Some of the receptors on the surface of cardiac muscle cells (cardiomyocytes) mediate the response of these cells to catecholamines by causing the production of the common second messenger cyclic adenosine monophosphate (cAMP). An example of such receptors are the β1- and β2-adrenergic receptors (βARs) that are heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors. Selective stimulation of these two receptor subtypes leads to distinct physiological and pathophysiological responses, but their precise location on the surface of cardiomyocytes has not been correlated with these responses. In an ingenious combination of techniques, Viacheslav Nikolaev, Alexey Moshkov, Alexander Lyon, Michele Miragoli, Pavel Novak, Helen Paur, Martin Lohse, Yuri Korchev, Sian Harding, and Julia Gorelik have mapped the function of these receptors for the first time [Reference Nikolaev, Moshkov, Lyon, Miragoli, Novak, Paur, Lohse, Korchev, Harding and Gorelik1]. (See Figure 1.)
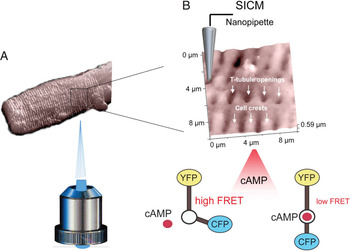
Figure 1: Principle of the SICM/FRET technique and its use to study βAR localization in cardiomyocytes. (A) SICM image of an adult rat cardiomyocytes acquired using a nanopipette from the top of the cell. The sample is positioned on an inverted epifluorescent microscope so that recordings of cellular fluorescence can be performed. (B) Inset shows a 10 × 10 μm scan of the cardiomyocyte surface with characteristic structural features (cell crests, Z-lines and T-tubule openings). The cell is expressing a FRET-based cAMP sensor Epac2-camps which reports changes in intracellular cAMP levels after local cell surface stimulation via a SICM nanopipette with β1AR or β2AR selective ligands applied either into a T-tubule opening or onto the cell crest. Binding of cAMP to the sensor causes a change in its conformation, which results in a longer distance between the fluorophores (CFP and YFP) and lower FRET signal.
The key was to combine a fluorescence resonance energy transfer (FRET)-based cAMP sensor with scanning ion conductance microscopy (SICM). The FRET sensor gave functional data that was correlated spatially with SICM. SICM is a specialized version of scanning probe microscopy in which a nano-pipette is used to visualize the three-dimensional surface topography of living cells. The resolution is equal to the inner diameter of the pipette (in the range of 50 to 100 nm). Nikolaev et al. imaged structural features of cardiomyocytes such as Z-grooves, cell crests located between the grooves, and the opening of transverse (T)-tubules on the surface; they correlated this with the different functions of the βAR subtypes. This was a breakthrough compared to immunocytochemical or electron microscopic detection of native βARs with antibodies that are limited by the low expression level of these proteins and by insufficient specificity of available antibodies.
Nikolaev et al. began by isolating cardiomyocytes from healthy adult rats and introducing an adenovirus vector into the cells that encoded a cytosolic cAMP sensor and then imaged the cell surface topography by SICM. Next, they positioned the pipette onto morphologically defined regions of the membrane and applied receptor ligands in a highly localized fashion. As they selectively stimulated the localized β1- and β2-adrenergic receptors, they measured cAMP synthesis and its accumulation in the cytosol by FRET microscopy. Control experiments determined that the ligands were locally delivered and that the topography was not significantly deformed. Furthermore, the stimulated area was in the size range of a T-tubule opening and well below the size of a sarcomere.
Selective stimulation of β1 receptors in both the region of the T-tubule or on the cell crest resulted in a robust decrease of FRET signal, reflecting the activation of cAMP synthesis by the receptors localized on both parts of the membrane. In sharp contrast, β2-cAMP was observed only after local stimulation of the T-tubule openings, but not after stimulation of the cell crest. This and additional data indicated that β2ARs are exclusively localized to the T-tubules, whereas β1ARs are present in both the cell crests and the T-tubules. Additional experiments with both β1 and β2AR knockout mice confirmed these localizations of the receptors.
Next, Nikolaev et al. studied whether βAR localization was altered in a rat model of chronic heart failure induced by myocardial infarction. Extensive experiments suggested that the redistribution of the β2AR-cAMP from the T-tubules to the cell crest in failing cardiomyocytes results in uncoupling of the β2ARs from the normal compartmentation of β2AR-cAMP signaling. Thus, in failing cells, activation of the β2ARs leads to cell-wide cAMP signal propagation patterns similar to the patterns observed for the β1ARs. Thereby the normally cardioprotective properties of the β2AR response may acquire characteristics of the β1AR response, contributing to the heart failure phenotype.
Nikolaev et al. have combined two techniques to functionally localize β1- and β2ARs on cardiomyocytes and reveal mechanisms leading to abnormal cAMP compartmentation in heart failure. These findings provide a deeper understanding of this common cardiac disease and facilitate the development of new therapeutic strategies. Furthermore, they have demonstrated the usefulness of combining techniques that provide both functional and spatial information that can have significant biological and clinical implications [2].



