ARHI due to sensorineural hearing loss is a common public health problem resulting in communication deficits and poor quality of life in the elderly (Dalton et al., Reference Dalton, Cruickshanks, Klein, Klein, Wiley and Nondahl2003). SIN perception, described as poor speech recognition in a noisy environment, is a common complaint in ARHI, particularly hearing loss in the higher frequencies. SIN perception accounts for the clarity component of hearing ability rather than just hearing sensitivity. Older individuals often have difficulty grouping and segregating a mixture of sounds in the environment (e.g., group of people speaking, music playing) into mental representations known as auditory streams; this process is known as auditory analysis and is a component of normal hearing ability (Bregman, Reference Bregman1990). ARHI is considered to be a common complex trait, in which genetic and environmental factors are likely to play a role (Karlsson et al., Reference Karlsson, Harris and Svartengren1997; Viljanen et al., Reference Viljanen, Era, Kaprio, Pyykko, Koskenvuo and Rantanen2007; Wolber et al., Reference Wolber, Steves, Spector and Williams2012). To date, studies on the environmental influences on hearing loss have either been of small sample size (Gates et al., Reference Gates, Schmid, Kujawa, Nam and D’Agostino2000; Kaksonen et al., Reference Kaksonen, Pyykko, Kere, Starck and Toppila2000; Lee et al., Reference Lee, Matthews, Mills, Dubno and Adkins1998) or have been restricted to predominantly male samples (Brant et al., Reference Brant, Gordon-Salant, Pearson, Klein, Morrell, Metter and Fozard1996; Gates et al., Reference Gates, Schmid, Kujawa, Nam and D’Agostino2000; Karlsson et al., Reference Karlsson, Harris and Svartengren1997). Moreover, most studies have used PTA as a measure for the hearing outcome. While PTA is the gold standard measure of hearing, and has been used successfully to identify genetic (Wolber et al., Reference Wolber, Girotto, Buniello, Vuckovic, Pirastu, Lorente-Cánovas and Williams2014) and epigenetic associations in ARHI (Wolber et al., Reference Wolber, Steves, Tsai, Deloukas, Spector, Bell and Williams2014), its capacity to measure everyday hearing disability is limited (Demeester et al., Reference Demeester, Topsakal, Hendrickx, Fransen, van Laer, Van Camp and van Wieringen2012); in particular, the ability to comprehend speech in a noisy environment (Dubno et al., Reference Dubno, Lee, Matthews and Mills1997; Vermiglio et al., Reference Vermiglio, Soli, Freed and Fisher2012). The SIN phenotype, therefore, has advantages in reproducing a better environment of greatest disability and may capture other subtraits that influence hearing. It is important to define precisely the role of environmental risk factors in ARHI because they may be amenable to modification, and public health strategies could be put in place to modify the impact of this disability in the aging population of the future.
We have investigated the influence of genetic and environmental factors on SIN in a predominantly female population sample of adult twin volunteers (TwinsUK). A heritability study was conducted, based on the classical twin model, to determine the role of genetic and environmental factors on SIN, using the web-based SIN hearing test (Action on Hearing Loss, 2006). Along with determining a formal heritability estimate of SIN, we also conducted a cross-sectional study to determine the influence of dietary factors, alcohol intake, and smoking on the SIN hearing ability.
Methods
Data Collection
Ethical approval for this study was obtained from St Thomas’ Hospital Research Ethics committee and consent was obtained from all participants. The subjects completed the web-based SIN hearing test either at home between March and September 2011 or at a Twin Research Department visit in June 2013, with a number of participants being assessed in both settings. The SIN test was first developed as a screening method in the Netherlands (Smits et al., Reference Smits, Kapteyn and Houtgast2004). We and others (Bosman & Smoorenburg, Reference Bosman and Smoorenburg1995; Smits et al., Reference Smits, Kapteyn and Houtgast2004; Smoorenburg, Reference Smoorenburg1992) have validated SIN against PTA, the gold standard measure of hearing loss, and shown them to be correlated between 0.62 and 0.8, with high sensitivity (89%) and specificity (80%) for hearing loss (Wolber et al., Reference Wolber, Steves, Spector and Williams2012). At home, subjects used their own PC to obtain online access to the web-based SIN test (Action on Hearing Loss, 2006) and performed the test in a quiet environment, without the use of headphones, with any hearing aids removed. At the visit, six computer stations with attached headphones were set up to reduce the influence of background noise. During the SIN test, digit combinations in the form of triplets (e.g., four–six–three) are presented to the subject at a constant sound intensity against a variable background noise (a hissing sound like analogue radio interference). Initially, subjects were asked to set the sound intensity at a comfortable level where the triplets could be identified correctly. Upon commencement of the test, noise intensity was increased or decreased by 2 decibels (dB) depending on whether the triplet input by the subject was correct or incorrect, respectively. The subjects’ SIN perception was generated by creating a ratio (known as the SIN ratio) of the speech sound intensity over white noise sound intensity. The SIN ratio ranges between +8dB and -16dB, where a positive value indicates that speech could only be heard at a low background noise and a negative value that indicates good speech recognition even at high masking noise. To enable simple interpretation, the SIN ratios were converted to test scores ranging from 0 to 11, with a low score indicating hearing impairment and high score indicating good speech recognition in a noisy environment. Hearing ability was categorized as good (score ≥8.5 or SIN ratio ≤-9), moderate (score <8.5 and ≥7.5 or SIN ratio >-9 and ≤-7) or low (score <7.5 or SIN ratio >-7) (Lutman et al., Reference Lutman, Hall and Athalye2006). Where volunteers had performed the test twice, the better score was used. The test uses randomized triplets, so there is no significant learning effect (Smits et al., Reference Smits, Kapteyn and Houtgast2004).
Environmental Exposures
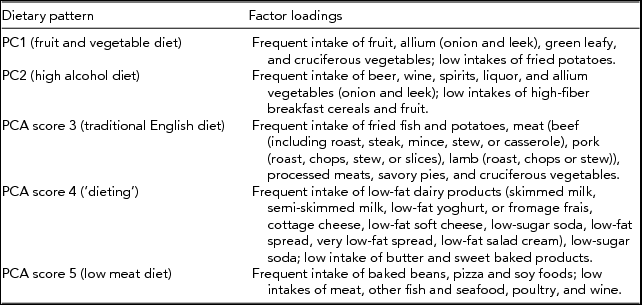
Participants completed questionnaires to capture demographic characteristics and details of previous exposure to loud noise. Data on dietary exposure had been obtained from FFQs. The validated FFQ had been completed and a clinical assessment was conducted at St Thomas’ Hospital. Dietary patterns identified using FFQs show stability and reproducibility over extended periods of time proving to be appropriate for cross-sectional analyses (Newby et al., Reference Newby, Weismayer, Akesson, Tucker and Wolk2006). The FFQ followed the format used in the EPIC study (Bingham et al., Reference Bingham, Welch, McTaggart, Mulligan, Runswick, Luben and Day2001) and has been validated against serum measures of nutrients (Bingham et al., Reference Bingham, Gill, Welch, Cassidy, Runswick, Oakes and Day1997). The FFQ covered 131 food items, which were combined into 54 food groups. Because of the correlated nature of dietary intake, our analysis focused on patterns of dietary intake that had been established previously through a principal components analysis (PCA) approach that distinguished five dietary patterns figuratively labeled by the dominating food type as: fruit and vegetable diet (PC1), alcohol intake (PC2), traditional English diet (PC3), ‘dieting’ (PC4), and low meat diet (PC5). Factor loadings of these five dietary patterns are shown in the appendix. Total cholesterol and HDL cholesterol levels were measured on venous blood collected at a clinical visit between 1992 and 2007; from this, cholesterol ratio was computed by dividing the total cholesterol levels by HDL cholesterol levels. Cholesterol ratio has been shown to measure the risk of cardiovascular disease in an individual with values above ratio of 5 indicating a high risk of acquiring cardiovascular disease (Arsenault et al., Reference Arsenault, Rana, Stroes, Despres, Shah, Kastelein and Khaw2009). Data on lifetime smoking exposure has been collected regularly in TwinsUK since 1993 on a self-report basis. Participants were classified as non-smokers, ex-smokers, or current smokers according to all available information. Levels of cotinine, a metabolite of nicotine, detectable in serum samples had been assessed as part of a large metabolomics screening panel as reported previously (Suhre et al., Reference Suhre, Shin, Petersen, Mohney, Meredith, Wagele and Gieger2011). This metabolite serves as a biomarker for exposure to tobacco smoke (both active and passive smoking) over several days (up to 1 week) with high sensitivity (96–97%) and specificity (99–100%; Jarvis et al., Reference Jarvis, Tunstallpedoe, Feyerabend, Vesey and Saloojee1987; Pojer et al., Reference Pojer, Whitfield, Poulos, Eckhard, Richmond and Hensley1984). It was available on a subset of twins having hearing data.
Statistical Analyses
Statistical analyses were conducted in PASW Statistics 18 (IBM Corporation, Armonk New York) and STATA version 13 (Stata-Corp LP, College Station, Texas).
To determine the test–retest reliability of the SIN test, the scores of individuals who had performed the test in both settings were compared using Bland–Altman comparison (Bland & Altman, Reference Bland and Altman1986) and Pitman test of difference in variance (Pitman, Reference Pitman1938).
Genetic Factors
SIN ratios used for this analysis were transformed to normality. Heritability studies aim to determine the proportion of phenotypic variance caused by genetic and environmental factors. Under the classical twin model, MZ twins are assumed to share all (100%) of their additive genetic variation (A), while DZ twin siblings share on average half (50%) of their segregated alleles. In addition, both MZ and DZ twin siblings fully share their common environment (C), including their time in uterus and family environment. It is further assumed that twin siblings are exposed to unique environmental factors (E) (Rijsdijk & Sham, Reference Rijsdijk and Sham2002).
The analytic approach applied maximum likelihood-based structural equation models to the phenotypic variance and covariance observed in the twin pairs using Mx software (Neale et al., Reference Neale, Boker, Xie and Maes2006). The full model, taking into account all three latent factors (ACE), was fitted first, using the observed phenotypic variance and covariances in SIN and age-adjusted SIN residuals. Three nested models were compared to the full ACE model, taking into account different causal factors: AE (additive genetics and unshared environmental factors), CE (shared and unshared environmental factors), and E (unshared environmental factors). The fit of submodels was compared to full ACE model using a likelihood ratio test. Akaike's information criterion (AIC; Akaike, Reference Akaike1974) was used to discriminate between non-nested models. Heritability estimates (with measurement errors) from the most suitable model were reported for SIN and age-adjusted SIN residuals for the complete study sample and a female-only subsample. Unpaired twins were included in the structural equation modeling to reflect the population variance in SIN ratio.
Environmental Factors
Online hearing test score values were converted to SIN ratio and transformed to achieve a normal distribution. Transformed SIN ratio, dietary variables, HDL levels, and cholesterol ratio levels were then standardized. Multivariable regression analyses were used to examine the influence of risk factors on standardized SIN perception. The association between SIN and variables of interest were examined initially in a regression model adjusted for age, age2, and sex (model 1) and in a second model (model 2) that included all environmental factors (dietary patterns and smoking) as well as age, age2, and sex. In both models, twin relatedness was taken into account using variance correction.
Results
Hearing data were available from 2,076 individuals, all of Northern European descent, of which 1,823 (87.8%) were female and 253 (12.2%) were male. The participants’ ages ranged from 18 to 87 years with a mean age of 54.4 (SD ± 13.4) years. There was no significant difference in age between the sexes (t = -1.29, p = .20). However, significant difference in hearing ability between the sexes was observed (t = -2.20, p = .028), with women demonstrating better SIN perception (mean SIN = -10.6, 95%CI = -10.3 to -10.1) than men (mean SIN = -9.81, 95% CI = -10.1 to -9.50). Overall, the prevalence of subjects with moderate loss in SIN perception was 10% and severe loss in SIN perception was 6.8%. The sample distribution and assigned hearing status are shown in Figure 1.

FIGURE 1 The distribution of hearing determined by SIN perception in TwinsUK.
The test–retest reliability analysis for the 57 individuals having repeated measurements in different settings showed a reasonable intraclass correlation (r 2 = 0.7,231), and the scores laid between the two limits of agreement on the Bland–Altman comparison (data not shown; mean score difference between visit test and home test = 0.229 + 1.96 SD). The Pitman test of difference in variance showed no difference between the two pairs of samples (p = 0.34), so SIN ratio scores obtained at the hospital visit were included in further analysis, and where an individual was measured twice, the better score was used.
Heritability estimates were obtained for the whole sample (n = 2,076) and a female-only (n = 1,823) subsample (Table 1) adjusted and unadjusted for age. Twin correlation (non-adjusted and age-adjusted) is shown in Table 2. For both the mixed gender and female-only sample, the AE model (taking into account additive genetic and unique environmental causal factors) provided the most suitable model fit for the observed variance in SIN residuals (Table 3). For the SIN test, additive genetic factors accounted for 40% of the variance and unshared environmental exposure accounted for 60% of the variance in SIN perception. When adjusted for age, the estimates obtained show that variance in SIN residuals is influenced primarily by environmental exposure not shared within twin siblings (E = 75%), while additive genetic factors account for only 25% of SIN variance (Table 3). Heritability estimates showed minimal differences between the mixed gender and female-only sample, demonstrating no evidence of gender specific differences in genetic influence. The high proportion of phenotypic variance in SIN perception explained by environmental exposure led us to examine putative environmental risk factors.
TABLE 1 Demographic and Phenotypic Summary of TwinsUK Sample Used in the Heritability Study
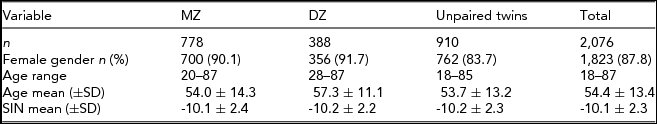
Summary of the demographic and phenotypic measures of all subjects used in the heritability study. The sample was divided into three subsamples by zygosity of the participants (MZ = monozygotic twins, DZ = dizygotic twins and unpaired twins). Each subsample was characterized by the number of individuals (n), female gender, age range, and mean age (±SD) at speech-in-noise test. Furthermore, transformed speech-in-noise (SIN) ratios are summarized as mean ± SD from the mean.
TABLE 2 Non-Adjusted and Age-Adjusted Twin Correlations
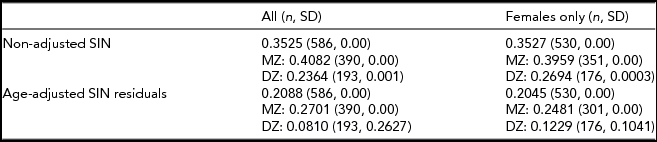
Non-adjusted and age-adjusted monozygotic (MZ) and dizygotic (DZ) twin correlation estimates. n = sample size; SD = standard deviation.
TABLE 3 SIN Heritability Estimates Based on the Classical Twin Model
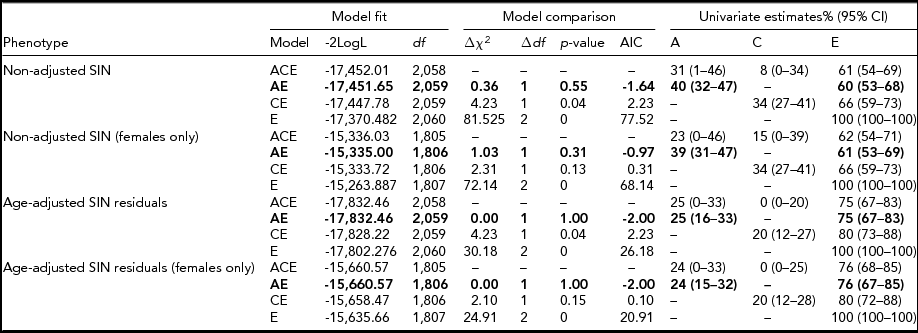
Model fit was based on maximum likelihood estimation. The full ACE model was compared to reduced nested models (AE, CE, and E). Model comparison was established in a likelihood ratio test, with the significance of this test (p value) being based on a chi-square statistic (∆χ2) with 1 or 2 degrees of freedom (∆df) and the Akaike's information criterion (AIC). For each phenotype the ACE model fit and nested models with a better model fit (highlighted in bold) are shown. Variances explained by the specific causal factors (A = additive genetics, C = shared environment, and E = unshared environment) are given with 95% confidence intervals (95% CI) for each model.
The environmental factors assessed have been summarized in Table 4. The risk factors for SIN perception impairment were considered individually in univariate regression (model 1) and together in multiple regression (model 2), as shown in Table 5. For model 2, participants (n = 940) having complete data on hearing, diet, smoking, and alcohol intake were included. Age was a highly significant predictor of hearing ability and accounted for 20.7% of the variance in SIN (β = -6.08 × 10−04, 95% CI = -7.86 × 10−04 to -4.30 × 10−04, p = 3.2 × 10−11). Examination of dietary factors revealed that PC3 (traditional English diet) showed a weak negative association with SIN hearing ability in model 1 (β = -0.0615, 95% CI = -0.125 to 1.58 × 10−03, p = .056). Since the dietary patterns (and cholesterol) were standardized, one standard deviation increase in PC3 predicts a 0.0615 standard deviation decrease in SIN value. However, when adjustment for smoking and the other four dietary factors was made in model 2, PC3 was significantly associated with loss in SIN perception (β = -0.0677, 95% CI = -0.131 to -0.00397, p = .037). As PC3 describes a diet rich in fried food, we examined cholesterol levels. We found that while cholesterol ratio (the ratio of serum total cholesterol and serum HDL cholesterol) was not significantly associated with hearing ability (n = 1,658, β = -0.0201, p = .40), HDL cholesterol level showed a strong positive association with SIN hearing ability (n = 1,659, β = 0.0703, 95% CI = 0.0231 to 0.117, p = .004), suggestive of a protective effect.
TABLE 4 Demographic and Phenotypic Summary Statistics of TwinsUK Sample
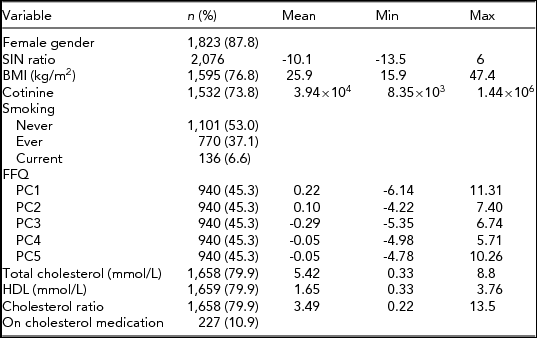
Summary statistics for each variable are given as mean and range except where stated. n represents the sample size for each variable with total possible sample = 2,076.
TABLE 5 Risk Factors for Hearing Ability: Univariate Regression and Multiple Regression Models
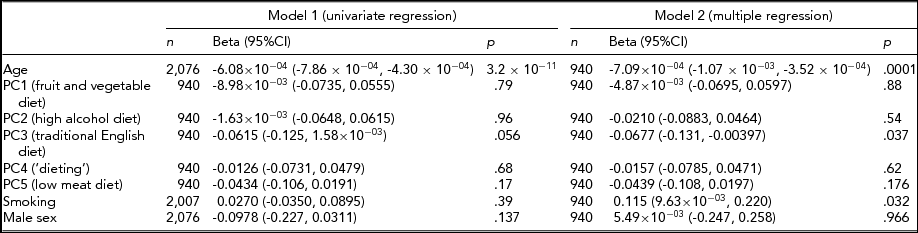
Model 1 (univariate analysis) included the risk factor shown adjusted for age, age2, sex, and twin relatedness. Model 2 (multiple regression) included all risk factors shown and was adjusted for age, age2, sex, and the twin relationship. PC represents principal component of diet data from FFQ; n = sample size; beta = the effect size, 95%C = 95% confidence interval, p = probability.
Smoking status was not associated with loss in SIN perception when adjusted for age and sex in model 1; however, in model 2, a weakly significant positive association was detected (β = 0.115, 95% CI = 9.63 × 10−03 to 0.220, p = .032). To explore these results in more detail, serum cotinine levels were investigated; 1,532 individuals of the original sample had been tested for cotinine levels and 142 (9.27%) participants showed detectable levels of cotinine in their bloodstream. The self-reported smoking variable was validated using the measured cotinine data and showed that those twins reporting smoking did indeed have highly significant detectable levels of cotinine in their blood (p = 3.3 × 10−54). However, no association could be detected between cotinine and SIN values in model 1 (β = -0.0962, 95% CI = -0.250 to 0.0574, p = .22) and in model 2 (β = -0.0202, 95% CI = -0.234 to 0.194, p = 0.85) in our limited subset (n = 142). As diet and smoking are related, we examined the PC3 dietary pattern in smokers and non-smokers and found it not to differ (t = -0.60, p = .55).
Discussion
This study is the first of its kind performed to examine SIN in a large sample of twins. The volunteers registered with TwinsUK have been shown to be representative of a singleton UK population (Andrew et al., Reference Andrew, Hart, Snieder, de Lange, Spector and MacGregor2001). Although heritability was estimated to be 40% for SIN, age adjustment reduced the heritability to 25% with unshared environmental factors accounting for 75% of variance in SIN. Age is thus a significant confounding factor in hearing ability and the variance in SIN is determined mainly by environmental factors unique to, i.e., not shared among, the twin siblings. Heritability estimates showed no significant difference for a mixed gender or purely female sample, indicating no difference in SIN heritability between males and females.
Our heritability estimates were lower than reported previously by Viljanen et al. (Reference Viljanen, Era, Kaprio, Pyykko, Koskenvuo and Rantanen2007) who determined the heritability of speech perception in female twins from the Finnish Twin Study on Aging. This study used a smaller sample size (103 MZ and 114 DZ female twin pairs) and speech recognition was assessed using phonetically balanced bisyllabic Finnish words without background sounds (Palva, Reference Palva1952). In the Finnish sample, the AE model provided the best explanation for the observed data and a heritability of 66% (95% CI = 55–74%) was demonstrated. Non-shared environmental exposure in the Finnish twin siblings accounted for 34% of the variance in speech perception. Speech perception in the Finnish sample was measured in a quiet environment, whereas the SIN results presented here were measured against a background hissing noise, like interference on the radio. The contrasting results of the two twin studies suggests that the speech perception test may be more similar — and more genetically mediated — to the pure tone audiogram, than the SIN test. Alternative explanations including the differing genetic contribution of different sample populations cannot be excluded however.
The influence of environmental risk factors was studied in a wide age range (18–87 years) from the well-characterized TwinsUK cohort, which has been shown to reflect the general singleton population for many lifestyle factors and traits (Andrew et al., Reference Andrew, Hart, Snieder, de Lange, Spector and MacGregor2001). For age, although the decrease in SIN perception every year appears to be small (β = -6.08×10−04), over the course of several decades this reduction in SIN perception accumulates in a large overall effect of ARHI leading to a high prevalence of hearing symptoms in the elderly (Mao et al., Reference Mao, Zhao, Pu, Wang, Zhang and He2013; Smeeth et al., Reference Smeeth, Fletcher, Ng, Stirling, Nunes, Breeze and Tulloch2002). Subsequent models were therefore adjusted for age and sex, as a significant difference in SIN scores was observed with women demonstrating better speech understanding in noise than men — as reported previously (Dubno et al., Reference Dubno, Lee, Matthews and Mills1997; Gates et al., Reference Gates, Cooper, Kannel and Miller1990).
Influence of the five principal components of diet derived from the FFQ, PC3 (traditional English diet) was found to be associated with an increased likelihood of loss in understanding speech in noisy environment. As PC3 (traditional English diet) contained fried fish and potatoes and processed meat products, we postulated a role for cholesterol. Cholesterol ratio is a useful index commonly used to predict cardiovascular risk in individuals (Lemieux et al., Reference Lemieux, Lamarche, Couillard, Pascot, Cantin, Bergeron and Despres2001; Ridker et al., Reference Ridker, Rifai, Cook, Bradwin and Buring2005). HDL cholesterol is also a commonly measured biomarker but represents the ‘good’ form of cholesterol which promotes cellular cholesterol efflux from lipid-laden molecules, endothelial function and repair, and is also involved in anti-inflammatory and anti-oxidant mechanisms (Barter et al., Reference Barter, Nicholls, Rye, Anantharamaiah, Navab and Fogelman2004; Chapman et al., Reference Chapman, Le Goff, Guerin and Kontush2010; Kontush & Chapman, Reference Kontush and Chapman2006; Navab et al., Reference Navab, Ananthramaiah, Reddy, Van Lenten, Ansell, Fonarow and Fogelman2004; Tso et al., Reference Tso, Martinic, Fan, Rogers, Rye and Barter2006). We did not detect an association between cholesterol ratio and SIN, but high HDL cholesterol levels were strongly correlated with better SIN perception suggesting a role for the so-called ‘good cholesterol’ (Chapman et al., Reference Chapman, Le Goff, Guerin and Kontush2010). These findings are in agreement with Suzuki et al. (Reference Suzuki, Kaneko and Murai2000) who have also shown a protective effect of HDL on hearing ability as measured by PTA (n = 924), and Spankovich and Le Prell (Reference Spankovich and Le Prell2013) who found high cholesterol and fat intake to be strongly associated with hearing loss as assessed by PTA (n = 2,366). Simpson et al. (Reference Simpson, Matthews and Dubno2013) conducted a longitudinal study and were unable to find a correlation between cholesterol ratio or triglycerides and hearing loss. Conversely, Jones and Davis (Reference Jones and Davis2000) found a positive correlation between hypercholesterolemia and hearing threshold levels in a clinical population presenting to a neuro-otology clinic. It is noteworthy that unlike the study by Jones & Davis (Reference Jones and Davis2000), where patients present with different types of hearing loss that may be difficult to control for, our study has the advantage of a relatively healthy population sample.
An unexpected positive association was initially observed that did not hold up to further scrutiny (e.g., using cotinine). Given the link with cholesterol and putative mechanism of atheroma, it is surprising that we did not detect an influence of smoking on hearing ability despite a reasonable sample size. However, the sample of 136 current smokers was small; the limited number of smokers likely reduced the power to detect an association in this relatively health conscious group of volunteers. A recent cross-sectional study by Dawes et al. (Reference Dawes, Cruickshanks, Moore, Edmondson-Jones, McCormack, Fortnum and Munro2014) with a very large sample size (n = 164,770) from the UK Biobank found both active and passive smoking to have a negative effect on hearing ability as measured by the SIN test. One advantage of our study is that the validity of self-reported smoking status was confirmed using cotinine. Further lack of association between cotinine and a deleterious effect on hearing ability is consistent with a previous study (Nondahl et al., Reference Nondahl, Cruickshanks, Dalton, Schubert, Klein, Klein and Tweed2004) but both this study and ours is likely limited by small sample size.
No influence of moderate alcohol intake on SIN perception was detected (p = .96). Rosenhall et al. (Reference Rosenhall, Sixt, Sundh and Svanborg1993) and Nash et al. (Reference Nash, Cruickshanks, Klein, Klein, Nieto, Huang and Tweed2011) have previously found an association between alcohol abuse and ARHI (via PTA). But some cross-sectional studies have suggested that there may be a protective effect of moderate alcohol consumption (Fransen et al., Reference Fransen, Topsakal, Hendrickx, Van Laer, Huyghe, Van Eyken and Van Camp2008; Itoh et al., Reference Itoh, Nakashima, Arao, Wakai, Tamakoshi, Kawamura and Ohno2001; Popelka et al., Reference Popelka, Cruickshanks, Wiley, Tweed, Klein, Klein and Nondahl2000), with Dawes et al. (Reference Dawes, Cruickshanks, Moore, Edmondson-Jones, McCormack, Fortnum and Munro2014) demonstrating a protective effect throughout all levels of alcohol consumption (mild, moderate, and heavy) in the volunteers at UKBioBank who, like most volunteers, are likely to be moderate drinkers, rather than heavy. The findings presented here in a predominantly female sample are consistent with a prospective study of 531 men showing no association between moderate alcohol intake and hearing loss (Brant et al., Reference Brant, Gordon-Salant, Pearson, Klein, Morrell, Metter and Fozard1996).
There were a number of limitations to our study. The FFQ was performed before the hearing data collection, between 1993 and 2001, and may be limited in measuring the dietary intake, with diet diaries and dietary recalls being more reliable (Olafsdottir et al., Reference Olafsdottir, Thorsdottir, Gunnarsdottir, Thorgeirsdottir and Steingrimsdottir2006). However, FFQs have been shown to be more consistent in terms of assessing dietary intake over an extended period of time, thus being more representative of the individuals’ dietary habits (Teucher et al., Reference Teucher, Skinner, Skidmore, Cassidy, Fairweather-Tait, Hooper and MacGregor2007) and the temporal relationship may have improved our ability to detect an influence of diet on hearing. Furthermore, dietary patterns derived from FFQs can account for the colinearity of foods consumed together at meals (Teucher et al., Reference Teucher, Skinner, Skidmore, Cassidy, Fairweather-Tait, Hooper and MacGregor2007). Another limitation was the self-report smoking status data collection, which may be biased due to underreporting in a health conscious group, and limited due to the effects of selective mortality of the cohort. This relationship may be better understood by using quantitative methods for measuring exposure to tobacco smoke, thus providing the opportunity to observe a dose–response relationship.
One particular strength of this study is that it looked at a large sample of twins, allowing us to perform the first heritability analysis of SIN perception. Furthermore, this cohort consisted of participants younger than many of those examined in other studies, and with a higher proportion of women. Twin modeling has shown that 75% of the variance in SIN measures may be explained by environmental factors. Unlike most previous studies exploring the effect of environmental factors on hearing sensitivity measured through PTA, our study looked at the clarity aspect of hearing ability using SIN — a measure which may capture better the disabling aspects of the impairment. Our results support the notion that SIN is influenced by environmental factors and that the genetic factors are closely linked to age, much like osteoporosis (Moayyeri et al., Reference Moayyeri, Hammond, Hart and Spector2012) and Alzheimer's disease (Rao et al., Reference Rao, Degnan and Levy2014). The frequent consumption of fried and processed foods may have a detrimental effect on hearing ability. If this association is confirmed in other studies, government-led or social/voluntary organization-led public health strategies relating to healthy diet promotion for ARHI prevention in future may be established. Furthermore, in audiology departments, patients are often asked about exposure to smoking but not alcohol consumption or a healthy diet; hence, these findings may change the approach to general history taking as well.
Acknowledgments
SM was supported by an Action on Hearing Loss Summer Studentship grant. The web-based SIN test was made available by Action on Hearing Loss. The hearing data collection was supported by Action on Hearing Loss and the Hastings Catenian Association. We would like to thank all volunteers from the TwinsUK register, who kindly contributed to this study Twins UK. The study was funded by the Wellcome Trust; European Community's Seventh Framework Programme (FP7/2007–2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London.
Disclosure of Interests
The authors declare that they have no conflict of interest.
Appendix Distribution of Frequent Consumption of Foods for the Five Dietary Patterns