Dementia is one of the most common and most devastating diseases of late life; approximately 4.6 million new cases of dementia are estimated to occur worldwide every year and the number of people affected is predicted to double every 20 years to 81.1 million by 2040. Reference Ferri, Prince, Brayne, Brodaty, Fratiglioni and Ganguli1 People with mild cognitive impairment are at increased risk of developing dementia, although the conversion rates reported range from 1% to 25% or more per year. Reference Bruscoli and Lovestone2 Prediction of progression to dementia – conversion – is of considerable clinical importance. To date there is no variable other than cognitive impairment itself that unequivocally increases the risk of conversion. Diabetes mellitus is associated with cognitive dysfunction. Type 2 diabetes mellitus has been related to accelerated cognitive decline in elderly people, development of mild cognitive impairment and increased risk of dementia, including both Alzheimer's disease and vascular dementia. Reference Yaffe, Blackwell, Kanaya, Davidowitz, Barrett-Connor and Krueger3–Reference Das, Bose, Biswas, Dutt, Banerjee and Hazra7 With the global number of people with diabetes set to rise over the next few decades, Reference Zimmet, Alberti and Shaw8 the evidence that diabetes is a risk factor for dementia is of considerable public health importance. However, although the association between diabetes mellitus and risk of dementia is robust, it is not yet clear whether diabetes mellitus increases the risk of conversion from mild cognitive impairment to dementia. As early identification strategies are frequently targeted at people with mild impairment this is of clinical relevance – faced with a patient with mild cognitive impairment, the clinician needs to be able to judge the risk of conversion to dementia relative to the chances of the impairment remaining mild and causing no functional difficulty. We aimed to determine the association between diabetes mellitus and conversion to dementia in individuals with mild cognitive impairment over a follow-up period of 4 years in a prospective community-based study.
Method
White European men and women aged 65 years or above with no psychiatric or neurological disorder were recruited as part of a longitudinal cohort study. As previously described, Reference Ellul, Archer, Foy, Poppe, Boothby and Nicholas9,Reference Hye, Lynham, Thambisetty, Causevic, Campbell and Byers10 study participants with no dementia were recruited through local primary care practices in south London. Potential candidates identified from the general practice registers were contacted by post and invited to participate. Those who responded positively were assessed either in their own home or at the practice surgery. Following baseline assessment, participants were assessed annually from 2001 through 2007.
Diagnosis of cognitive impairment
Cognitive assessments at baseline were conducted with the Mini-Mental State Examination (MMSE) and the neuropsychological battery from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Reference Folstein, Folstein and McHugh11,Reference Morris, Mohs, Rogers, Fillenbaum and Heyman12 The National Adult Reading Test (NART) was performed to rate premorbid IQ. Reference Nelson13 Participants were considered to have mild cognitive impairment if they satisfied Petersen's original classification: Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen14
-
(a) memory complaint, preferably corroborated by an informant;
-
(b) objective memory impairment;
-
(c) normal general cognitive function;
-
(d) intact activities of daily living;
-
(e) no diagnosis of dementia.
A diagnosis of mild cognitive impairment was made if the MMSE score was 25–27 and the participant's scores were 1.5 standard deviations below the published norms for any of the CERAD domains (relative to age, education and gender). Reference Lopez, Jagust, DeKosky, Becker, Fitzpatrick and Dulberg15
Diabetes mellitus
Participants (and informants) were asked whether the participant had ever been diagnosed by a physician as having diabetes mellitus. They were also asked about all medications used on a daily basis. Antidiabetic medication use was validated by reviewing the general practitioners' prescription slips and medication boxes. Participants were classified as having diabetes mellitus if there was a report of physician diagnosis of the disorder with evidence of use of oral antidiabetic medications or insulin and information from the general practitioner. This was determined at baseline and every follow-up assessment.
Other confounding factors
History of hypertension, stroke, transient ischaemic attack (TIA) and coronary heart disease (myocardial infarction, angina and coronary artery bypass grafting) was ascertained at each assessment point. The medical history and medication use were further validated by reviewing the general practitioners' prescription slips and medication boxes. These too were determined at baseline and every follow-up assessment.
History of smoking (current and lifetime) and heavy alcohol drinking any time during lifetime was obtained from self-report. A shorter version of the Geriatric Depression Scale (GDS) was used for evaluating depressed mood, Reference Yesavage, Brink, Rose, Lum, Huang and Adey17 with a score above 5 being a potential indicator of current depression.
Venous blood was obtained for DNA extraction and genotyping for the apolipoprotein E (APOE) alleles using standard methods. Reference Wenham, Price and Blandell16 The APOE haplotype was determined using two allelic discrimination assays (rs7412 and rs429358) based on fluorogenic 5' nuclease activity, the Taq polymerase single nucleotide polymorphism genotyping assay (TaqMan, Applied Biosystems Inc., www.appliedbiosystems.com).
Diagnosis of dementia
At follow-up a modified version of the Telephone Interview for Cognitive Status (TICS–M) Reference De Jager, Budge and Clarke18 and the GDS were administered over the telephone. If the TICS–M score was 22 or less, a full face-to-face assessment including evaluation of dementia was conducted. The assessment process has been described and fully referenced elsewhere, Reference Foy, Nicholas, Hollingworth, Boothby, Williams and Brown19 but in brief, participants and informants were interviewed using a structured interview including the Cambridge Examination for Mental Disorders of Older People (CAMDEX), Reference Roth, Tym, Mountjoy, Huppert, Hendrie, Verma and Goddard20 the MMSE for cognition, assessment of function using the Blessed Dementia Scale and the Bristol Activities of Daily Living Scale, Reference Blessed, Tomlinson and Roth21,Reference Bucks, Ashworth, Wilcock and Siegfried22 the Neuropsychiatry Inventory for non-cognitive symptoms, Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein23 the Cornell Scale for Depression in Dementia, Reference Alexopoulos, Abrams, Young and Shamoian24 and the Webster Disability Scale for motor symptoms. Reference Webster25 Dementia syndromes were diagnosed using a validated algorithm and this assessment process has previously been shown to be highly accurate with respect to post-mortem diagnosis. Reference Foy, Nicholas, Hollingworth, Boothby, Williams and Brown19
All participants gave informed consent. The study was approved by the joint Institute of Psychiatry and South London & Maudsley National Health Service (NHS) Trust ethical committee.
Statistical analysis
Data were recorded and analysed with SPSS version 15.0 for Windows. The incidence rates of dementia per person-year were calculated as the number of new cases divided by the number of person-years at risk. Association of patient characteristics at baseline and conversion to dementia was tested using the chi-squared test and Student's t-test. The longitudinal data were analysed using Cox proportional hazard models both unadjusted and with additional adjustment for survival status. The backward stepwise method was used, which includes all the independent variables in a baseline regression model and successively removes the variables that contribute least to the model. In this way, a model is identified that provides the ‘best fit’ in terms of finding variables that are significant in predicting the dependent variable.
Results
Among the individuals identified from primary care practices who agreed to participate, 165 were considered to have possible mild cognitive impairment, of whom 103 fulfilled Peterson's criteria. The mean age of this cohort (n=103) at baseline was 79.4 years (s.d. = 6.3) and 66 (64%) were women. They had 10.6 years of education (s.d. = 2.6) with a mean baseline MMSE score of 26.3 (s.d. = 1.1). Of the 95 participants who had APOE genotyping, 30 (32%) had at least one APOE4 allele. Thirty-one (29%) of the 92 participants assessed for mood with the GDS were positive for current depression.
The cohort was followed from 2001 through 2007. During this period 17 of the 103 participants (16%) died, 6 (6%) were lost to follow up, 3 (3%) were not approached as they had moved too great a distance from the study site and 16 (16%) refused to continue participating in the study. In total, 61 participants were successfully followed for 4 years (59%). They were comparable in sociodemographic profile, risk factors and clinical parameters to those who did not complete follow up, except for being younger (mean 2 years). Sixteen (16%) participants had a diagnosis of diabetes, with a mean duration of 9.3 years (s.d. = 5.4). Among the 61 participants who successfully completed the follow-up for 4 years there were 19 (31%) incident cases of dementia, 2 (3%) participants improved in their cognition, reverting to normal cognitive levels, and 40 (59%) remained stable. The mean annual conversion rate to dementia was 8%. The dementia diagnoses were predominantly Alzheimer's disease (84%; probable, n = 11, possible, n =5); mixed Alzheimer's disease and vascular dementia (5%; n =1); vascular dementia (5%; n = 1); and dementia of Lewy body type (5%; n =1).
The baseline characteristics of participants with and without diabetes mellitus are compared in Table 1. Compared with participants without diabetes, those with the disorder had a greater prevalence of current depression, a history of heavy drinking, and increased rates of stroke and/or TIA and coronary heart disease. Table 2 shows the baseline characteristics of the participants who progressed to develop dementia compared with those who did not. Those who converted to dementia were older, had a greater prevalence of diabetes, a longer duration of diabetes and a higher prevalence of stroke and/or TIA. Among those who converted to dementia, 7 participants had diabetes, of whom 6 developed Alzheimer's disease.
Table 1 Comparison of sociodemographic variables, cognitive parameters and medical history of participants with mild cognitive impairment with or without diabetes
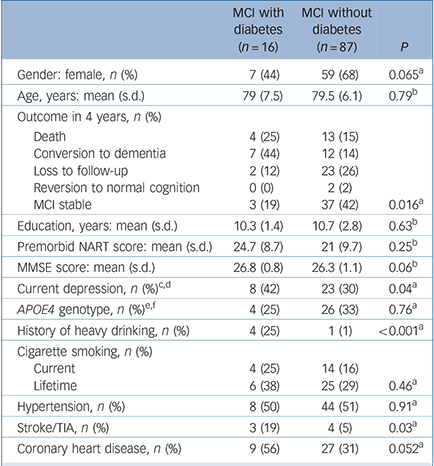
| MCI with diabetes (n = 16) | MCI without diabetes (n = 87) | P | |
|---|---|---|---|
| Gender: female, n (%) | 7 (44) | 59 (68) | 0.065a |
| Age, years: mean (s.d.) | 79 (7.5) | 79.5 (6.1) | 0.79b |
| Outcome in 4 years, n (%) | |||
| Death | 4 (25) | 13 (15) | |
| Conversion to dementia | 7 (44) | 12 (14) | |
| Loss to follow-up | 2 (12) | 23 (26) | |
| Reversion to normal cognition | 0 (0) | 2 (2) | |
| MCI stable | 3 (19) | 37 (42) | 0.016a |
| Education, years: mean (s.d.) | 10.3 (1.4) | 10.7 (2.8) | 0.63b |
| Premorbid NART score: mean (s.d.) | 24.7 (8.7) | 21 (9.7) | 0.25b |
| MMSE score: mean (s.d.) | 26.8 (0.8) | 26.3 (1.1) | 0.06b |
| Current depression, n (%)c,d | 8 (42) | 23 (30) | 0.04a |
| APOE4 genotype, n (%)e,f | 4 (25) | 26 (33) | 0.76a |
| History of heavy drinking, n (%) | 4 (25) | 1 (1) | <0.001a |
| Cigarette smoking, n (%) | |||
| Current | 4 (25) | 14 (16) | |
| Lifetime | 6 (38) | 25 (29) | 0.46a |
| Hypertension, n (%) | 8 (50) | 44 (51) | 0.91a |
| Stroke/TIA, n (%) | 3 (19) | 4 (5) | 0.03a |
| Coronary heart disease, n (%) | 9 (56) | 27 (31) | 0.052a |
Table 2 Comparison of sociodemographic variables, cognitive parameters and medical history of participants with mild cognitive impairment categorised by conversion to dementia
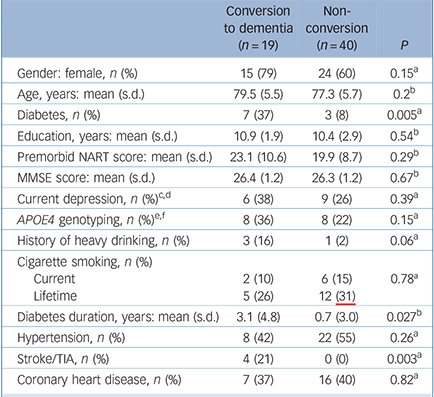
| Conversion to dementia (n = 19) | Non-conversion (n = 40) | P | |
|---|---|---|---|
| Gender: female, n (%) | 15 (79) | 24 (60) | 0.15a |
| Age, years: mean (s.d.) | 79.5 (5.5) | 77.3 (5.7) | 0.2b |
| Diabetes, n (%) | 7 (37) | 3 (8) | 0.005a |
| Education, years: mean (s.d.) | 10.9 (1.9) | 10.4 (2.9) | 0.54b |
| Premorbid NART score: mean (s.d.) | 23.1 (10.6) | 19.9 (8.7) | 0.29b |
| MMSE score: mean (s.d.) | 26.4 (1.2) | 26.3 (1.2) | 0.67b |
| Current depression, n (%)c,d | 6 (38) | 9 (26) | 0.39a |
| APOE4 genotyping, n (%)e,f | 8 (36) | 8 (22) | 0.15a |
| History of heavy drinking, n (%) | 3 (16) | 1 (2) | 0.06a |
| Cigarette smoking, n (%) | |||
| Current | 2 (10) | 6 (15) | 0.78a |
| Lifetime | 5 (26) | 12 (31) | |
| Diabetes duration, years: mean (s.d.) | 3.1 (4.8) | 0.7 (3.0) | 0.027b |
| Hypertension, n (%) | 8 (42) | 22 (55) | 0.26a |
| Stroke/TIA, n (%) | 4 (21) | 0 (0) | 0.003a |
| Coronary heart disease, n (%) | 7 (37) | 16 (40) | 0.82a |
All the variables were further analysed using Cox proportional regression models. Both unadjusted and adjusted models (Table 3) identified diabetes as the only significant predictor. The backward stepwise method showed presence of diabetes as a significant predictor for conversion to dementia, with a hazard ratio of 2.9 (95% CI 1.1–7.3).
Table 3 Hazard ratios for unadjusted and adjusted analyses

| Unadjusteda | Model 1b | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Diabetes | 2.9 (1.1-7.3) | 0.03 | 2.9 (1.1-7.3) | 0.03 |
| Age | 1.1 (0.9-1.1) | 0.23 | 1.0 (0.9-1.1) | 0.43 |
| Gender | 1.9 (0.6-5.8) | 0.25 | 2.3 (0.8-7.2) | 0.14 |
| Education | 1.0 (0.9-1.2) | 0.61 | NA | |
| Premorbid NART score | 1.0 (0.9-1.1) | 0.4 | NA | |
| Coronary heart disease | 1.1 (0.4-2.8) | 0.85 | NA | |
| Stroke/TIA | 3.7 (1.2-11.1) | 0.02 | 2.8 (0.9-9.3) | 0.09 |
| Drinking alcohol | 2.6 (0.7-8.8) | 0.1 | NA | |
| Smoking statusc | 0.8 (0.17-4.3) | 0.8 | NA | |
| APOE4 d | 0.6 (0.14-3.4) | 0.7 | NA | |
| MMSE score | 1.1 (0.7-1.6) | 0.72 | NA | |
| Duration of diabetes | 1.1 (1.0-1.2) | 0.08 | 0.9 (0.7-1.1) | 0.29 |
| Depression | 1.4 (0.5-3.9) | 0.5 | NA | |
Seven participants showed deterioration in cognition during follow-up on TICS–M and MMSE, but did not wish to have a complete assessment towards a formal dementia diagnosis. Although these individuals were likely to be converters they were excluded from the analysis. Including them in the analysis gave a group size of n = 26 for participants showing deterioration, and Cox regression analysis models demonstrated that diabetes continued to be the only variable associated with increased risk of progression to dementia (hazard ratio 2.5, 95% CI 1.1–5.7, P = 0.03). The two participants who reverted to normal cognition did not have diabetes, were 82.5 (s.d. = 3.6) years old, had a mean MMSE score of 26.5 (s.d. = 0.7) and had 12.5 (s.d. = 4.9) years of education. They continued to have normal cognition during the remaining period of the study.
Discussion
This prospective longitudinal study shows that diabetes is associated with a substantially increased risk of conversion of mild cognitive impairment to dementia. Our finding supports the hypothesis that diabetes is linked to cognitive dysfunction.
Incidence of dementia
The rate of progression to dementia in our study (8%) was slightly lower than but broadly consistent with previous research. Reference Bruscoli and Lovestone2,Reference Fischer, Jungwirth, Zehetmayer, Weissgram, Hoenigschnabl and Gelpi26,Reference Yaffe, Petersen, Lindquist, Kramer and Miller27 The most common diagnosis post-conversion was Alzheimer's disease (n = 16, 84%) and one person had a diagnosis of mixed Alzheimer's disease with vascular dementia. As in other studies, the conversion rates showed a high probability of receiving a diagnosis of Alzheimer's disease. Reference Fischer, Jungwirth, Zehetmayer, Weissgram, Hoenigschnabl and Gelpi26,Reference Yaffe, Petersen, Lindquist, Kramer and Miller27
Diabetes, other confounding factors and risk of conversion
Previous longitudinal studies of the risk of dementia in elderly people with diabetes have shown a consistent pattern with a hazard ratio of 1.5, with an overall increase in incidence of 50–100% relative to people without diabetes. Reference Biessels, Staekenborg, Brunner, Brayne and Scheltens5 Diabetes was associated with a higher risk of incident mild cognitive impairment in a population with a high prevalence of this disorder (HR = 1.4, 95% CI 1.1–1.8). Reference Luchsinger, Reitz, Patel, Tang, Manly and Mayeux4 Both cognitive impairment and diabetes may be important independent risk factors for dementia, which, when combined, confer even greater risk, as reflected in the almost 3 times greater potential hazard of developing any type of dementia (HR = 2.9, 95% CI 1.1–7.3) in our study. The risk remained consistent even after adjustment for gender, age, vascular risk factors such as stroke and/or TIA and coronary heart disease, and diabetes-specific features such as diabetes duration, which are important determinants for increased risk of dementia in people with diabetes. Reference Das, Bose, Biswas, Dutt, Banerjee and Hazra7
The prevalence of diabetes in our sample was consistent with the reports for a White population aged around 70 years, i.e. 10–20%. Reference Biessels, Deary and Ryan6 Longer duration of diabetes has been associated with an increased risk of developing mild cognitive impairment. Reference Roberts, Geda, Knopman, Christianson, Pankratz and Boeve28 The duration of diabetes was longer in members of our cohort who progressed to dementia, although this did not show an association when adjusted for other variables (see Table 3). A possible explanation could be the small number of participants. In our study, individuals with diabetes had greater prevalence of cardiovascular and cerebrovascular disease, i.e. stroke, TIA, angina, myocardial infarction and coronary artery bypass grafting. This is consistent with reports that people with diabetes have increased vascular risk factors, and it has been suggested that it is this that increases the risk of dementia. Reference Fillit, Nash, Rundek and Zuckerman29 However, although stroke or TIA was associated with increased risk of progression to dementia, this association did not remain when adjusted for presence and duration of diabetes, age and gender (see Table 3).
Apolipoprotein E genotype did not have an effect on the risk of conversion to dementia or Alzheimer's disease from cognitive impairment in our study. This was in accordance with a recent study which reported that carrying at least one APOE4 allele was a risk factor for the development of mild cognitive impairment, but that once impairment was established the gene had no effect on the risk of progressing to Alzheimer's disease. Reference Barabash, Marcos, Ancín, Vázquez-Alvarez, de Ugarte and Gil30
Limitations
The main limitation of this study was that we did not have measures of glycaemia to ascertain undiagnosed diabetes or to determine the degree of diabetic control. This almost certainly leads to an underestimate of the prevalence of diabetes. However, any such underestimate is unlikely to alter the conclusions substantially because of the longitudinal study design. If anything, an underestimate of underlying diabetes might act to reduce the size of the effect on conversion. Although our sample number was modest, recruitment from primary practices rather than memory clinics increases the representativeness of the study population. Our finding of a relatively low rate of conversion from mild cognitive impairment reflects this recruitment strategy and is in line with other studies with sample populations that approximate more closely to the general population than those drawn from secondary care specialist clinics. Reference Bruscoli and Lovestone2 Our cohort consisted of White European participants, and it would be interesting to repeat this study in different populations, as the prevalence of diabetes is higher in some ethnic groups. Reference Luchsinger, Reitz, Patel, Tang, Manly and Mayeux4
Potential mechanisms and implications
Diabetes might accelerate cognitive decline and conversion to dementia by a number of potential mechanisms. These include insulin resistance syndrome, disturbances in insulin homeostasis in the brain, hyperinsulinaemia, interplay with the insulin degrading enzyme involved in both insulin and amyloid proteolysis or effects of insulin signalling on tau metabolism and generation of advanced products of glycosylation. Reference Zimmet, Alberti and Shaw8 Whatever the mechanism, with an expected increase in prevalence of diabetes in people of all ages including older adults, Reference Zimmet, Alberti and Shaw8 the risk of developing dementia may increase. Identification of those at particular risk of progression might help to target early treatment – both pharmacological and social.
There are few data to guide evidence-based decision-making for patients with diabetes and cognitive impairment. Our study demonstrates that individuals with mild cognitive impairment and diabetes are at increased risk of developing dementia. This suggests the need for studies of improved diabetes control and related approaches as possible strategies for early intervention.
Funding
The study was funded by the Alzheimer's Research Trust and the UK Medical Research Council, and through the National Institute for Health Research Specialist Biomedical Research Centre for Mental Health at the South London & Maudsley National Health Service Foundation Trust.
Acknowledgements
We particularly thank Lee Wilding, research administrator, Section of Old Age Psychiatry, Institute of Psychiatry, London, for carrying out TICS–M telephone screening interviews for the majority of participants in the community.






eLetters
No eLetters have been published for this article.