined through the lowest Bayesian information criterion, lowest Akaike information criterion, highest relative entropy and theoretical and clinical utility of the model. Missing data were handled with full-information maximum likelihood.Reference Proust-Lima, Philipps and Liquet25 LCMM analyses were conducted with the R package lcmm (version 1.8.1) for the R software for Windows, version 3.5.3.Reference Proust-Lima, Philipps and Liquet25
We ran binary logistic regressions to assess factors associated with different ADHD trajectories of those with ADHD at 22 years of age. A significance level of 5% and two-tailed tests were considered in the analyses. SPSS for Windows version 18.0 was used in these analyses.
Results
ADHD prevalence
The estimated prevalence of ADHD (regardless of the age-at-onset criterion at 18 and 22 years of age) were 8.9% (393/4424), 9.7% (407/4211), 12.1% (492/4052) and 14.3% (540/3780) at 11, 15, 18 and 22 years of age, respectively. Taking into account all DSM-5 criteria, the prevalence rates observed at 18 and 22 years of age were 3.5% (142/4052) and 4.5% (169/3780), respectively.
Polythetic definitions to characterise neurodevelopmental and late-onset ADHD cases at 22 years of age
According to the criteria used, 540 individuals had ADHD syndrome at 22 years of age. From those, 507 had complete data and were included in the analysis. Three hundred and eight individuals had at least subthreshold ADHD at 11 years of age. From the remaining 199 individuals, 34 had at least subthreshold ADHD at 15 years of age. These ruled-out participants were considered as having a homotypic trajectory (Fig. 1).
Among the 165 remaining participants, 71 individuals had severe internalising or externalising problems at 11 or 15 years of age by parent-report, and were therefore considered to have a heterotypic trajectory, resulting in 94 participants. In this remaining group, two had low IQ (<78) and six had high IQ (>116). Among the 86 remaining participants, 48 had internalising or externalising problems at 18 or 22 years of age, and were excluded (ADHD possibly better explained by another psychiatric disorder). Finally, seven individuals in this group had important drug or alcohol use across their lifespan. After this procedure, 31 (6.1%) adults with ADHD at 22 years of age could be defined as bona fide adolescent- or adult-onset ADHD. We used the same criteria for refining the total sample in the non-ADHD group (3240 individuals), resulting in around 17% (514 individuals) of the sample that went on to be characterised as supernormal controls (Fig. 1).
LCMM trajectories of ADHD symptoms
When taking into account the entire cohort, the best model comprised three trajectories: 77.4% (stable-medium), 17.6% (descending-low) and 4.9% (ascending-high) (Fig. 2). Regarding trajectories of the group of individuals with ADHD at 22 years, the best solution was the one with two classes (Fig. 3). This model included 78.1% in the stable (neurodevelopmental) trajectory (n = 422), and 21.9% in the ascending trajectory (high probability of late-onset) (n = 118). For comparisons between different LCMM models, please refer to the Supplementary material available at https://doi.org/10.1192/bjp.2020.200.
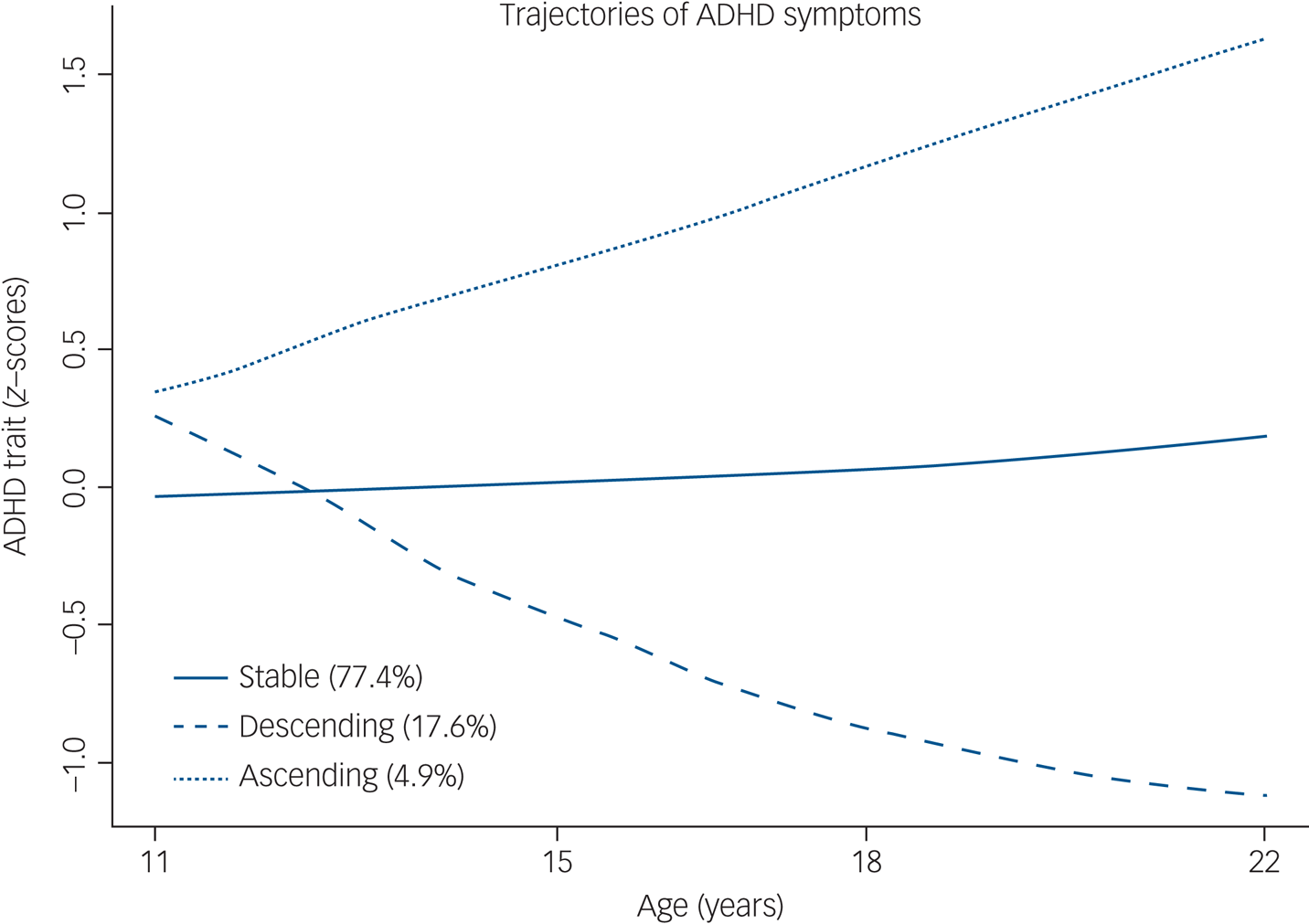
Fig. 2 ADHD trajectories in the entire cohort (n = 4676). ADHD, attention-deficit hyperactivity disorder.
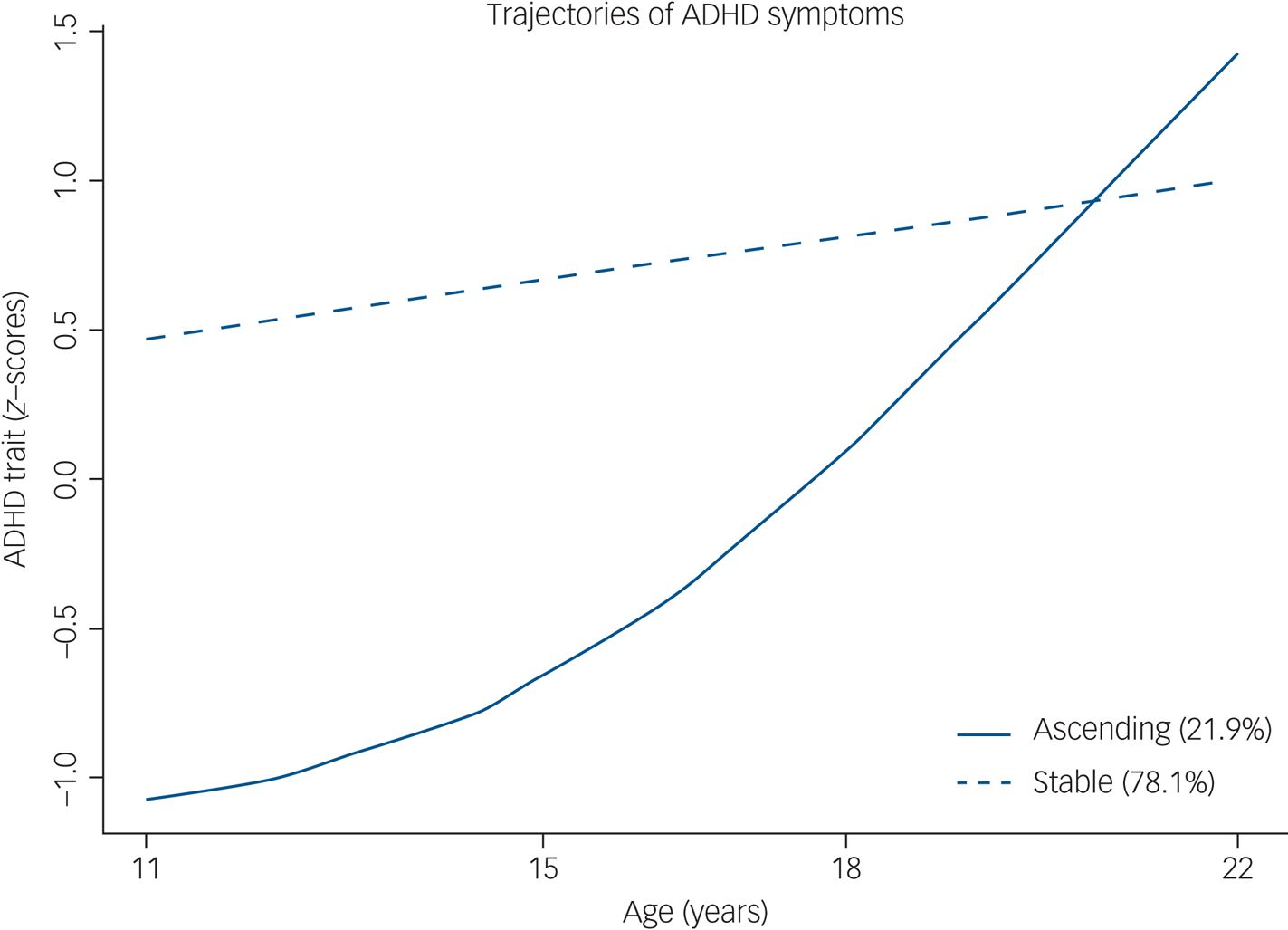
Fig. 3 ADHD trajectories in those with ADHD at 22 years of age (n = 540). ADHD, attention-deficit hyperactivity disorder.
To avoid bias caused by different raters, we performed the same latent trajectory analysis considering only self-reported information (data available for 11, 18 and 22 years of age). The best model fit was the one with three trajectories (see Supplementary material).
Factors associated with persistent or late-onset trajectories in those with ADHD at 22 years of age
The ascending trajectory was associated with female gender (71% of the individuals in this trajectory) (odds ratio, 0.42; P = 0.001) and higher IQ (odds ratio, 1.04; P = 0.004) (Table 1).
Table 1 Characteristics associated with the ascending trajectory (stable trajectory as the reference category)
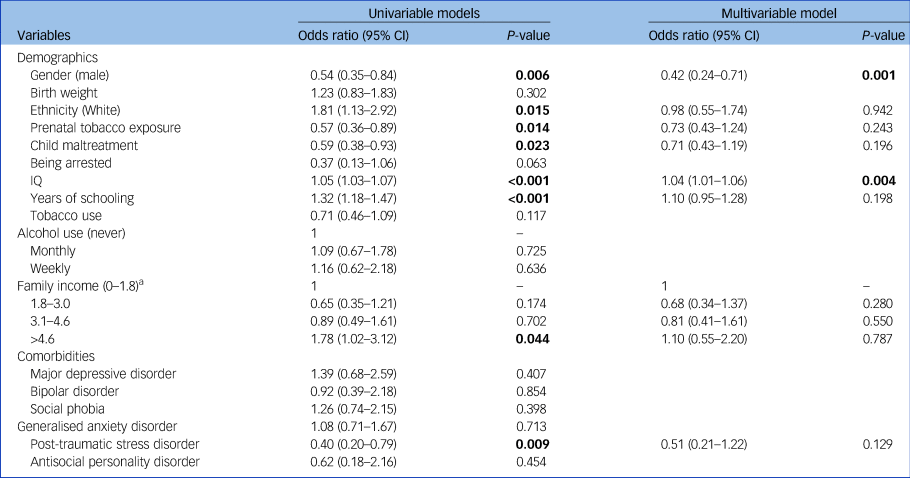
P-value items <0.05 are marked in bold.
a. Family income was measured in units of 'minimum wage' per month (1 minimum wage = USD$200.00).
Discussion
This is the first study of ADHD that used polythetic and latent-trajectory analytic approaches in data from a birth cohort. Our results provided empirical data on the predominant neurodevelopmental nature of ADHD since at least 78% of the individuals diagnosed at 22 years of age had a chronic and persistent trajectory of symptoms. On the other hand, our results also showed the existence of adult ADHD cases with an ascending ADHD trajectory after puberty that was constituted predominantly by women and individuals with higher IQ.
According to the multi-step polythetic approach, most adult ADHD cases (67%) had chronic symptoms since childhood or early adolescence and had a homotypic neurodevelopmental trajectory, as previously reported.Reference Sibley, Rohde, Swanson, Hechtman, Molina and Mitchell15 These results also confirm one of the possible causes of dubious late-onset ADHD related to the presence of subthreshold symptoms instead of a full disorder in childhood. Thus, our results are in line with previous findings showing that subthreshold ADHD symptoms in childhood predict ADHD in late adolescence,Reference Lecendreux, Konofal, Cortese and Faraone8 and that children with subthreshold symptoms are at higher risk for adverse outcomes later in life.Reference Kirova, Kelberman, Storch, DiSalvo, Woodworth and Faraone26 When considering the heterotypic trajectory, in which other psychiatric disorders precede ADHD, 43% of the ADHD cases not classified as homotypic actually had the heterotypic trajectory, which is in accordance with previous findings from a high-risk cohort of children.Reference Manfro, Santoro, Polanczyk, Gadelha, Pan and Bressan13
Also, even excluding individuals with a potentially false-positive, late-onset diagnosis owing to other psychiatric disorders that could better explain ADHD symptoms, around 20% of cases initially classified as late onset could still be considered as ‘true’ late-onset cases. This result is in line with the findings of Sibley et alReference Sibley, Rohde, Swanson, Hechtman, Molina and Mitchell15 regarding ADHD cases with onset in adulthood in the individuals from the MTA study normative control group. In this sense, removing potential non-late-onset cases provided the most conservative estimate of the actual rate of late-onset cases, at 6%. However, this is one of the most disputable exclusion criterion, since high rates of comorbidities are the rule rather than the exception in the ADHD clinic. The distinction between ‘primary’ and ‘secondary’ disorder is a highly questionable and subjective diagnostic procedure, resulting from the lack of biological markers for ADHD.Reference Parnas27 In this regard, a genomic study found that psychiatric disorders share common genetic underpinnings, as is the case of ADHD and major depression.Reference Anttila, Bulik-Sullivan, Finucane, Walters, Bras and Duncan28 Furthermore, when we applied the same rule-out criteria in non-ADHD individuals, only 17% of the population remained, indicating how overexclusive the polythetic method might be.
Through the use of a latent analysis, we observed, for the first time, the existence of an ascending trajectory of symptoms. This trajectory represented the course of almost 22% of adults with ADHD at 22 years of age. Previous population-based studies characterised the ADHD trajectories assessing the whole population, but did not consider ADHD cases separately.Reference Riglin, Collishaw, Thapar, Dalsgaard, Langley and Smith18,Reference Döpfner, Hautmann, Görtz-Dorten, Klasen and Ravens-Sieberer19 In those studies, a three-trajectory solution was usually present, with individuals belonging to high, medium and low symptom trajectories, similar to those obtained in our study when using the entire population. In our analysis, the ascending (late-onset) trajectory was associated with female gender and higher IQ. Thus, the emergence of ADHD symptoms in late adolescence or early adulthood in women could explain the more balanced gender ratio observed in adults in clinical and community studies of the disorder.Reference Kooij, Buitelaar, van den Oord, Furer, Rijnders and Hodiamont3,Reference Vitola, Bau, Salum, Horta, Quevedo and Barros4 In a previous study that assessed the retrospective recall of childhood ADHD symptoms in adulthood, using data from the same Pelotas birth cohort, male gender was associated with the false-negative recall of childhood symptoms,Reference Breda, Rohde, Menezes, Anselmi, Caye and Rovaris29 reinforcing the idea that men with ADHD in adulthood come from a stable trajectory even when they do not recall having ADHD symptoms in childhood. Furthermore, our findings support the hypothesis that women are diagnosed later than men.Reference Young, Adamo, Ásgeirsdóttir, Branney, Beckett and Colley30 Late-onset trajectories obtained from self-reported information were similar to those derived from parent-reports. These findings suggest that undetected ADHD symptoms in women might not be a determinant cause of false late-onset ADHD cases.
The association between late-onset ADHD and higher IQ is in line with previous findings,Reference Agnew-Blais, Polanczyk, Danese, Wertz, Moffitt and Arseneault10,Reference Cooper, Hammerton, Collishaw, Langley, Thapar and Dalsgaard14 supporting the idea that intelligence could ‘mask’ or protract the onset of symptoms or impairment until higher cognitive demands occur in life.Reference Kosaka, Fujioka and Jung31 Our results on the persistent trajectory are also complementary to findings showing that ADHD persistence into adulthood was associated with male gender and low IQ.Reference Agnew-Blais, Polanczyk, Danese, Wertz, Moffitt and Arseneault10,Reference Kessler, Adler, Barkley, Biederman, Conners and Faraone32 Still, in univariate analyses, the persistent trajectory was nominally associated with prenatal tobacco exposure, an environmental factor potentially associated with higher genetic loading for ADHD.Reference Wang, Hu, Chen, Xue and Du33 This set of results supports accuracy of the LCMM analysis to determine trajectories of clinically relevant subpopulations, as such adults with ADHD.
Limitations
The current findings should be interpreted with the acknowledgment of some limitations. Community samples as the Pelotas cohort might not be representative of other populations, and generalisation could represent an important limitation. Furthermore, in the last assessment, the 1993 Pelotas cohort had a retention rate of 73.6%.Reference Amorin23 However, this rate is higher than the ones observed in follow-up studies from low- and middle-income countries, and the final sample in 2015 is representative of the original cohort.Reference Richter, Victora, Hallal, Adair, Bhargava and Fall34 Although more women were retained than men (79.9% v. 72.6%), the absolute gender proportion was fairly balanced (53.2% v. 46.8%) and in line with other population studies.Reference Kooij, Buitelaar, van den Oord, Furer, Rijnders and Hodiamont3,Reference Vitola, Bau, Salum, Horta, Quevedo and Barros4 Furthermore, ADHD assessments at 11 and 15 years of age were performed through a screening questionnaire, and not through complete clinical evaluations. However, this approach seems to be a feasible option in epidemiological studies.Reference Sibley, Swanson, Arnold, Hechtman, Owens and Stehli35 The assessments were also not performed in the same manner in childhood and adulthood, with assessments at 11 and 15 years of age performed with parent-reported SDQ and assessments in adulthood performed with self-rated ASRS. The strategy used to minimise the effect of different raters was to run latent trajectories with available self-report information on ADHD at 11, 18 and 22 years of age. The resulting trajectories were similar to the ones using collateral information at 11 and 15 years of age and self-report information at 18 and 22 years of age. The best latent model of self-reported information comprised three trajectories with one late-onset or ascending pathway observed in 32.6% of those individuals with ADHD at 22 years of age (see Supplementary material). The effect of using different instruments to measure ADHD in childhood (SDQ) and adulthood (ASRS) is minimised with the use of LCMM.Reference Proust-Lima, Philipps and Liquet25 We also compared, through a confusion matrix, the latent trajectory classification with only self-reported information at 11, 18 and 22 years of age to those extracted using both third-party and self-reported information. This analysis identified 66 (56%) individuals from 118 of the original ascending trajectories (Fig. 3) with low levels of ADHD symptoms at 11 years of age in both self- and parent-report assessments, reinforcing the finding that at least 12% of the cases had a later onset of ADHD.
Our results suggest that clinicians might consider a probability of a neurodevelopmental trajectory even when evaluating patients who do not recall childhood symptoms. Still, the fact that women and individuals with higher IQ had a higher probability of late-onset ADHD indicates that more work is needed to disentangle if the absence of detected symptoms in childhood is related to a compensatory mechanism or an actual absence of symptoms. Finally, future studies should focus more on using individual-based and latent approaches when assessing the course of ADHD.
In summary, both polythetic and latent trajectory analyses support the view that the majority of adults with ADHD had a chronic, stable neurodevelopmental trajectory of the disorder. On the other hand, our results also confirm the existence of late-onset ADHD in around a fifth of cases, owing to an age-dependent ascension of symptoms.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjp.2020.200.
Data availability
The questionnaires and interviewer guides are available in electronic formats in Portuguese (available at http://www.epidemio-ufpel.org.br/site/content/coorte_1993/questionarios.php). Further details can be found on the Cohort website (http://www.epidemio-ufpel.org.br/site/content/coorte_1993/index.php).
The data that support the findings of this study are available upon reasonable request. Applications regarding collaborations or to gain access to the 1993 cohort data should be made by contacting the corresponding author or completing the application form for the Pelotas Birth Cohorts (available at http://www.epidemio-ufpel.org.br/site/content/estudos/formularios.php).
Funding
This research was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and scholarships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS-PRONEX), Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), Porto Alegre, Brazil. This article is based on data from the 1993 Pelotas birth cohort study, conducted by the Postgraduate Program in Epidemiology at Universidade Federal de Pelotas with the collaboration of the Brazilian Public Health Association (ABRASCO). The Wellcome Trust supported the 1993 birth cohort study through grant 086974/Z/08/Z from the program entitled Implications of Early Life and Contemporary Exposures on Body Composition, Human Capital, Mental Health, and Precursors of Complex Chronic Diseases in Three Brazilian Cohorts (1982, 1993, and 2004). The European Union, National Support Program for Centers of Excellence and the Brazilian Ministry of Health supported previous phases of the study.
Author contributions
V.B. conceived the study idea. The design of the analyses was determined by V.B. and E.H.G., with inputs from E.S.V., L.A.R., A.C. and D.L.R. Analyses were performed by V.B. and E.S.V., with statistical advice from D.L.R. A.M.B.M. and L.A. were responsible for management of data acquisition. L.A.R. and C.H.D.B. were important critical reviewers for intellectual content. All authors contributed to the interpretation of data and drafting of the manuscript, approved the final version for publication, and agreed to be accountable for all aspects of the work.
Declaration of interest
E.H.G. has served as a speakers’ bureau/advisory board for Shire Pharmaceuticals in the past 3 years. He also received travel awards from Shire and Novartis for taking part in two psychiatric meetings. L.A.R. reported receiving honoraria, serving on the speakers’ bureau/advisory board, and/or acting as a consultant for Eli-Lilly, Janssen-Cilag, Novartis/Sandoz, Medice and Shire in the past 3 years; receiving authorship royalties from Oxford Press and ArtMed; and receiving travel awards from Shire for his participation in the 2017 World Federation of ADHD meetings and from Novartis to take part in the 2018 American Psychiatric Association meeting. The ADHD and juvenile bipolar disorder out-patient programmes chaired by him received unrestricted educational and research support from the following pharmaceutical companies in the past 3 years: Janssen-Cilag, Novartis/Sandoz and Shire. All other authors report no financial relationships with commercial interests.








eLetters
No eLetters have been published for this article.