Implications
This study reviews several management strategies that affect the amount of nitrogen (N) used per kg of meat produced. Genetic selection, castration and slaughter weight have an effect. Diet is still the most important factor affecting N efficiency, however. When the diet is adapted to the animal requirements, a whole body N efficiency close to 60% seems attainable for group-fed pigs. This would improve the environmental sustainability of pork production. When evaluating the effect of management strategies on N efficiency, all of these concepts need to be taken into account to avoid false conclusions.
Introduction
In contemporary pig production, ~6.3 kg N is used to raise an 8-kg piglet to a 110-kg finishing pig (own calculations based on current Belgian feeds and breeds, Table 1). While ~46% of this N is retained in the animal, the other 54% is excreted, mainly through feces and urine. Part of the excreted N can be re-used as a fertilizer but part of it is either lost to the air as ammonia (NH3), nitrous oxide (N2O) and N oxide (NOx) emissions or lost to ground and surface waters via leaching and runoff of nitrate (NO3) and other N compounds (Leip et al., Reference Leip, Weiss, Lesschen and Westhoek2014). Increasing the efficiency of converting plant protein into animal protein would decrease the environmental burden per kg pork and may improve the sustainability of pig production.
Table 1 Estimation of nitrogen input and nitrogen efficiency of a hybrid sow and her offspring (27 piglets per year over 2.4 cycles) in a commercial Belgian farm (Hybrid sow×Piétrain boar)

1 Estimation based on commercially published feed recommendations and average performances reported by Government of Flanders (Belgium).
2 Measured nitrogen content of gilts of different BW.
Nitrogen efficiency decreases ~3% (from 46% to 43%) when the sow’s production of piglets is included in the calculation (Table 1). In piglet production, N is retained in piglets (200 g N per 8 kg piglet, Table 1) and in the sow (BW gain). Most of the N is excreted to the urine and feces, yielding a N efficiency level around 24%. Relative to the fattening phase, the effect of the piglet production on N efficiency is small. For a sow producing 27 piglets per year, the sow and her piglets consume around 28.8 kg N per year, while the 27 piglets consume around 170.1 kg N to grow from 8 to 110 kg (Table 1). This may explain why efforts to reduce N excretion mainly focus on the fattening phase and why this paper focuses exclusively on the fattening phase.
Nitrogen efficiency in pork production has improved through the application of scientific knowledge gained since the 1980s; further improvements can be expected by implementing recent knowledge. The aim of the current paper is to critically evaluate existing knowledge and assumptions on strategies to maximize N efficiency in pork production. We first discuss three major management strategies frequently linked with improved N efficiency: genetic selection, castration and slaughter weight. With every management strategy, adapted feeding is important for a correct evaluation of N efficiency. Because nutrition is the most important factor affecting N excretion, we then discuss nutritional strategies to maximize N efficiency. Although individual adapted feeding may yield benefits and represents a paradigm shift in pig production that some authors claim to be necessary (Andretta et al., Reference Andretta, Pomar, Rivest, Pomar and Radunz2016), most pigs throughout the world are still housed and fed in groups. Improving the feeding practices for group-fed animals may therefore have the highest impact now and in the near future. Therefore, the main focus of this paper is on increasing efficiency in group-fed pigs. Based on the currently available scientific knowledge we estimate which level of N efficiency may be achieved with these group-fed pigs in the short term.
Calculating and expressing protein efficiency
On a fattening pig farm, piglets and nutrients can be considered as inputs. Outputs can be defined in different ways: kg live weight, kg carcass, kg lean meat or kg nutrients (e.g. kg N).
Theoretically, economic and environmental optimization of nutrient efficiency should be done per ‘animal unit’ on the farm while accounting for all trade-offs between inputs and outputs. Within a group, animals vary in their individual requirements and performances (Ferguson et al., Reference Ferguson, Gous and Emmans1997; Pomar et al., Reference Pomar, Kyriazakis, Emmans and Knap2003). Under most practical (group housing) circumstances, the individual differences between pigs cannot be measured or managed. Optimization of individual nutrient efficiency, although desirable, may not be feasible in current practice. Nevertheless, nutrient efficiency can still be improved by taking measures at the population (barn) level.
Although stochasticity should be considered when adapting livestock management strategies (Pomar et al., Reference Pomar, Kyriazakis, Emmans and Knap2003), reasoning at the level of the ‘average’ animal results in robust yet simple calculations. This ‘average’ pig is a theoretical animal whose requirements, performances and efficiency can be directly calculated from measured performances at either pen or farm level.
In this ‘average’ pig, whole body N efficiency can be defined as the amount of N retained in the body divided by the amount of N ingested by the animal. Because the major aim of raising pigs is to produce meat, a more functional approach is to use the amount of N needed to produce one kg lean meat. Because protein needs are expressed relative to lysine (LYS) (see below), we propose using standardized ileal digestible (SID) LYS/kg lean meat as a functional measure for N efficiency.
Management strategies to improve nitrogen efficiency
Genetic selection
Genetic selection may affect N efficiency of group-fed pigs via two mechanisms: first, by directly selecting for increased efficiency, and second, by selecting for homogeneous groups of pigs.
Improving feed energy efficiency is a major objective of current animal breeding programs (Shirali et al., Reference Shirali, Doeschl-Wilson, Knap, Duthie, Kanis, van Arendonk and Roehe2012). Traditionally, improving energy efficiency was obtained by selecting for a lower feed conversion ratio. However, this approach may result in a reduction of feed intake, which in turn may limit further improvement of growth (Shirali, Reference Shirali2014). Therefore, residual feed intake (RFI) has been used as measure of feed efficiency, which is theoretically independent of lean growth (Shirali, Reference Shirali2014). Residual feed intake is the difference between the amount of feed (energy) the animal eats and the amount it is expected to eat based on requirements for maintenance and production (Lefaucheur et al., Reference Lefaucheur, Lebret, Ecolan, Louveau, Damon, Prunier, Billon, Sellier and Gilbert2011).
Without changing the diet, it is clear that pigs with a low feed conversion ratio (FCR) or low residual feed intake (LRFI) consume less, and hence excrete less N per kg of gain. However, while it seems logical to assume that dietary protein efficiency can be beneficially affected by genetic selection, selection has primarily focused on energy efficiency rather than on protein efficiency. Hammond (Reference Hammond1947) stated that when environmental conditions limit the development of a character, it is not possible to select for genes that can be expressed when not hindered by environmental factors. Therefore, most breeding programs provide a diet designed to allow the animal to express its full genetic potential. When animals are fed diets where protein and amino acid (AA) level do not limit growth, increased protein efficiency is at best a side effect of selection for improved energy efficiency.
Regardless, that side effect does appear to be a reality: Moehn et al. (Reference Moehn, Ball, Fuller, Gillis and de Lange2004) observed that the rate of inevitable lysine catabolism decreases with increasing pig growth potential. A recent study reports that LRFI pigs have better protein efficiency than high RFI pigs (Cruzen et al., Reference Cruzen, Harris, Hollinger, Punt, Grubbs, Selsby, Dekkers, Gabler, Lonergan and Huff-Lonergan2013). The authors state that decreased muscle protein turnover may be an important reason for improved feed efficiency in LRFI pigs, based on measured enzyme activity. Still, in experiments with genetically different pig lines on feeds that were shown to limit growth, the marginal efficiency of protein use did not differ between breeds (Kyriazakis et al., Reference Kyriazakis, Dotas and Emmans1994; Susenbeth et al., Reference Susenbeth, Dickel, Diekenhorst and Hohler1999). Marginal efficiency can be defined as the proportion of each increment in protein intake that is retained in the body. Trials with at least two (preferably more) protein levels are needed for this. Caution is needed when interpreting differences in muscle metabolism between pigs from different genetic lines that are fed only one type of diet, because the amount of AA intake relative to their requirement may differ considerably and may therefore evoke different responses. A higher degree of protein restriction should result in more efficient use of the feed, accompanied by lower muscle protein turnover. When selecting for increased N efficiency, it might be good to use diets with AA concentrations that limit growth, as we hypothesize that these diets should favor animals that use protein more efficiently. Apart from the marginal efficiency of N use described, a higher proportion of muscle N in the body would probably be linked to the amount of meat per kg of N input and the ratio of muscle to maintenance N. Thus, the amount of SID lysine/kg feed should be higher, but the amount of SID lysine intake/kg lean meat is probably lower in lean compared with fat pigs.
Direct selection for increased N efficiency is an option but selection for lower variance in feed intake and protein accretion potential may also be a useful strategy to improve N efficiency of group-fed pigs. Ibanez-Escriche et al. (Reference Ibanez-Escriche, Varona, Sorensen and Noguera2008) stated that environmental variance of slaughter weight at 175 days in pigs may be partly genetically determined. The heritability estimates for the SD of BW at birth and at 3 weeks of age are around 0.1, hence worth selecting for (Canario et al., Reference Canario, Lundgren, Haandlykken and Rydhmer2010). With lower variation, more animals in the group can be fed adequately, thus decreasing inefficiencies in group-fed pigs (see below).
Castration
In most countries, castration of male piglets has been common practice until recently. Now societal pressure is leading many farmers to raise entire male pigs or immunocastrates (Millet et al., Reference Millet, Gielkens, De Brabander and Janssens2011a). The pig sector in the EU has committed to ban surgical castration of male piglets by 2018. Boars have higher protein deposition capacity than either gilts or barrows. In terms of feed consumption, immunocastrates can be considered boars until the second vaccination (Millet et al., Reference Millet, Gielkens, De Brabander and Janssens2011a), after which their feed intake drastically increases. Differences in N efficiency between barrows and boars are especially visible when using a reductionist approach with one type of diet characterized by adequate AA levels. For example, Van den Broeke et al. (Reference Van den Broeke, Leen, Aluwé, Ampe, Van Meensel and Millet2016) performed a study where four types of animals (boars, gilts, barrows, immunocastrates) received the same diets, formulated to fulfill the AA requirements of boars. In doing so, barrows consumed dietary protein in excess of their requirements, which was reflected in higher serum urea levels in barrows compared with boars. Similarly, a tremendous increase in serum urea level was observed after the second GnRH dosis in immunocastrates, in accordance with their increased feed intake, which also resulted in protein intake in excess of their requirements. While differences in nutrient requirements between genders are well established, little is known about gender-specific differences in marginal protein efficiency. In accordance with Moehn et al. (Reference Moehn, Ball, Fuller, Gillis and de Lange2004) who observed that the rate of inevitable lysine catabolism decreases with increasing pig growth potential, higher marginal lysine efficiency can be expected in boars compared with barrows. Moreover, as boars are leaner than barrows (Quiniou and Noblet, Reference Quiniou and Noblet1995) the ratio of muscle protein to total body protein and the ratio of protein for growth v. protein for maintenance may also be higher in boars v. barrows. Therefore, one could expect a general decrease in the level of SID lysine intake per kg lean meat when raising entire males v. barrows.
Slaughter weight
Shirali et al. (Reference Shirali, Doeschl-Wilson, Knap, Duthie, Kanis, van Arendonk and Roehe2012) stated that N excretion per BW gain rises with increasing BW. The question arises whether this is a result of decreasing marginal efficiency or non-adapted feeding. Ghimire et al. (Reference Ghimire, Pomar and Remus2016) observed no significant difference in lysine efficiency between growing and finishing pigs. Moehn et al. (Reference Moehn, Gillis, Moughan and de Lange2000 and Reference Moehn, Ball, Fuller, Gillis and de Lange2004) stated that inevitable lysine catabolism and the marginal efficiency of using available lysine is independent of BW. In contrast, according to the National Research Council (NRC, 2012), empirical results suggest that the marginal efficiency of using SID lysine intake for protein deposition decreases with increasing BW, from 0.68 at 20 kg to 0.57 at 120 kg BW. Furthermore, maintenance AA requirements increase with increasing BW. Both imply a higher need for AA per kg of lean gain as BW increases.
While reduction of slaughter weight seems to lead to improved N efficiency, there is a trade-off between the input of piglets and feed (Van Meensel et al., Reference Van Meensel, Lauwers and Van Huylenbroeck2010). Lower slaughter weights imply a higher feed efficiency but also a higher number of piglets to produce 1000 kg of pork, and a higher number of sows. Increasing the slaughter weight decreases the number of fattening rounds per year and thus lowers the number of pigs, but it does imply increased feed costs (both economic and environmental). Increased BW also increases carcass yield (Wagner et al., Reference Wagner, Schinckel, Chen, Forrest and Coe1999; Correa et al., Reference Correa, Faucitano, Laforest, Rivest, Marcoux and Gariepy2006; Serrano et al., Reference Serrano, Valencia, Fuentetaja, Lazaro and Mateos2008) without a clear effect on lean meat percentage (Correa et al., Reference Correa, Faucitano, Laforest, Rivest, Marcoux and Gariepy2006; Serrano et al., Reference Serrano, Valencia, Fuentetaja, Lazaro and Mateos2008). Therefore, pigs that are too light or too heavy may both require higher amounts of AA per kg lean gain. In simulations of Morel and Wood (Reference Morel and Wood2005), where N excretion was taken into account (in contrast to economic optimization alone) the optimal slaughter weight decreased, especially in fat genotypes (115.2 kg with economic optimization alone v. 96.6 kg when placing a large emphasis on reducing N excretion). Note, however, that the authors assumed only one type of finisher feed independent of slaughter date.
Based on the above information, the effect of increased slaughter weight on N efficiency may be overestimated in practice, caused primarily by other factors such as excess protein supply in comparison with the requirements at higher BWs.
Nutritional strategies: feeding for maximal N efficiency in group-fed pigs
The three abovementioned management strategies (genetic selection, castration and slaughter weight) affect the amount of SID lysine per kg lean meat. When using a reductionist approach (i.e. the same diet for all experimental pigs) to study the effects of these management factors, a large part of the observed differences in studies can be attributed to the diet. Obviously, when feeding barrows the same diet as gilts or when feeding 150 kg pigs the same diet as 100 kg pigs, the intrinsic differences will be exaggerated. Therefore, it is important to feed the right diet for each type of animal, both in practice and in experimental studies. To optimise N efficiency, several nutritional concepts should be taken into account. Briefly, the amount of excreted N and the route of excretion (fecal or urinary) depends on the amount of ingested N, the fraction of absorbed N, the animal’s AA requirements and the AA balance in the diet.
Meeting the requirements of group-fed pigs throughout different stages of growth
Phase feeding (adapting the dietary AA content to the physiological needs of an animal during its different life stages) is a recognized strategy to lower N inputs and outputs while maintaining maximal performance (Han et al., Reference Han, Lee, Kim, Kim, Kim and Paik2000). Terms such as one-phase, three-phase and multiphase feeding all share the same aim of providing the pig with sufficient nutrients at each time point (Figure 1a). As the number of phases increases, theoretically the amount of ingested protein will decrease and will therefore better match the animal’s nutritional requirements over time. Pomar et al. (Reference Pomar, Pomar, Dubeau, Joannopoulos and Dussault2014) estimated a 12% reduction in N excretion by switching from three-phase feeding to daily multiphase feeding in individually fed pigs. Of course, the amount of reduction depends on the control treatment to which it is compared. In their study, whole body N efficiency was 37% on three-phase feeding and 40% on the daily multiphase feeding strategy. Andretta et al. (Reference Andretta, Pomar, Rivest, Pomar and Radunz2016) reached up to 57% N efficiency in individually fed pigs given a daily-phase feeding program designed to meet 80% of the estimated nutritional requirements, compared with 45% in a three-phase feeding program. In a recent trial at Flanders Research Institute for Agriculture, Fisheries and Food (ILVO, Melle, Belgium), 54% N efficiency was obtained in group-fed boars in a three-phase feeding system with diets formulated in line with commercial practice (Van den Broeke et al., Reference Van den Broeke, Leen, Aluwé, Van Meensel and Millet2017). However, there is a large difference between theoretical and practical phase-feeding systems. While, in theory, the AA levels proposed for three-phase feeding are sufficient at the beginning and in excess at the end of the feeding phase (Figure 1a), in the commercial three-phase feeding system nutrients are limiting at the beginning and in excess at the end of the feeding phase (Figure 1b).
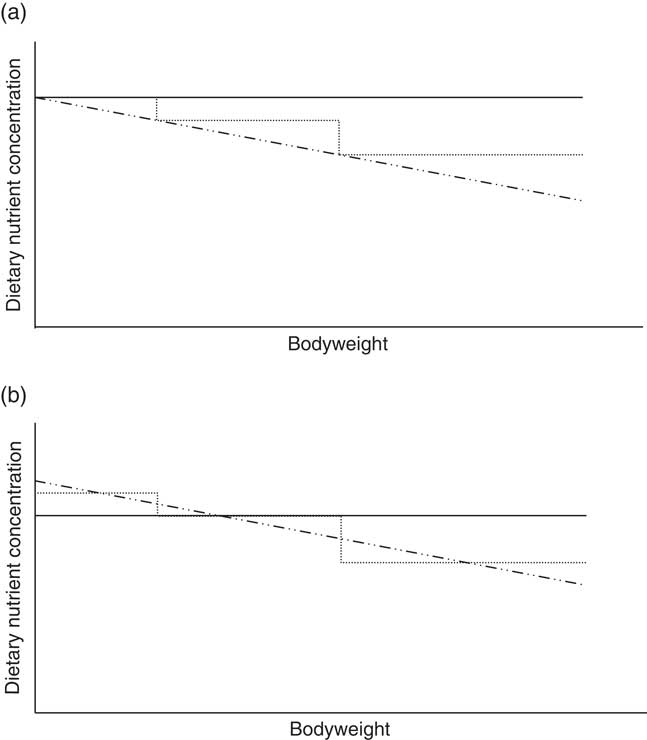
Figure 1 Schematic illustration of the dietary nutrient concentration of three feeding systems differing in the subdivision of feeding phases: one-phase (—), three-phase (.....) and multiphase (–..–) feeding. The figure on the top (a) shows the theoretical concept, while the figure on the bottom (b) shows the translation into practice.
As stated above, group housing is common practice for piglets and fattening pigs on farms. Pigs are fed and housed per age group. However, individual pigs of the same age group also differ in protein deposition capacity and hence may differ in AA requirement. This variation is important when formulating recommendations for feeding pigs in groups and may explain differences in research results on individual or group level. When feeding to evoke optimal responses of a group of pigs, many of the pigs receive excess nutrients. Therefore, even when feeding below the requirement for optimal performance of a group in a commercial three-phase feeding strategie, a considerable number of the pigs in the group are still likely to have their nutritional needs met at all times. Furthermore, compensatory growth mechanisms may be at play: several authors report that pigs subjected to early dietary AA restrictions may compensate and decrease N excretion during both the restriction and re-alimentation phases (Fabian et al., Reference Fabian, Chiba, Frobish, McElhenney, Kuhlers and Nadarajah2004; Millet et al., Reference Millet, Langendries, Aluwé and De Brabander2011b; Millet and Aluwé, Reference Millet and Aluwé2014). Because results among studies do not always agree (De Greef et al., Reference De Greef, Kemp and Verstegen1992; Chiba et al., Reference Chiba, Kuhlers, Frobish, Jungst, Huff-Lonergan, Lonergan and Cummins2002), it is difficult to generate general recommendations for maintaining profitability while minimizing N excretion through short-term dietary protein deficiencies. Despite clear compensatory growth responses after AA restriction in gilts (Millet et al., Reference Millet, Langendries, Aluwé and De Brabander2011b) and barrows (Millet and Aluwé, Reference Millet and Aluwé2014), the best (numerical) feed efficiency was still observed in the pigs that had never been restricted, while the highest lysine efficiency was seen in piglets that were fed an AA restricted diet throughout their life. In these studies, the lowest amount of total lysine per kg lean meat gain reached was 45.4 g in barrows and 42.1 g in gilts. Similar improvements at low lysine levels were found by Ghimire et al. (Reference Ghimire, Pomar and Remus2016), where higher efficiency of lysine utilization was observed at lower levels of lysine intake. Moehn et al. (Reference Moehn, Ball, Fuller, Gillis and de Lange2004) only observed a reduced rate of lysine catabolism at the lowest lysine intake level (40% below requirements). Those two studies used data on individual piglets. As stated above, feeding pigs below the group optimum may increase lysine efficiency by decreasing the variance in lysine utilization. In conclusion, daily multiphase feeding adapted to the individual animal’s needs is probably the most efficient in terms of N efficiency. For animals housed in groups, phase feeding appears to be required for optimizing N efficiency on group level. When combined with periods of temporary AA restriction, this efficiency can be further improved.
Optimal standardized ileal digestible lysine : digestible crude protein ratio
The increasing availability of feed grade AA makes it possible to decrease the CP content in the diet. While it is generally accepted that animals need AA rather than CP, the question remains whether there is a lower limit to protein provision. Wu (Reference Wu2014) stated that minimal levels are also required for non-essential AA. But N itself can also be limiting: Mansilla et al. (Reference Mansilla, Columbus, Htoo and de Lange2015) showed that N absorbed from the large intestine can be used when animals are fed diets deficient in dispensable AA nitrogen. Some studies have been performed on the optimal essential : total N ratio (Mitchell et al., Reference Mitchell, Becker, Harmon, Norton and Jensen1968; Heger et al., Reference Heger, Mengesha and Vodehnal1998; Lenis et al., Reference Lenis, van Diepen, Bikker, Jongbloed and van der Meulen1999). However, essential AA are only essential up to the point where they no longer limit growth. Essential AA in excess can be deaminated and utilized for the synthesis of non-essential AA (Lenis et al., Reference Lenis, van Diepen, Bikker, Jongbloed and van der Meulen1999). Therefore, the ratio of SID lysine to apparent total tract digestible (ATTD) CP may be a more helpful measure to improve N efficiency; 25 years ago, Henry and Dourmad (Reference Henry and Dourmad1993) suggested that the crude lysine : protein ratio should not exceed 0.065 to 0.068. This ratio was suggested to limit the risk for deficiencies in non-essential AA or in essential AA that were not taken into account. Since then, a large body of work has further clarified AA requirements. As knowledge increases about the requirement of all the essential AA, it may become possible to decrease dietary CP content even further. Recently, several studies have tested decreases of CP level while maintaining (SID) lysine (LYS) level (Table 2). When the corresponding SID LYS : CP ratio was calculated, the maximal ratio varied between 0.062 and 0.070 (total lysine: CP between 0.068 and 0.076). Using this maximum in feed formulation may enable a decrease in CP during the growing-finishing period. If this ratio is used during the piglet phase (first weeks after weaning), the CP level may determine the SID LYS level. Indeed, a decreased CP level in piglet rations helps to maintain intestinal health (Nyachoti et al., Reference Nyachoti, Omogbenigun, Rademacher and Blank2006). In two recent trials (Millet et al., Reference Millet, Aluwé, Le Gall, Corrent, Lambert, De Sutter, Ampe and De Campeneere2017), performances improved linearly with an SID LYS level between 8.5 and 13.5 and a corresponding CP level varying between 201 and 210 g/kg. In contrast, when CP was fixed at 180 g/kg, the SID LYS level for optimal FCR was 11.4 based on a linear plateau model and 12.9 based on a quadratic plateau model. Assuming that CP was limiting performance at the highest SID LYS levels, this would yield a maximal SID LYS : CP level of 0.064 or 0.072 (Millet et al., Reference Millet, Aluwé, Le Gall, Corrent, Lambert, De Sutter, Ampe and De Campeneere2017, Table 2). Further research is needed to determine the maximal SID LYS : CP level that does not negatively affect performance in different growth phases. Given the current knowledge, a ratio of 0.063 (0.07 LYS : CP) may be a safe choice. As N absorbed from the large intestine can also be used (Mansilla et al., Reference Mansilla, Columbus, Htoo and de Lange2015), ideally ATTD CP should be determined in these trials and the maximum expressed as SID LYS : ATTD CP. Assuming an ATTD CP digestibility of 80%, with the studies mentioned in Table 2, the calculated SID LYS : ATTD CP in the studies mentioned in Table 2 would be between 0.077 and 0.087.
Table 2 Maximal dietary standardized ileal digestible (SID)Footnote 1 lysine : CP ratios reported in scientific literature that can be used without negative effects on performance

1 The first five studies have been performed with a fixed SID LYS content and varying CP level.
2 Values in italics have been estimated based on following assumptions: SID LYS : LYS= 0.9; apparent total tract digestible (ATTD) CP/CP=0.8.
3 In the study of Millet et al. (Reference Millet, Aluwé, Le Gall, Corrent, Lambert, De Sutter, Ampe and De Campeneere2017), CP was fixed at 180 g/kg and SID LYS varied.
Optimal amino acid balance
Animal protein requirements are based on intake of a complete set of AA instead of CP. AAs given in excess are deaminated and the resulting urea is excreted in the urine (van Milgen and Dourmad, Reference van Milgen and Dourmad2015). Decreasing the dietary CP content while maintaining optimal SID AA concentrations has been proven successful to reduce N input per kg of lean meat gain. This can be obtained by combining highly digestible AA sources and formulation of feeds for an optimal AA composition. Single AA deficiencies lead to inefficient use of the other AA, which are in turn deaminated and excreted in the urine, causing suboptimal growth. This knowledge has led to the well-known concept of ‘ideal protein,’ which varies with physiological state and level of productivity of the animal (NRC, 2012) and has been extensively discussed elsewhere (Boisen et al., Reference Boisen, Hvelplund and Weisbjerg2000; van Milgen and Dourmad, Reference van Milgen and Dourmad2015). Now that feed grade crystalline AA are available, feed can be formulated close to the ideal AA pattern to be used in research and commercial practice. Although the ideal protein concept is clear, the methods to deduce the optimal balance between AA are still under discussion and different methodologies still yield (slightly) different results.
Estimation of maximal nitrogen efficiency attainable in group-fed pigs
Estimations of the maximal N efficiency for producing marketable pork meat were calculated from data presented in Table 3. With 45 g of LYS/kg lean meat gain as value we observed in several studies (Millet et al., Reference Millet, Langendries, Aluwé and De Brabander2011b; Millet and Aluwé, Reference Millet and Aluwé2014) and 0.07 as a safe LYS : CP ratio, we calculate an N efficiency of 57% for pigs between 8 and 110 kg (total lysine was used in accordance with other studies; Table 3). With further research, the amount of lysine per kg lean meat may decrease and the LYS : CP ratio may be increased, leading to an even higher efficiency. The availability of different protein sources or commercially available AA may interfere with reaching a 0.07 LYS : CP ratio while maintaining a correct AA balance. On the other hand, when applying the management choices of precision feeding, genetic selection and raising entire male pigs, the amount of lysine per kg lean meat gain can probably be reduced even further. More research is needed before this can be achieved. Furthermore, economic studies related to the management choices and the cost of technology are required. Although the proposed efficiency is much higher than the 33% reported for growing pigs under practical circumstances in the Netherlands, France and Denmark (Dourmad et al., Reference Dourmad, Seve, Latimier, Boisen, Fernandez, van der Peet-Schwering and Jongbloed1999) and the 46% estimated for contemporary Belgian pig production (Table 1), N efficiency close to 60% appears to be achievable in the near future in group-fed fattening pigs.
Table 3 Estimate of nitrogen efficiency attainable with group-fed pigs

1 Grams of lysine needed per kg of lean meat growth that have been observed in studies at ILVO. This is probably an overestimation of the minimum.
2 Maximal dietary lysine : CP ratio that still allows maximal performance reported in literature ranges between 0.068 and 0.079. In this calculation, 0.070 was chosen as a conservative estimate.
3 Nitrogen content of a 110 kg pig minus nitrogen content of an 8 kg piglet.
Acknowledgments
Thanks to Miriam Levenson for language correction.






