Onset of menarche is believed to represent an important psychosocial and/or biological transition point for the female rise in depression, Reference Patton, Hibbert, Carlin, Shao, Rosier and Caust1 with studies reporting higher levels of depressive symptoms in girls who have experienced menarche compared with those who have not. Reference Patton, Hibbert, Carlin, Shao, Rosier and Caust1–Reference Hayward, Gotlib, Schraedley and Litt3 It has also been suggested that the timing of menarche relative to one's peers may be a critical factor for the emergence of depressive symptoms, Reference Rierdan and Koff4,Reference Caspi and Moffitt5 with studies reporting that earlier timing of menarche is associated with increased depressive symptoms Reference Hayward, Gotlib, Schraedley and Litt3–Reference Kaltiala-Heino, Marttunen, Rantanen and Rimpelä7 and depression. Reference Stice, Presnell and Bearman8 However, to our knowledge, only three of these studies prospectively examined the emergence of depressive symptoms Reference Caspi and Moffitt5,Reference Ge, Conger and Elder6 or depression Reference Stice, Presnell and Bearman8 from late childhood into adolescence; two were limited by a relatively small sample size Reference Caspi and Moffitt5,Reference Ge, Conger and Elder6 and one examined a sample comprising 11- to 15-year-olds for a period of only 2 years. Reference Stice, Presnell and Bearman8 Two of these studies Reference Caspi and Moffitt5,Reference Stice, Presnell and Bearman8 are limited by retrospective self-reports of age at menarche, raising the risk of recall bias, particularly among older females where more time has elapsed since the onset of menarche. Only in one study Reference Ge, Conger and Elder6 were self-reports of age at onset of menarche validated against year-by-year menstruation experience using four waves of data collection, but this investigation was based on a small sample. Only one of these studies adjusted for potential confounding factors that are associated with both earlier timing of menarche and increased levels of depressive symptoms. Reference Stice, Presnell and Bearman8 Socioeconomic disadvantage and adverse life events (e.g. family breakdown) are well-established risk factors for depressive symptoms in adolescence Reference Hazel, Hammen, Brennan and Najman9,Reference Lewinsohn, Roberts, Seeley, Rohde, Gotlib and Hops10 and have also been linked to earlier timing of menarche. Reference Belsky, Steinberg, Houts, Friedman, DeHart and Cauffman11 Body mass index (BMI) is also associated with level of depressive symptoms Reference Cortese, Falissard, Angriman, Pigaiani, Banzato and Bogoni12,Reference Needham and Crosnoe13 and timing of menarche. Reference Lee, Appugliese, Kaciroti, Corwyn, Bradley and Lumeng14
The current study, based on a large contemporary birth cohort from south-west England, examines whether girls who experience earlier timing of menarche than their peers have higher levels of depressive symptoms in adolescence. We aim to address the limitations of previous studies by using a large sample size, repeated measurements of depressive symptoms from late childhood into adolescence, frequent contemporaneous reports of age at onset of menarche and by adjusting for potential confounding factors.
Method
Participants
The sample comprised participants from the Avon Longitudinal Study of Parents and Children (ALSPAC), an ongoing population-based study investigating a wide range of environmental and other influences on the health and development of children. Reference Golding, Pembrey and Jones15 The primary sources of data collection are via self-completion questionnaires and direct assessment at research clinics. Ethical approval for the study was obtained from the local research ethics committees and the study is monitored by the ALSPAC Law and Ethics Committee. More information on the ALSPAC study is available on the website: www.bristol.ac.uk/alspac/.
Pregnant women resident in the former Avon Health Authority in south-west England, having an estimated date of delivery between 1 April 1991 and 31 December 1992, were invited to take part, resulting in a cohort of 14 541 pregnancies and 13 988 children (including 6764 girls) alive at 12 months of age. When the oldest children were approximately 7 years of age, an attempt was made to increase the size of the initial sample with eligible cases that did not join the cohort at the outset. As a result, when considering variables collected from this point onwards there are data available for more than the 14 541 pregnancies mentioned above. The number of active new cases not in the initial sample that are currently represented in the data resource is 542 (294 females). Hence, the starting sample for the current study was n = 7058 (6764 + 294), comprising 3426 girls who provided data on age at onset of menarche. Figure 1 shows how the various samples considered were derived from the starting sample (see online supplement for further details on derivation of these samples).
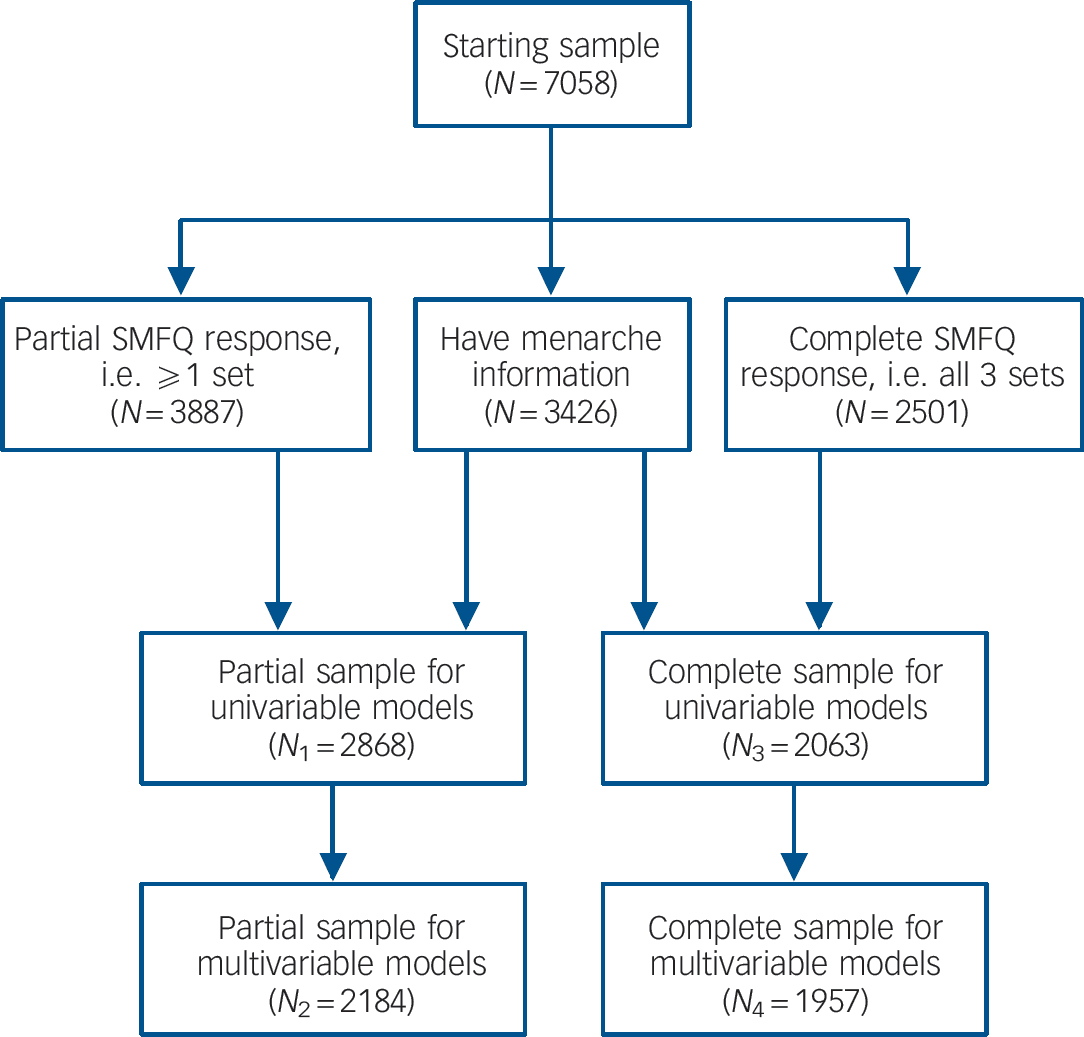
Fig. 1 Flow chart depicting sample derivation. SMFQ, Short Mood and Feelings Questionnaire.
Measures
Depressive symptoms
The Short Mood and Feelings Questionnaire (SMFQ) Reference Angold, Costello, Pickles, Messer, Winder and Silva16 enquires about the occurrence of low mood and psychological correlates (low self-esteem and self-worth) that should define the potential population continuum of subthreshold/screening symptoms of depression (hereafter referred to as depressive symptoms). Responses on three levels (‘true’, ‘sometimes’, ‘not at all’) are made for each symptom, based on experiences over the past 2 weeks. The SMFQ has been administered to the study children on three occasions during visits to research clinics at mean ages 10 years 8 months, 12 years 10 months and 13 years 10 months (hereafter referred to as 10.5, 13 and 14 years). The internal construct validity of a single continuum of severity of depressive symptoms has been supported in a UK community sample in which the items were subjected to unidimensional item-response modelling after simple binary recoding. Reference Sharp, Goodyer and Croudace17
Timing of menarche
Age at onset of menarche was derived primarily from a series of six postal questionnaires pertaining to pubertal development administered approximately annually to the mother from the time their daughter was 8 years old. The relevant questions were: ‘Has your daughter started her menstrual periods yet’? If yes: ‘How old was your daughter when she had her first period’? As one would expect, the actual respondent(s) changed as the study child became older. Earlier questionnaires were completed mainly by the study mother (e.g. 84% at age 8 years), but once the study children reached their teens, around half of questionnaires were completed by the study child or study child/mother together. These data were bolstered by information collected by a questionnaire administered during a research clinic at age 13 years, which asked: ‘Have you started your periods yet’? If yes, ‘When did you have your first period’?
For the majority of girls, the source of data on age at menarche was the questionnaire (n = 3160, 92%). The first-reported age at onset was used, as these data ought to be the most accurate and least affected by recall bias. The mean age at onset of menarche for girls in the ALSPAC cohort was 12 years 6 months (s.d. = 1.04, range 7.6–15), corresponding closely to that reported in other large contemporary studies based on samples from the USA and Western European countries. Reference Parent, Teilmann, Juul, Skakkebaek, Toppari and Bourguignon18 A three-level categorical variable summarising timing of menarche was derived from these data. Our primary interest was in early onset; here we define early to be before the age of 11 years 6 months (1 standard deviation below the mean age of 12.5 years; 16.7% of the sample). Previous studies have defined early menarche in a similar way. Reference Stice, Presnell and Bearman8,Reference Tam, de Zegher, Garnett, Baur and Cowell19 A late-onset group was derived in a similar manner, with 17.7% of the girls in this sample experiencing menarche at or after 13 years 6 months. The remaining group (65.6%) was defined as normative onset and employed as a reference group in our analyses.
Potential confounders of the association between early menarche and depressive symptoms
Absence of biological father
Questionnaires given to mothers at regular intervals since the birth of the study child ask whether their partner is the natural father of the study child and if not, how old the study child was when the natural father stopped living with the family. From this data we derived a categorical variable (father present; father left when the study child was 5 years or over; father left during the first 5 years of study child's life) because there is evidence that family breakdown during the first 5 years of life is the most sensitive period for influencing subsequent timing of menarche. Reference Belsky, Steinberg, Houts, Friedman, DeHart and Cauffman11
Socioeconomic disadvantage
Mothers have been asked to report on the occurrence of major financial problems at regular intervals since the birth of the study child, through self-completion questionnaires. We derived a categorical variable with four levels (no financial problems; financial problems only experienced when study child was ≤5 years; financial problems experienced when study child was 5–9 years; financial problems experienced from 0 to 9 years) because there is evidence among adolescents that continued exposure to adversities more proximal to the onset of depression largely accounts for the association between early adversity (first 5 years of life) and depression. Reference Hazel, Hammen, Brennan and Najman9
Body mass index
For the majority of girls (89.5% of those who provided menarche data), BMI was obtained from height and weight measurements taken systematically during a research clinic attended at age 9 years. The remaining girls reported height and weight in a questionnaire administered at age 9 years (correlation coefficient for clinic and questionnaire BMI 0.846, P<0.001, n = 1454; mean BMI 18.1, s.d. = 3.1, range 11.5–39.6). A categorical BMI variable with 3 levels (lowest BMI <15; mid-range BMI 15–20; highest BMI >20) was derived because studies have reported both linear (increased levels of depressive symptoms in adolescents with a higher BMI) Reference Stice, Presnell and Bearman8,Reference Needham and Crosnoe13 and curvilinear relationships (higher levels of depressive symptoms in both heavier-than-average and underweight girls) Reference Cortese, Falissard, Angriman, Pigaiani, Banzato and Bogoni12 between BMI and depressive symptoms.
Statistical analysis
First, we compared the depressive symptom sum-scores at each of the three time points (T 1 = 10.5 years; T 2 = 13 years; T 3 = 14 years) across groups defined by timing of menarche (early, normative and late) using simple ANOVA. Second, the association between timing of menarche and depressive symptoms was assessed within a structural equation model using Mplus version 5.2 run on Windows XP (www.statmodel.com/ugexcerpts/shtml).
There are two main reasons for favouring a structural equation model approach based on the individual SMFQ items. Using a structural equation model allows derivation of a normally distributed latent trait underlying the observed SMFQ scores. In contrast, the majority of previous studies using the SMFQ have used a binary measure of depressive symptoms because summing the responses to the SMFQ items yield a highly skewed scale. For example, a cut-point of 11 on the SMFQ has been applied in some studies based on community samples. Reference Angold, Erkanli, Silberg, Eaves and Costello20,Reference Patton, Olsson, Bond, Toumbourou, Carlin and Hemphill21 However, there is a lack of agreement in the literature with regard to a suitable cut-off for the SMFQ, and such a cut-point would be somewhat arbitrary in our data-set without our own assessment of external validity. Although a categorical approach is valuable for clinical practice and applications, there is evidence that depression may be better represented as a continuum Reference Kraemer, Noda and O'Hara22 and that a dimensional approach should be favoured over a categorical approach for hypothesis testing. Reference Hankin, Fraley, Lahey and Waldman23 Second, the use of item responses within a structural equation model setting permits us to examine the extent of measurement invariance across the three time points. The establishment of some degree of measurement invariance through constraining the model parameters across time is an important first step prior to the examination of longitudinal change in any latent construct.
The measurement model
Depressive symptoms at the three time points were modelled as three correlated continuous latent factors using a confirmatory factor analysis (CFA) model with each factor being measured by 12 categorical items from the SMFQ (Fig. 2). Unconditional models (SMFQ in the absence of covariates) were derived initially for those who had completed the 12 SMFQ items at all three time points (complete set of 36 SMFQ items, n = 2501) and repeated using the larger sample comprising those who had complete SMFQ data from at least one time point (n = 3887). Further details on the derivation of the measurement model can be found in the online supplement.
The structural model
Dummy variables representing timing of menarche were added to the CFA model described above to assess differences in the levels of the latent factor of depressive symptoms at each of the three time points. We report the differences in symptom levels in terms of standard deviation effect sizes (Cohen's d) taking the metric from the first time point (10.5 years) (see online supplement). Regression-style adjustments were then made for the confounders. In a second model, the effect of timing of menarche on depressive symptoms was further adjusted for the previous depressive symptom measure. Similar to an ANCOVA model, this allows us to assess how change in depressive symptoms between time points relates to timing of menarche.
Results
Table 1 shows how timing of menarche and the confounding variables vary across four subsamples of the data-set as depicted in Fig. 1. There are clear trends apparent in these figures, for example the prevalence of girls reporting early-onset menarche changed as the sample reduced in size. Hence we decided to assess any change in the conclusions across these different samples.
Table 1 Comparison of timing of menarche and the confounding variables in different samples

| n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Starting sample (N = 7058) | Partial sample for univariable models (N 1 = 2868) | Partial sample for multivariable models (N 2 = 2184) | Complete sample for univariable models (N 3 = 2063) | Complete sample for multivariable models (N 4 = 1957) | ||||||
| Onset of menarche | ||||||||||
| Early | 572 | (16.7) | 450 | (15.7) | 324 | (14.8) | 304 | (14.7) | 288 | (14.7) |
| Normative | 2246 | (65.6) | 1902 | (66.3) | 1423 | (65.2) | 1351 | (65.5) | 1273 | (65.1) |
| Late | 608 | (17.7) | 516 | (18.0) | 437 | (20.0) | 408 | (19.8) | 396 | (20.2) |
| Father absence | ||||||||||
| Father present | 3972 | (75.0) | 2159 | (79.4) | 1771 | (81.1) | 1635 | (81.7) | 1602 | (81.9) |
| Father left during first 5 years | 961 | (18.2) | 381 | (14.0) | 268 | (12.3) | 240 | (12.0) | 234 | (12.0) |
| Father left when child was ≥5 | 361 | (6.8) | 188 | (6.9) | 145 | (6.6) | 127 | (6.3) | 121 | (6.2) |
| Major financial problems | ||||||||||
| None | 3692 | (67.5) | 1875 | (67.8) | 1502 | (68.8) | 1391 | (69.0) | 1349 | (68.9) |
| Before the age of 5 years | 1173 | (21.5) | 566 | (20.5) | 435 | (19.9) | 399 | (19.8) | 387 | (19.8) |
| Between 5–9 years | 201 | (3.7) | 105 | (3.8) | 77 | (3.5) | 76 | (3.8) | 72 | (3.7) |
| Throughout 0–9 years (persistent) | 400 | (7.3) | 218 | (7.9) | 170 | (7.8) | 151 | (7.5) | 149 | (7.6) |
| Body mass index | ||||||||||
| Up to 15 | 614 | (14.0) | 340 | (12.6) | 288 | (13.2) | 264 | (13.1) | 259 | (13.2) |
| 15 to 20 | 2876 | (65.5) | 1768 | (65.5) | 1442 | (66.0) | 1347 | (66.6) | 1301 | (66.5) |
| Over 20 | 902 | (20.5) | 592 | (21.9) | 454 | (20.8) | 412 | (20.4) | 397 | (20.3) |

Fig. 2 Confirmatory factor analysis model. Fac, factor; BMI, body mass index.
Crude assessment of the association between timing of menarche and depressive symptom sum-scores
Before moving on to the symptom scales derived from the measurement model, our initial analysis used the sample (N 3 = 2063) with complete data on depressive symptoms and timing of menarche.Figure 3 displays mean symptom scores on the SMFQ by timing of menarche. The ANOVA results showed evidence for differences in level of depressive symptoms by timing of menarche at both T 2 (13 years; F = 8.06, P<0.001) and T 3 (14 years; F = 11.97, P<0.001), but not at T 1 (10.5 years; F = 0.19, P = 0.823).
Timing of menarche – unadjusted differences in latent depressive symptoms
Using our chosen measurement model (see online supplement), we assessed the unadjusted association between timing of menarche and depressive symptoms (Table 2) and compared results across the four subsamples. Results indicate differences in depressive symptoms across the three levels of the menarche variable, with all estimates referenced against the group with normative timing of menarche. The depressive symptoms scale has been standardised to the metric of the first time point (10.5 years), hence differences are in standard deviations and the magnitude of estimates can be compared across time points. Findings were consistent with the crude data (Fig. 3) and conclusions were unaffected by the sample chosen. There is good evidence for differences in level of depressive symptoms according to timing of menarche at both 13 and 14 years. At 13 years the early menarche group has moderately higher symptom levels than the other two groups. By 14 years there is clear, although again moderate, separation between all three groups.
Table 2 Unadjusted estimates of differences in depressive symptoms across timing of menarche groups

| N 1 = 2868a | N 2 = 2184b | N 3 = 2063c | N 4 = 1957d | |||||
|---|---|---|---|---|---|---|---|---|
| Timing of menarche | d (95% CI) | P | d (95% CI) | P | d (95% CI) | P | d (95% CI) | P |
| T 1 (10.5 years) | 0.89 | 0.81 | 0.79 | 0.78 | ||||
| Early | 0.02 (–0.11 to 0.15) | 0.05 (–0.10 to 0.20) | 0.05 (–0.10 to 0.20) | 0.05 (–0.10 to 0.21) | ||||
| Normative | 0.00 ref | 0.00 ref | 0.00 ref | 0.00 ref | ||||
| Late | 0.03 (–0.09 to 0.15) | 0.02 (–0.12 to 0.15) | 0.03 (–0.11 to 0.16) | 0.03 (–0.11 to 0.16) | ||||
| T 2 (13 years) | <0.001 | 0.003 | 0.004 | 0.010 | ||||
| Early | 0.26 (0.13 to 0.40) | 0.27 (0.11 to 0.42) | 0.26 (0.10 to 0.42) | 0.24 (0.08 to 0.41) | ||||
| Normative | 0.00 ref | 0.00 ref | 0.00 ref | 0.00 ref | ||||
| Late | – 0.02 (–0.15 to 0.11) | – 0.02 (–0.16 to 0.12) | 0.00 (–0.14 to 0.14) | – 0.00 (–0.15 to 0.14) | ||||
| T 3 (14 years) | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Early | 0.24 (0.09 to 0.38) | 0.21 (0.07 to 0.38) | 0.23 (0.07 to 0.38) | 0.22 (0.06 to 0.38) | ||||
| Normative | 0.00 ref | 0.00 ref | 0.00 ref | 0.00 ref | ||||
| Late | – 0.18 (–0.31 to –0.05) | – 0.21 (–0.35 to –0.07) | – 0.22 (–0.36 to –0.08) | – 0.23 (–0.37 to –0.09) | ||||

Fig. 3 Depressive symptom sum-scores at three time points across groups defined by timing of menarche (early, normative and late). SMFQ, Short Mood and Feelings Questionnaire.
Timing of menarche – adjusted differences in latent depressive symptoms
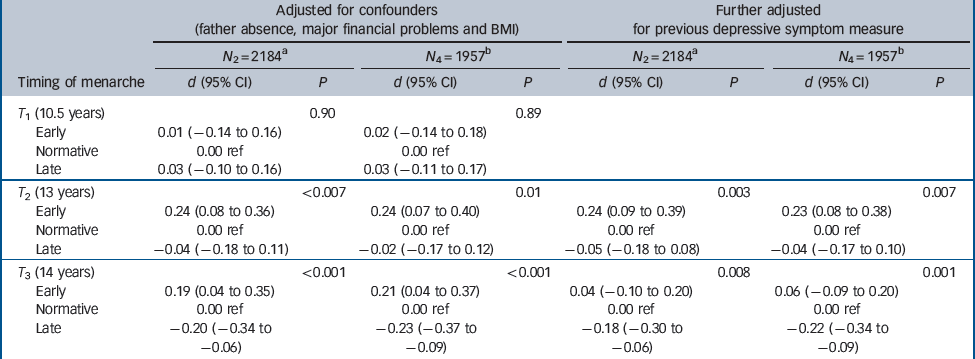
Table 3 shows the results for the adjusted models fitted in samples N 2 (n = 2184) and N 4 (n = 1957). We examined these different options to see if there was any impact on the conclusions. Following adjustment for potential confounders (socioeconomic disadvantage, father absence, high BMI) (samples N 2 and N 4), there was still a consistent association between timing of menarche and depressive symptoms.
Table 3 Adjusted estimates of differences in depressive symptoms across timing of menarche groups
| Adjusted for confounders (father absence, major financial problems and BMI) | Further adjusted for previous depressive symptom measure | |||||||
|---|---|---|---|---|---|---|---|---|
| N 2 = 2184a | N 4 = 1957b | N 2 = 2184a | N 4 = 1957b | |||||
| Timing of menarche | d (95% CI) | P | d (95% CI) | P | d (95% CI) | P | d (95% CI) | P |
| T 1 (10.5 years) | 0.90 | 0.89 | ||||||
| Early | 0.01 (–0.14 to 0.16) | 0.02 (–0.14 to 0.18) | ||||||
| Normative | 0.00 ref | 0.00 ref | ||||||
| Late | 0.03 (–0.10 to 0.16) | 0.03 (–0.11 to 0.17) | ||||||
| T 2 (13 years) | <0.007 | 0.01 | 0.003 | 0.007 | ||||
| Early | 0.24 (0.08 to 0.36) | 0.24 (0.07 to 0.40) | 0.24 (0.09 to 0.39) | 0.23 (0.08 to 0.38) | ||||
| Normative | 0.00 ref | 0.00 ref | 0.00 ref | 0.00 ref | ||||
| Late | – 0.04 (–0.18 to 0.11) | – 0.02 (–0.17 to 0.12) | – 0.05 (–0.18 to 0.08) | – 0.04 (–0.17 to 0.10) | ||||
| T 3 (14 years) | <0.001 | <0.001 | 0.008 | 0.001 | ||||
| Early | 0.19 (0.04 to 0.35) | 0.21 (0.04 to 0.37) | 0.04 (–0.10 to 0.20) | 0.06 (–0.09 to 0.20) | ||||
| Normative | 0.00 ref | 0.00 ref | 0.00 ref | 0.00 ref | ||||
| Late | – 0.20 (–0.34 to –0.06) | – 0.23 (–0.37 to –0.09) | – 0.18 (–0.30 to –0.06) | – 0.22 (–0.34 to –0.09) | ||||
We then examined differences in later symptoms across the timing of menarche groups, allowing for covariance for prior symptom level. Between 10.5 and 13 years there is an increase in level of depressive symptoms in the early menarche group compared with the others. Between 13 and 14 years there is no evidence for a further increase in depressive symptoms in the early menarche compared with the normative group. In contrast, the change in depressive symptoms is less marked in the late menarche group.
Discussion
Main findings
We found evidence for an association between earlier menarche and increased levels of depressive symptoms in mid-adolescence. The difference in level of depressive symptoms in girls with early compared with normative or late menarche emerged at around age 13 years and persisted into mid-adolescence (around 14 years). It is notable that girls with early menarche initially experienced the earliest rise in level of depressive symptoms from late childhood (10.5 years) to early adolescence (13 years) compared with the other groups, but between 13 and 14 years there was no further divergence in level of symptoms between the normative and early-onset groups. By age 14, there was separation between all three groups, with the early menarche group having the highest level of depressive symptoms, followed by normative, then late onset – an apparent dose–response relationship. Low levels of depressive symptoms in the late-onset group are consistent with a previous suggestion that later maturation may be protective against psychological distress in adolescent girls. Reference Ge, Conger and Elder6 Even after taking into account well-established shared risk factors for depressive symptoms and earlier menarche (socioeconomic disadvantage, father absence and high BMI), there is still evidence for an independent association between timing of menarche and depressive symptoms.
Strengths and limitations
The current study has a number of strengths, including a large sample size, longitudinal design, repeated measures of self-reported depressive symptoms from late childhood to adolescence, regular administration of questionnaires on pubertal development (thus reducing the chance of recall bias about age at onset of menarche) and availability of data on potential confounding factors. A limitation of this study relates to sample attrition and this has important implications for the internal validity of the study. Sample attrition in ALSPAC is strongly associated with socioeconomic disadvantage. However, our results were consistent over the different samples employed, so we feel that the conclusions are robust in spite of the differential drop out.
Possible explanations for the link between early menarche and increased level of depressive symptoms
The overall findings suggest that the increase in reported levels of depressive symptoms occurs at a younger age in girls who experience earlier menarche and underlines the importance of pubertal timing as a risk factor for the emergence of depressive symptoms in this sample of adolescents. Consistent with previous studies, the current findings lend support to the early timing hypothesis, which predicts that girls who mature earlier than their peers are more vulnerable to psychological distress. Reference Caspi and Moffitt5,Reference Ge, Conger and Elder6 To explain the link between early menarche and psychological distress it has been suggested that early maturation in girls is associated with psychosocial sequelae that may increase the risk for developing depressive symptoms in adolescence. The transition into puberty is a critical developmental period associated with biological, cognitive and social changes, Reference Conger, McCarty, Yang, Lahey and Kropp24 including increased conflicts with parents, development of romantic relationships, changes in body image and fluctuating hormone levels. These changes may have a more adverse impact on girls who mature at an earlier age than their peers. From a developmental deviance perspective, early maturing girls may feel isolated and faced with demands that are inconsistent with their level of cognitive and emotional development. Reference Caspi and Moffitt5,Reference Conger, McCarty, Yang, Lahey and Kropp24,Reference Petersen, Taylor and Adelson25
An epigenetic model of risk for depression Reference McGowan and Kato26,Reference Meaney27 may provide a further interpretative framework for the current findings. There is increasing evidence for gene–environment interactions associated with individual differences in depressive risk. Reference Caspi and Moffit28 Increased environmental stressors associated with early maturation may lead to the emergence of depressive symptoms in genetically liable individuals.
The stressful change hypothesis is an alternative theory that could potentially explain the link between pubertal timing and psychological distress. This theory predicts that puberty itself is a stressful event and that all girls eventually experience some level of distress, regardless of their timing of maturation. It could be argued that we found higher levels of depressive symptoms in girls who experienced earlier onset of menarche because they have spent longer in the time period of high risk than those with later menarche. Reference Patton, Hibbert, Carlin, Shao, Rosier and Caust1 However, according to the stressful change hypothesis, the highest level of depressive symptoms should occur around the time of onset of menarche and symptoms are likely to be transient. Reference Ge, Conger and Elder6 This theory is not supported by our findings because the increased level of depressive symptoms in girls with early menarche compared with the other groups persisted well beyond the onset of menarche into mid-adolescence.
Another potential explanation for the link between pubertal timing and psychological distress is the off-time hypothesis, which predicts that girls experiencing earlier maturation and those maturing later than their peers will suffer increased levels of distress compared with those developing ‘on time’. It has been suggested that difficulties encountered by girls experiencing late onset of menarche may not emerge until a later age. Reference Patton, Hibbert, Carlin, Shao, Rosier and Caust1 For example, there is some evidence for an increased level of depressive symptoms in high-school girls Reference Bisaga, Petkova, Cheng, Davies, Feldman and Whitaker29 and in women aged 31 years Reference Herva, Jokelainen, Pouta, Veijola, Timonen and Karvonen30 who experienced late-onset menarche (15 years and older), but these findings are based on retrospective reports of age at menarche.
It is still unclear from the current results whether early menarche is associated with persistent adverse consequences for emotional development beyond the mid-adolescent period. Only a few studies have examined whether early maturation has a long-term impact on rates of depression, but the findings of these studies are equivocal. There is some evidence for higher rates of depressive disorder in late adolescence and early adulthood among females who matured earlier than their peers, Reference Graber, Lewinsohn, Seeley and Brooks-Gunn31,Reference Graber, Seeley, Brooks-Gunn and Lewinsohn32 but these studies were based on retrospective self-reports of whether maturation was early, on time or late compared with peers. Others suggest that the negative impact of early puberty may diminish from mid-adolescence into early adulthood Reference Connolly, Paikoff, Buchanan, Adams, Montemayor and Gullotta33,Reference Dick, Rose, Viken and Kaprio34 or that girls who mature later may show a ‘catch-up’ effect Reference Stattin and Magnusson35 and eventually experience similar levels of psychological distress to those with early or normative onset menarche after sufficient time has unfolded.
Implications and future directions
These findings lend support to the hypothesis that early timing of menarche may be an important aetiological factor in the emergence of depressive risk, since timing was related to levels of depressive symptoms in girls during adolescence. Future research should be aimed at identifying the mechanism of action of early menarche on risk of depressive symptoms. School- and family-based programmes aimed at early intervention and prevention could target early maturing females.
Funding
The UK Medical Research Council (grant no. ), the Wellcome Trust (grant no. ) and the University of Bristol provide core support for The Avon Longitudinal Study of Parents and Children. This research was funded by the Economic and Social Research Council (Developmental trajectories of depressive symptoms from late childhood to early adolescence: impact of gender, puberty and adversity – ). T.C. is supported by a Career Scientist Award in Public Mental Health from the UK Department of Health and by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLARHC) for Cambridgeshire and Peterborough.
Acknowledgements
This study is based on the Avon Longitudinal Study of Parents and Children (ALSPAC). We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.









eLetters
No eLetters have been published for this article.