Maternal anemia is a common pregnancy complication worldwide. The World Health Organization (WHO) defines maternal anemia as a hemoglobin (Hb) concentration of less than 11 g/dL (WHO, 2011). Globally, 36.5% of pregnant patients have anemia (WHO, 2022). Maternal anemia increases the risk of low birth weight (LBW), preterm birth, perinatal mortality, stillbirth, and maternal mortality (Breymann et al., Reference Breymann, Bian, Blanco-Capito, Chong, Mahmud and Rehman2011; Jung et al., Reference Jung, Rahman, Rahman, Swe, Islam, Rahman and Akter2019; Rahman et al., Reference Rahman, Abe, Rahman, Kanda, Narita, Bilano, Ota, Gilmour and Shibuya2016).
Twin pregnancies are at a higher risk for maternal and neonatal complications (Gibson et al., Reference Gibson, Castleman, Meher and Kilby2020). Recently, twin gestations are increasing worldwide because of the development and application of assisted reproductive technology. The proportion of twin gestations constitutes 2%−4% of total pregnancies (Ananth et al., Reference Ananth and Chauhan2012; Fell et al., Reference Fell and Joseph2012; Russell et al., Reference Russell, Petrini, Damus, Mattison and Schwarz2003). However, the prevalence of anemia and adverse maternal and neonatal outcomes in twin pregnancies have been rarely studied. The present study aimed to investigate the prevalence of anemia in twin pregnancies, and to examine whether maternal anemia is associated with adverse maternal and neonatal outcomes in twin gestations. We further evaluated changes in outcomes based on successful anemia treatment.
Materials and Methods
Study Subjects and Design
This was a retrospective study in a tertiary hospital in China. The study was approved by the ethics committee of the hospital, and was conducted in accordance with the ethical standards of the Declaration of Helsinki. Since this was a retrospective study and had minimal risk for patients, a waiver of informed consent was approved by the ethics committee of our hospital. All twin pregnancies were eligible for inclusion. Pregnancies complicated by twin-to-twin transfusion syndrome, second trimester deliveries (< 28 weeks gestation), and substance use were excluded. In our hospital, patients routinely received folic acid during pregnancy. Based on the criteria of Hb <11 g/dL as pregnancy anemia, the twin pregnant women were divided into two groups: patients with Hb <11 g/dL were in the anemic group and patients with Hb ≥11 g/dL served as controls. Anemic patients were treated with supplemental iron therapy and the serum ferritin levels were measured for the remainder of the pregnancy. After early diagnosis and treatment, patients with anemia in the second trimester were further subcategorized by whether they had normal Hb in the third trimester. We also compared the maternal and neonatal outcomes between the recovered and unrecovered subgroups.
Data Collection
Baseline characteristics and comorbidities were analyzed for all eligible participants. This included maternal age, mode of conception, chorionicity, parity, and history of cesarean. Perinatal outcomes assessed included incidence of preterm birth, gestational diabetes (GDM), preeclampsia, preterm premature rupture of membranes (PPROM), cesarean delivery, and postpartum hemorrhage. Neonatal outcomes evaluated included birth weight, incidence of low birth weight (LBW), small for gestational age (SGA), birth weight discordance, congenital malformations, Apgar score at 1 min and at 5 min, neonatal intensive care unit (NICU) admission rate and perinatal mortality.
Definitions
Severity of anemia was categorized as mild (Hb 10.0−10.9 g/dL), moderate (Hb 7.0−9.9 g/dL) and severe (Hb <7.0 g/dL) based on the WHO recommendation (WHO, 2011). Chorionicity was determined by ultrasound at 10–14 weeks of gestation. SGA was defined as birth weight below the sex-specific 10th percentile of weights for gestational age (Kibel et al., Reference Kibel, Kahn, Sherman, Kingdom, Zaltz, Barrett and Melamed2017). LBW was defined as birth weight <2500 g (Hughes et al., Reference Hughes, Black and Katz2017). Perinatal death was defined as the occurrence of intrauterine death and neonatal death. Birth weight discordance was defined as a 20% difference in birth weight between the larger and the smaller twin and calculated by the following equation: (larger actual weight–smaller actual weight)/larger actual weight (American College of Obstetricians and Gynecologists [ACOG], 2021b; Qiao et al., Reference Qiao, Zhao, Jiang, Xu, Yang, Bao, Xie and Ying2020).
Statistical Analysis
Categorical variables were represented as number and percentage. The rates or proportions of different group were compared by the chi-squared test or the Fisher exact test (if the variable contained fewer than five measurements). Student’s t test was used for differences between continuous variables.
Univariable and multivariable logistic regression models were used to determine the association of maternal and neonatal characteristics with anemia. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each variable. Unadjusted ORs and adjusted ORs were reported. Linear regression analysis was used to estimate mean birth weight and gestational week.
A p < .05 or confidence interval excluding 1.0 was considered statistically significant. All analyses were performed using the Statistical Package for Social Science (SPSS Inc., Chicago, IL, version 20).
Results
Prevalence of Anemia and Demographic Characteristics of Participants
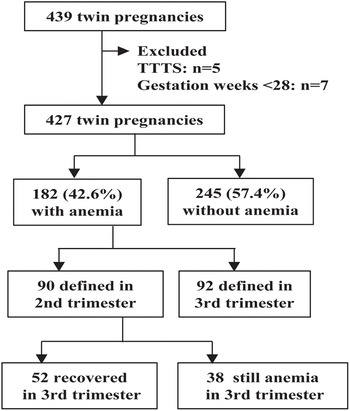
Of the total 427 women with twin pregnancy, 182 were diagnosed with anemia, with an overall anemia prevalence of 42.6% (95% CI [37.9, 47.3]). Of the anemic pregnant patients, 90 (49.5%) were diagnosed in the second trimester, and 92 (50.5%) were diagnosed in the third trimester (Figure 1). The anemia was mild in 127 (69.8%), moderate in 55 (30.2%), and severe in none of the 182 anemic patients.
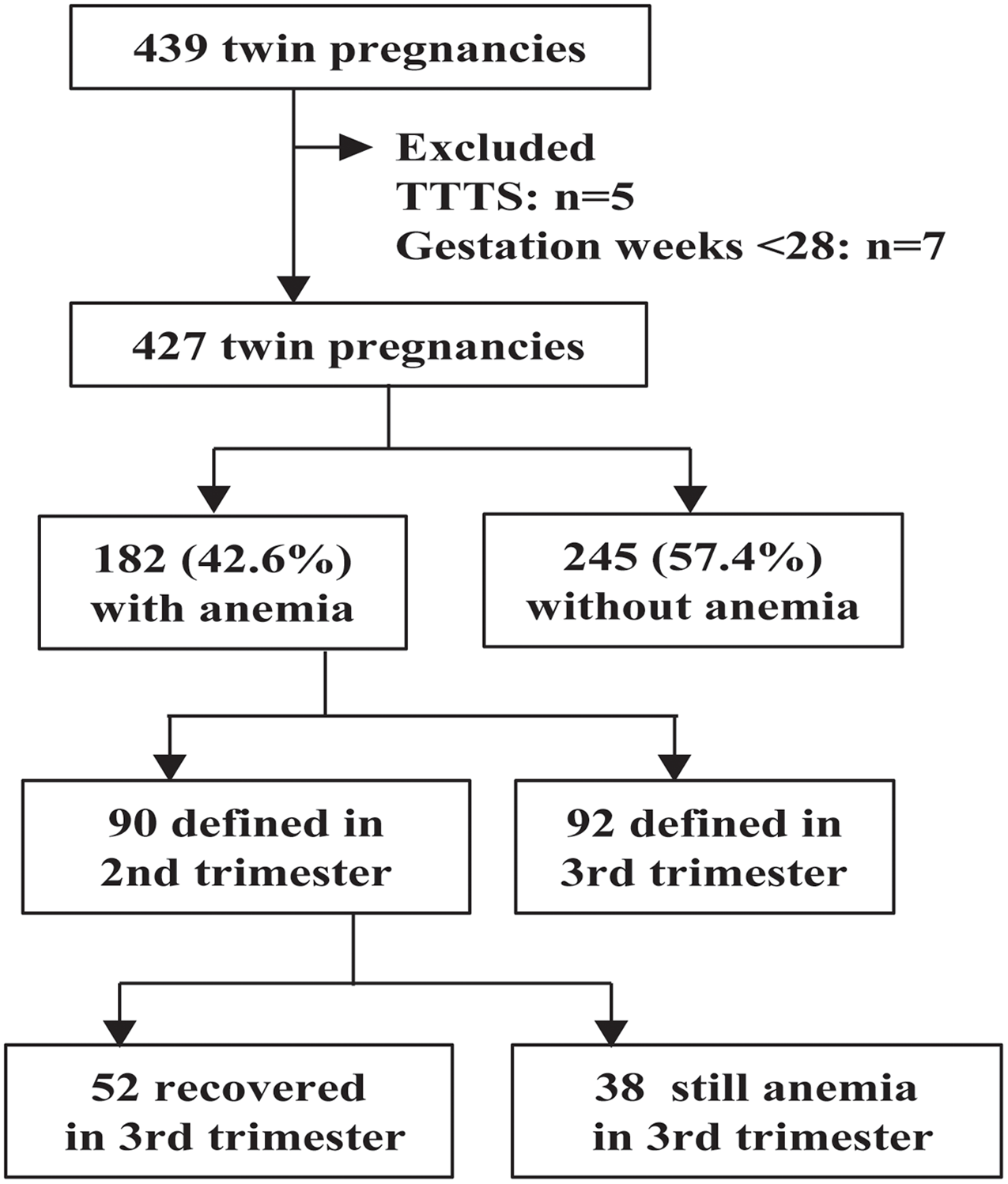
Figure 1. Flow diagram of study participants.
Note: TTTS, twin-to-twin transfusion syndrome.
The maternal demographic characteristics are shown in Table 1. None of the patients in the cohort were younger than 18 years old. Maternal ages and chorionicity were comparable between the anemia and control groups. Patients with anemia were more likely to have higher parity (p = .012), spontaneous conception (p = .028), and history of cesarean (p = .02). The two subgroups of twin pregnant women diagnosed with anemia in the second trimester had similar demographics (Table S1).
Table 1. Demographic characteristics of twin pregnancies with and without anemia
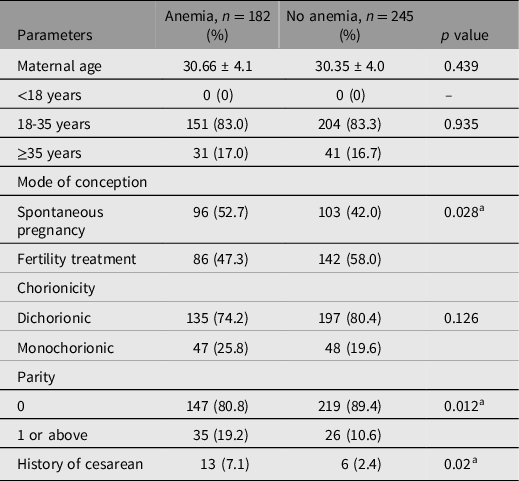
Note: Data are presented as number (percentage) or mean ± standard deviation.
a Denotes statistical significance.
Maternal Outcomes of Twin Pregnancies With Anemia
Tables 2 and 3 show the maternal outcomes in the two groups. There were no maternal deaths in the cohort. Patients with anemia were less likely to have GDM, compared with nonanemic patients (11.0% vs. 18.0%, p < .05). After adjusting for confounders, there was no significant difference in the odds of GDM, preeclampsia, PPROM, postpartum hemorrhage, cesarean delivery, or preterm birth (Table 3).
Table 2. Adverse maternal outcomes in twin pregnant women with and without anemia
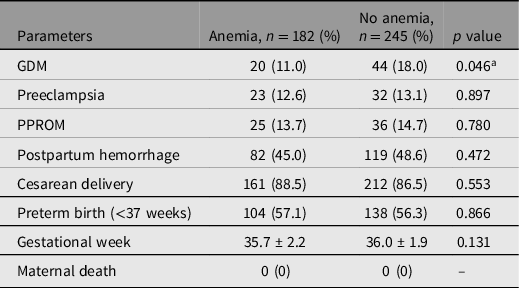
Note: Data are presented as number (percentage) or mean ± standard deviation. GDM, gestational diabetes mellitus; PPROM, preterm premature rupture of membranes.
a Denotes statistical significance.
Table 3. Association of maternal anemia with maternal outcomes in twin pregnancies
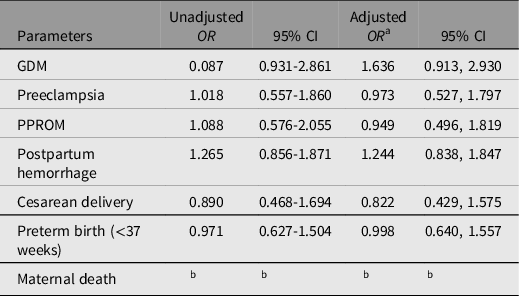
Note: OR, odds ratio; CI, confidence interval; GDM, gestational diabetes mellitus; PPROM, preterm premature rupture of membranes.
a Multivariate logistic regression was adjusted for mode of conception, parity and history of cesarean.
b Not reported as minimum number of events not met.
Neonatal Outcomes of Twin Pregnancies With Anemia
Tables 4 and 5 show the detailed neonatal outcomes of infants born to anemic and nonanemic patients. A higher rate of Apgar score ≤7 at 1 min (4.4% vs. 1.8%, p = .028) was observed in twins from the anemic group. Perinatal death was significantly higher among offspring born to anemic patients (1.9% vs. 0.2%, p = .012). Notably, newborn infants of patients with anemia were more likely to be admitted to NICU (27.2% vs. 20.2%, p < .05); this finding persisted after adjusting for confounders (adjusted OR, 1.478; 95% CI [1.07, 2.044]).
Table 4. Neonatal outcomes in twin pregnant women with and without anemia
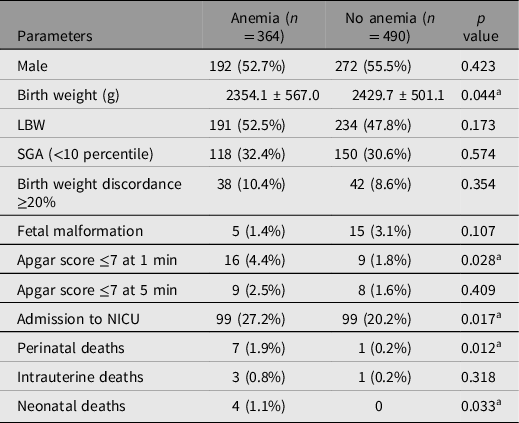
Note: Data are presented as number (percentage) or mean ± standard deviation. LBW, low birth weight; SGA, small for gestational age; NICU, neonatal intensive care unit.
a Denotes statistical significance.
Table 5. Unadjusted and adjusted odds ratios for neonatal outcomes of patients with anemia
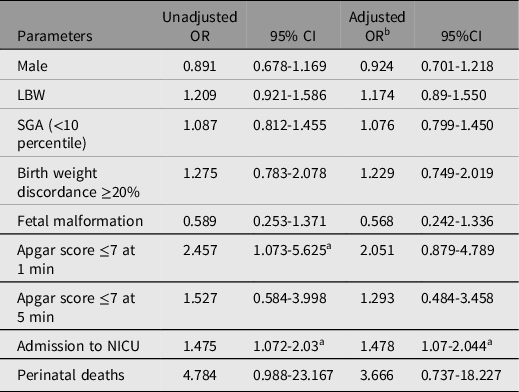
Note: OR, odds ratio; CI, confidence interval; LBW, low birth weight; SGA, small for gestational age; NICU, neonatal intensive care unit.
a Indicates significant difference;
b Multivariate logistic regression was adjusted for mode of conception, parity and history of cesarean.
Maternal and Neonatal Outcomes of Recovered and Unrecovered Anemic Pregnant Women
The 90 patients diagnosed with anemia during the second trimester were treated with iron supplements, and 52 had normal Hb in the third trimester and the remaining 38 still had anemia. There was a significant reduction in the odds of PPROM (OR, 0.233; 95% CI [0.067, 0.814]) among the successfully treated patients. As shown in Table 6, the recovered group had lower rates of preterm birth (32.7% vs. 55.3%, p = .032) and LBW (36.5% vs. 51.3%, p = .048) than the unrecovered group. Furthermore, NICU admission rate (13.5% vs. 30.3%, p = .006; OR, 0.388; 95% CI [0.186, 0.809]) was significantly lower in newborns of anemic patients who recovered (Tables 6 and 7). In the linear regression, gestational week and birth weight of newborns significantly increased in the recovered subgroup (β, 0.954 week; 95% CI [0.114, 1.794] and β, 171.01 g; 95% CI [9.894, 332.126] respectively).
Table 6. Adverse maternal and neonatal outcomes of recovered and unrecovered patients diagnosed with anemia in the second trimester
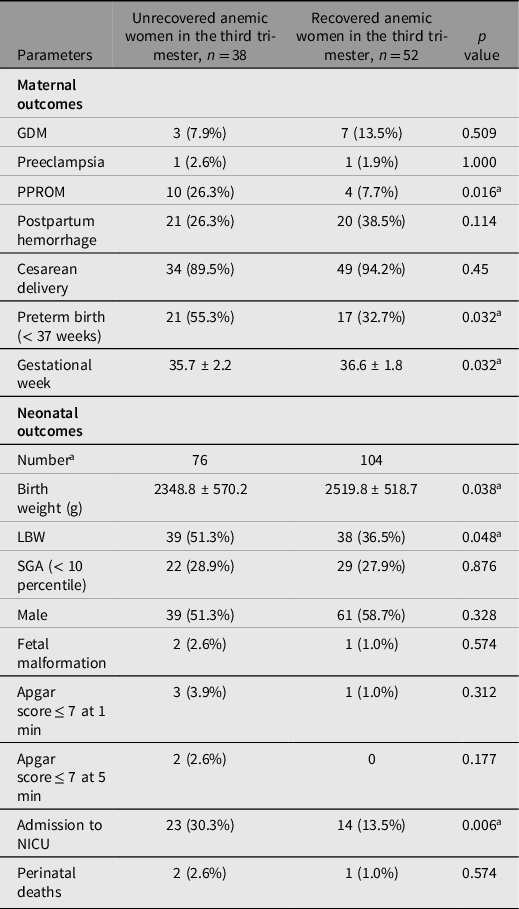
Note: Data are presented as number (percentage) or mean ± standard deviation. GDM, gestational diabetes mellitus; PPROM, preterm premature rupture of membranes; LBW, low birth weight; SGA, small for gestational age; NICU, neonatal intensive care unit.
a Denotes statistical significance.
Table 7. Maternal and neonatal odds ratios for adverse outcomes among patients diagnosed with anemia in the second trimester
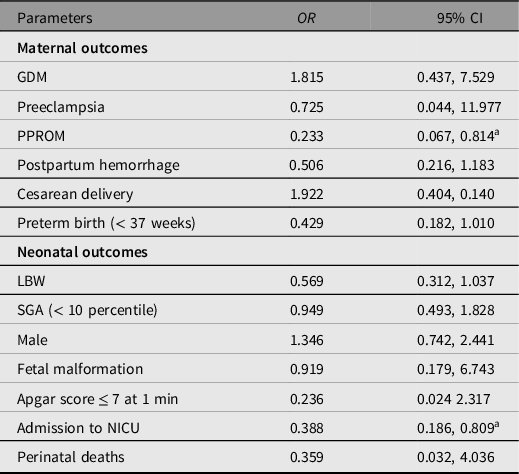
Note: OR, odds ratio; CI, confidence interval; GDM, gestational diabetes mellitus; PPROM, preterm premature rupture of membranes; LBW, low birth weight; SGA, small for gestational age; NICU, neonatal intensive care unit.
a Denotes statistical significance.
Discussion
Principal Findings
Our study showed that the prevalence of anemia was 42.6% in twin pregnancies from a tertiary hospital in China. Although there was no severe anemia in the present study, twin pregnant females with mild or moderate anemia were more likely to experience adverse neonatal outcomes, such as a low 1-minute Apgar score, lower birth weight, admission to NICU and perinatal death compared with nonanemic twin pregnant women. Notably, correction of anemia in the third trimester significantly improved neonatal outcomes of anemic patients with twin gestations.
Comparison With Other Studies and Clinical Implications
In this study, we reviewed the Hb levels of women with twin gestations in prenatal tests, and anemia was diagnosed when Hb value in any trimester was lower than 11g/dL (WHO, 2011). Similar to the prevalence of 44.6% in multiple pregnancies in New York (Ru et al., Reference Ru, Pressman, Cooper, Guillet, Katzman, Kent, Bacak and O’Brien2016), the 42.6% prevalence of anemia observed in twin pregnancy is much higher than the prevalence of 27.2% in singleton pregnancies previously reported by our center (Lan et al., Reference Lan, Li, Zhang, Chen, Hu and Wang2016), which suggested that women pregnant with twins may be at particularly high risk of anemia. According to WHO guidelines, a prevalence above 40% is a severe public health problem (WHO, 2017). Considering the high prevalence, anemia among twin pregnancy requires more attention.
Despite high rates of maternal anemia, there are few studies on patients with twin gestations, and they were not identified as an at-risk obstetric population by the last published American College of Obstetricians and Gynecologists Practice Bulletin on anemia in pregnancy (ACOG, 2021a). In the present study, twin-pregnant women with anemia was associated with higher risk of a low 1-minute Apgar score and lower birth weight, which also concurs with that found in singleton pregnancy (Figueiredo et al., Reference Figueiredo, Gomes-Filho, Silva, Pereira, Mata, Lyrio, Souza, Cruz and Pereira2018; Smith et al., Reference Smith, Teng, Branch, Chu and Joseph2019; Tunkyi & Moodley, Reference Tunkyi and Moodley2018; Young et al., Reference Young, Oaks, Tandon, Martorell, Dewey and Wendt2019;). Moreover, we observed increased perinatal deaths and NICU admission among twins born to anemic patients. Therefore, anemia in twin pregnancy is a factor for adverse neonatal outcomes.
Although anemia is known to exacerbate risks of preterm birth (Breymann et al., Reference Breymann, Bian, Blanco-Capito, Chong, Mahmud and Rehman2011; Jung et al., Reference Jung, Rahman, Rahman, Swe, Islam, Rahman and Akter2019; Rahman et al., Reference Rahman, Abe, Rahman, Kanda, Narita, Bilano, Ota, Gilmour and Shibuya2016), our study did not find a statistical difference in the incidence of preterm birth between the anemic and nonanemic twin pregnancies. This is consistent with the results of a previous study in twin pregnancies in Israel (Kosto et al., Reference Kosto, Okby, Levy, Sergienko and Sheiner2016). Preterm birth is highly prevalent in patients who are carrying twin fetuses (Chauhan et al., Reference Chauhan, Scardo, Hayes, Abuhamad and Berghella2010; Roman et al., Reference Roman, Ramirez and Fox2022), which may overlap with the rate of preterm birth caused by anemia.
In singleton pregnancies, patients with anemia may experience more postpartum hemorrhage, cesarean delivery, preeclampsia and PPROM than patients without anemia (Kanu et al., Reference Kanu, Hamner, Scanlon and Sharma2022; Smith et al., Reference Smith, Teng, Branch, Chu and Joseph2019). However, compared to twin pregnant women without anemia, we did not find increased rates of these complications among anemic patients.
We noted that more than half of the anemic patients were recovered following iron therapy. The successful treatment was associated with an increase in gestational age and birth weight and a considerable reduction of NICU admission. The findings highlight the importance of early diagnosis and treatment of anemia in twin pregnancies.
Strengths and Limitations
The strengths of this study include the focus on the effect of anemia in twin pregnancies and the elaborate study design of subgroups. This study has several limitations. First, we did not investigate the iron status or detailed iron supplement of patients. Second, data were collected retrospectively, and some information such as types of anemia and prepregnancy body mass index was missing. Third, our study did not evaluate the effects of severe anemia (Hb <7 g/dL) on twin pregnancy.
Conclusions
In this study, we found that 42.6% of females with a twin pregnancy had anemia. Anemia was associated with adverse neonatal outcomes. Correction of anemia in the third trimester significantly improved the pregnancy outcomes. The findings highlight the importance of early diagnosis and treatment of anemia for pregnant women who are carrying twins. Further studies are needed to validate the present findings.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2023.33.
Financial support
This study was supported by the National Natural Science Foundation of China (82071666), the Science and Technology Department of Jiangsu Province (BK20221169), the Health Commission of Nanjing City (ZKX20021), Jiangsu Province, China. The funders had no role in study design, data collection and analysis, preparation and writing of the manuscript and its submission.
Competing interests
The authors declare that they have no competing interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the ethics committee of the hospital (2021-080-01).