Case Report
Prenatal Course
A 34-year-old woman (G2P1) with a monochorionic (MC) twin pregnancy was followed biweekly from 14 weeks onwards at our obstetrical outpatient clinic. No clinical or ultrasound signs of twin–twin transfusion syndrome (TTTS), or selective fetal growth restriction (sFGR) were detected (Benoit & Baschat, Reference Benoit and Baschat2014; Khalil et al., Reference Khalil, Beune, Hecher, Wynia, Ganzevoort, Reed, Lewi, Oepkes, Gratacos, Thilaganathan and Gordijn2019). Middle cerebral artery peak systolic velocity (MCA-PSV) Doppler measurements were performed routinely and were not indicative of twin anemia polycythemia sequence (TAPS), as defined by delta MCA-PSV > 0.5 multiples of the median (MoM; Tollenaar et al., Reference Tollenaar, Lopriore, Middeldorp, Haak, Klumper, Oepkes and Slaghekke2018). Apart from mild cholestasis of pregnancy, the pregnancy was uncomplicated. The last estimated fetal weights (EFW) were 1951 g for Twin 1 and 2069 g for Twin 2, with an EFW discordance (calculated as (EFW large twin – EFW small twin)/EFW large twin) of 6%.
Delivery
Labor was induced at 36 + 2 weeks of gestation. Artificial rupture of membranes was performed at 15:10. Oxytocin stimulation was started at 16:40 due to a lack of contractions thus far. At 19:00, the midwife checked on the patient on account of self-reported increased contractility. Twin 1 was quickly delivered at 19:15 after precipitous labor. She had an Apgar score of 10/10 and weighed 2465 g. Umbilical cord blood gases showed an arterial pH of 7.08 with a base-excess (BE) of −12 and venous pH 7.5 with a BE of −6. The obstetrician was called and arrived a few minutes later. The cord of Twin 1 was clamped approximately 5 min after birth. Simultaneously, cardiotocogram (CTG) registration failed and an ultrasound was made, showing a deep bradycardia of Twin 2 with very limited cardiac output and suboptimal fetal position. An emergency caesarian section was performed, and Twin 2 was born at 19:35 with an Apgar score of 6/7/8 (1750 g) with a venous blood gas pH of 7.36 and a BE of −3 drawn from the umbilical cord. Arterial blood gas pH measurement failed due to an insufficient quantity. The cord was clamped immediately after birth. Twin 2 was limp and pale and did not breathe on her own. Five insufflation breaths were administered, after which continuous positive airway pressure (CPAP) was started. Heart rate was normal throughout the initial resuscitation. Twin 2 was transferred to the neonatal intensive care unit (NICU) with CPAP.
Neonatal Course
At the NICU, Twin 2 received a fluid bolus (NaCl 0.9% 10 ml/kg) due to mild hypotension with a mean blood pressure (BP) of 34 mmHg. Heart rate was 110–150 beats/min. Capillary blood gas revealed a lactate of 5.0 mmol/L. CPAP was stopped within a few hours after birth. Neonatal cerebral ultrasound did not show any abnormalities. The first full blood count 3 h after birth was clotted and could not be analyzed. The second blood count drawn approximately 9 h after birth showed a hemoglobin (Hb) level of 10.2 g/dL and a reticulocyte count of 38 ‰. Approximately 1 month after birth, Twin 2 was admitted to a peripheral hospital for an erythrocyte transfusion. Twin 1 had an uneventful neonatal course. Her Hb level at birth was 20.1 g/dL and her reticulocyte count 40 ‰.
Inter-twin Hb difference was 9.9 g/dL, and reticulocyte count ratio (calculated as the reticulocyte count of Twin 2/reticulocyte count of Twin 1) was 1.1. Birth-weight discordance (calculated as birth weight large twin – birth weight small twin/birth weight large twin × 100) was 29%.
Examination of the Placenta
The placenta was injected with colored dye according to standard protocol (Lopriore et al., Reference Lopriore, Slaghekke, Middeldorp, Klumper, van Lith, Walther and Oepkes2011; see Figure 1) using blue and pink dye for, respectively, the arteries and veins of Twin 1, and green and yellow dye for the arteries and veins of Twin 2. Both twins had a central cord insertion. The maternal side of the placenta did not show a color difference, as described for TAPS (Tollenaar et al., Reference Tollenaar, Zhao, Middeldorp, Oepkes, Slaghekke and Lopriore2017). A chorangioma with a diameter of 4−5 cm was present on the fetal surface of the placental share of Twin 2. After color injection, the chorangioma turned predominantly yellow, suggesting the presence of primary veins. The placenta was equally shared (50%−50%) after calculation using Image J version 1.52a. One arterio-arterial (AA) anastomosis was observed with a diameter of 1.4 mm. Eight minuscule arterio-venous (AV) and seven veno-arterial (VA) anastomoses from Twin 1 to Twin 2 were counted. Furthermore, fetal vessels were diffusely congested and the placental weight of Twin 2 was increased due to the presence of the chorangioma, which was isolated, large (dimensions: 4.8 cm × 4.2 cm × 2.8 cm) filled with blood and weighed 60 g. Without the chorangioma, the placental weight of Twin 2 was within the 50th−75th percentiles (Pinar et al., Reference Pinar, Sung, Oyer and Singer1996). Microscopic examination revealed parenchymal abnormalities (chronic villitis of unknown etiology, perivillous fibrinoid depositions and chronic intervillositis) in the placental share of Twin 2. Aside from a low grade chronic villitis and focal perivillous fibrin, there we no abnormalities in the placental share of Twin 1. Its weight fell within the 25th percentile.
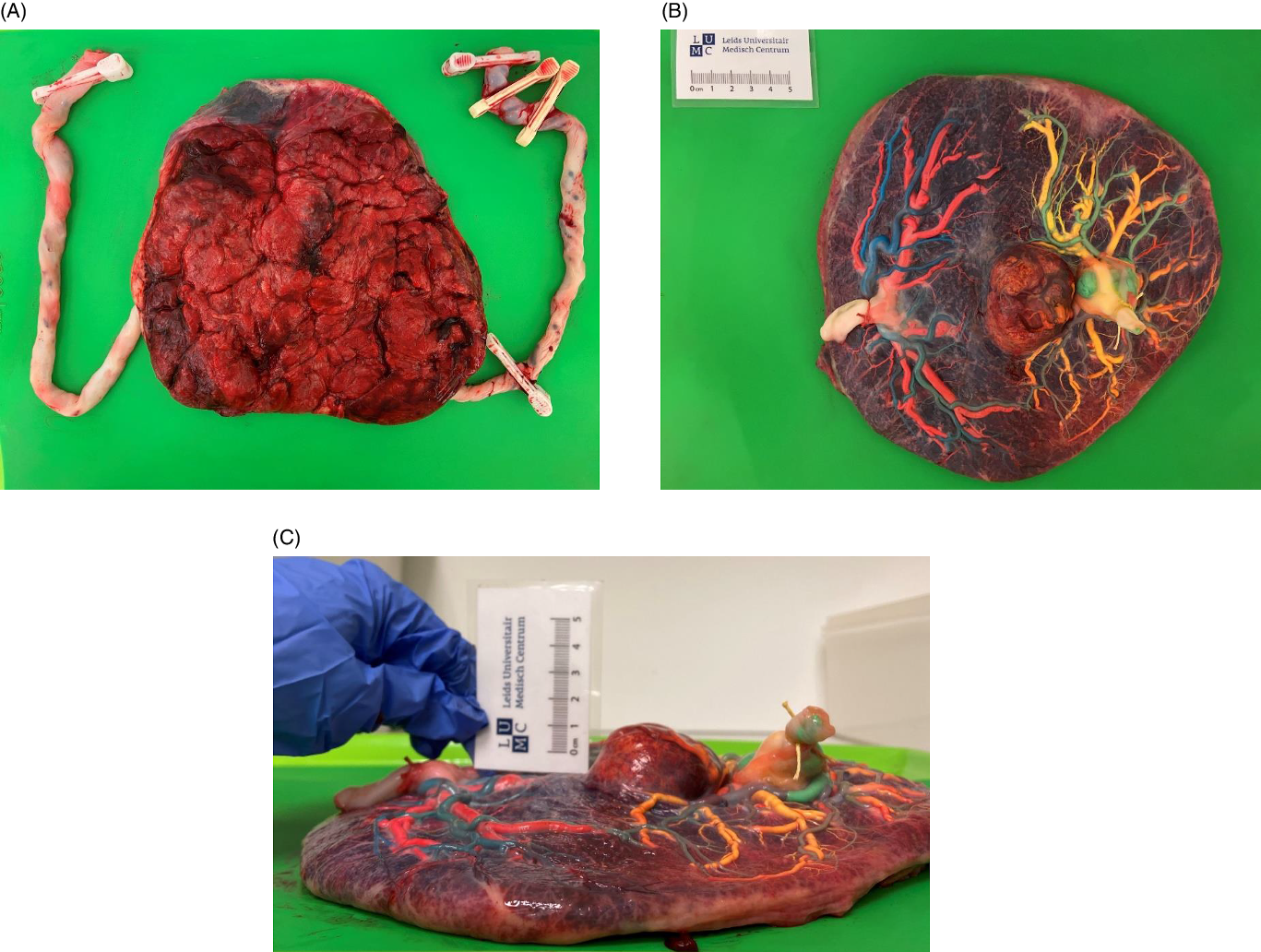
Figure 1. Macroscopic placental evaluation; A) maternal side of the placenta; B) fetal side of the placenta after color dye injection; C) horizontal view of the placenta showing the mass of the chorangioma
Discussion
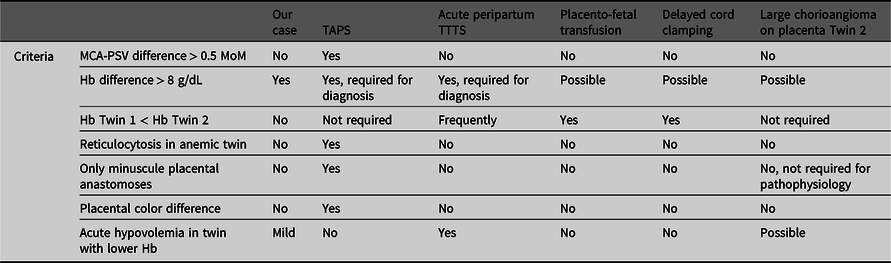
We report on a MC twin pair with an uncomplicated antenatal course, but a large postnatal intertwin Hb difference. Hb differences in MC twins are not uncommon and generally develop due to unbalanced transfusion through vascular anastomoses in the shared placenta. In this case, the exact cause for the intertwin Hb difference is not entirely understood (see Table 1). Several differential diagnoses can be considered.
Table 1. Overview of causes of potential hemoglobin differences in monochorionic twins in comparison with the characteristics of the presented case.
TAPS
In general, the most probable cause of large intertwin Hb differences in MC twins is TAPS. TAPS arises due to chronic distorted feto-fetal transfusion through minuscule placental anastomoses leading to chronic anemia in the donor twin and chronic polycythemia in the recipient twin, without signs of twin oligo-polyhydramnios sequence (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Kanhai, Walther and Vandenbussche2007). In our case, TAPS would be unlikely, since the antenatal Doppler ultrasound MCA-PSV measurements were normal. Moreover, at birth, the reticulocyte count ratio was low (1.1), indicating an acute transfusion rather than a chronic one. In addition, color dye injection of the placenta did not show the characteristic aspect of TAPS placenta with only minuscule anastomoses, and no color difference on the maternal side was noted between the two placenta shares (Tollenaar et al., Reference Tollenaar, Zhao, Middeldorp, Oepkes, Slaghekke and Lopriore2017).
Acute Peripartum TTTS
A possible explanation for the large Hb difference might be acute peripartum TTTS, following a rapid transfusion of blood from one twin (the donor) to the other (the recipient) during labor. Placentas in acute peripartum TTTS are characterized by the presence of at least one (large) superficial AA or VV anastomosis (with low resistance), allowing rapid intertwin transfer of blood (Lopriore et al., Reference Lopriore, Holtkamp, Sueters, Middeldorp, Walther and Oepkes2014). CTG registration during labor might show a sinusoidal pattern and fetal tachycardia, as a reflection of acute anemia and fetal hemorrhage. In our case, a sinusoidal pattern was not identified on CTG during delivery of Twin 1. Unfortunately, CTG registration for Twin 2 failed after Twin 1 was born, but ultrasound showed a significant fetal bradycardia with limited cardiac output. At birth, donors can be in acute hypovolemic shock, needing rapid fluid resuscitation and respiratory support. In our case, Twin 2 had hypotension needing fluid resuscitation, suggesting a sudden loss of blood. In line with the pathophysiology of acute TTTS, the reticulocyte count ratio was low and the placenta showed an AA anastomosis (>1 mm) that could have allowed for sudden feto-fetal transfusion. The exact pathophysiologic mechanisms in acute peripartum TTTS explaining the course of events leading to the acute intertwin transfusion are still not well understood. Supposedly, variations in BP due to uterine contractions or relative fetal positioning can generate a pressure gradient, affecting the transfer of blood between the twins. Clear evidence for this theory is currently lacking. In acute peripartum TTTS, the anemic twin is usually the first-born twin. In our case, the anemic twin was the second born child (Lopriore et al., Reference Lopriore, Holtkamp, Sueters, Middeldorp, Walther and Oepkes2014). However, it is possible that if Twin 2 was a direct conduit of the force applied by uterine contractions to expel Twin 1, this may create a pressure gradient between Twin 2 and Twin 1, with higher pressures in Twin 2.
Placento-Fetal Transfusion
Another potential mechanism of postnatal Hb differences in MC twins is placento-fetal transfusion (Verbeek et al., Reference Verbeek, Zhao, Te Pas, Middeldorp, Hooper, Oepkes and Lopriore2016). During vaginal delivery, after cord clamping of the first-born, blood from both placental shares is thought to be transfused to the circulation of the second-born twin through superficial vascular anastomoses. In analogy with acute peripartum TTTS, the mechanism of this transfusion during delivery is also elusive, and it is speculated that it is brought about by intertwin BP differences due to uterine contractions or fetal positioning (Verbeek et al., Reference Verbeek, Zhao, Middeldorp, Oepkes, Hooper, Te Pas and Lopriore2017). However, in line with the supposed pathogenesis of placento-fetal transfusion in MC twins, one would expect the second-born twin to have an increased Hb as opposed to the first-born twin. This does not hold for our case, as Twin 2 presented with a lower Hb.
Delayed Cord Clamping
Differences in timing of cord clamping can also potentially result in Hb differences. Early cord clamping (<30 s after birth) limits time for placento-fetal transfusion and is associated with lower Hb levels, whereas with delayed cord clamping (DCC) the opposite occurs, leading to higher Hb levels. In twins, obstetricians may tend to perform early cord clamping in the first-born twin in order to quickly shift the focus to the imminent delivery of the second twin. When the second twin is born, there is more time for DCC, leading to increased placental transfusion and thereby subsequent higher Hb levels (Ghirardello et al., Reference Ghirardello, Di Tommaso, Fiocchi, Locatelli, Perrone, Pratesi and Saracco2018; McDonald et al., Reference McDonald, Middleton, Dowswell and Morris2014; Mercer, Reference Mercer2001; Rabe et al., Reference Rabe, Gyte, Diaz-Rossello and Duley2019; Verbeek et al., Reference Verbeek, Zhao, Middeldorp, Oepkes, Hooper, Te Pas and Lopriore2017). In our case, the opposite occurred, as DCC was unintentionally performed in Twin 1 (cord clamped after 5 min) whereas the cord of Twin 2 was clamped early. One could speculate that this DCC allows for more placento-fetal transfusion towards Twin 1 and higher Hb levels, whereas early cord clamping in Twin 2 may have resulted in a lower Hb level. The higher birth weight in Twin 1 (2465 g) compared to the EFW of 1951 g suggests that increased blood volume could have led to a higher birth weight than antenatally anticipated. In contrast, as Twin 2 had a lower birth weight (1750 g) than the EFW (2069 g), this could result from a decreased blood volume and explain the discrepancy in EFW and actual birth weight. In MC twins, the optimal timing of cord clamping is not known and could be different compared to singletons or dichorionic twins, due to the presence of vascular anastomoses. Whether the strategy of DCC is also beneficial in MC twins or could be associated with an increased risk of acute peripartum intertwin transfusion requires further investigation.
Placental Chorangioma
Chorangiomas, a benign tumor of the placenta, are rare and occur in approximately 1% of pregnancies (Amer & Heller, Reference Amer and Heller2010). Large chorangiomas (>4 cm in diameter), as seen in this case, are even more rare and occur in 0.1−0.3% of pregnancies (Fan & Skupski, Reference Fan and Skupski2014). The atypical presence of the chorangioma also raises the possibility that the chorangioma played a role in causing the lower Hb in Twin 2. Antenatally, the chorangioma may have served as an additional reservoir for blood, as reflected by the increased placental weight of Twin 2 (Amer & Heller, Reference Amer and Heller2010; Bernischke et al., Reference Bernischke, Burton and Baergen2012). During delivery, contractions might have caused the chorangioma to be emptied (into the circulation of Twin 2) due to compression. Subsequently, when pressure is relieved (e.g., in between contractions or at birth), the chorangioma starts to refill, retaining blood from Twin 2 and hence causing a lower Hb. With regard to the dimensions of the chorangioma, it can be estimated, using the mathematical formula for calculating the volume of an ellipse (4/3 × ∏ × a × b × c, with a, b and c = elliptic radii) to contain a blood volume of ±29 mL. As Twin 2 had a circulating volume of approximately 140 mL (80 mL/kg), a peripartum loss of 29 mL into the chorangioma is a significant amount (21% of the total circulating volume). This is also consistent with the finding of a lower birth weight in Twin 2 (1750 g) compared to its EFW (2069 g). However, while it may explain the low Hb level in Twin 2, it does not readily explain the higher Hb in Twin 1.
In conclusion, the true mechanism of the Hb difference between MC twins as observed in our case remains elusive. We speculate that the most probable cause is a combination of DCC in Twin 1 leading to high Hb level, the chorangioma in Twin 2 leading to a low Hb level, and complex intrapartum pressure gradients leading to acute intertwin transfusion. Our case illustrates the potential impact of the connected circulations in MC twins and the importance of (routine) placental injection to unravel the mechanisms behind Hb differences in MC twins.
Conflict of Interest
The authors report no conflict of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.




