In general, all patients with schizophrenia could be considered treatment resistant, since full remission is unusual in this disorder. In order to create specific treatment algorithms for patients that are more difficult to treat, it is necessary to define treatment resistance. Reference KaneKane (1996) specified the following criteria for treatment resistance:
-
• available medications and other treatments are not useful in alleviating the target symptoms of schizophrenia (not only the positive and negative symptoms, but also disorganised or violent/aggressive behaviour, thought disorder and suicidal ideation);
-
• occurrence of adverse side-effects of medication;
-
• non-adherence to current treatment;
-
• presence of comorbid conditions such as substance misuse;
-
• failure of maintenance and relapse despite seemingly adequate doses of antipsychotics.
In addition, arbitrary defining criteria include failure to respond to at least two neuroleptic drugs equivalent to 600mg chlorpromazine per day for more than 4 weeks (Reference Juarez-Reyes, Shumway and BattleJuarez-Reyes et al, 1995). In clinical trial and audit settings, the most frequently used criteria are those adopted by Reference Kane, Honigfeld and SingerKane et al(1988) for their seminal trial of clozapine v. chlorpromazine in treatment-refractory schizophrenia. In this trial, patients were classified as resistant to treatment if:
-
1 they had not demonstrated improvement after three periods of treatment with neuroleptics (from two or more different chemical classes) in the previous 5 years equivalent to 1000mg per day of chlorpromazine for 6 weeks;
-
2 patients had had no episodes of good functioning in the previous 5 years.
Using these criteria, Kane et al found the incidence of treatment resistance in schizophrenia to be 20%.
Management with clozapine
This article focuses principally on the pharmacological management of treatment resistance. However, before initiating clozapine therapy, some basic principles should be adhered to, such as assessing non-adherence, re-evaluating the diagnosis, considering organic contributions to the diagnosis and assessing comorbidity (Reference MorrisonMorrison, 1996).
The evidence for clozapine as a superior treatment for schizophrenia is consistent and is the subject of a number of reviews and meta-analyses (Reference KaneKane, 1992; Reference MeltzerMeltzer, 1992; Reference Brambilla, Barale and CaverzasiBrambilla et al, 2002; Reference Iqbal, Rahman and HusainIqbal et al, 2003). Primary references for clozapine’s superior efficacy are cited in these reviews, but pivotal publications include a clinical trial of its use in treatment-resistant schizophrenia (Reference Kane, Honigfeld and SingerKane et al, 1988) and a meta-analysis of randomised controlled trials of clozapine (Reference Wahlbeck, Cheine and EssaliWahlbeck et al, 1999).
Another further useful practical guide to the drug’s use in treatment-resistant schizophrenia is a review by Reference Pantelis and LambertPantelis & Lambert (2003). In this, the authors suggest that treatment resistance is related to several symptom domains (Table 1) and they offer a three-phase algorithm for the pharmacological management of incompletely recovered (treatment-resistant) patients (Fig. 1).
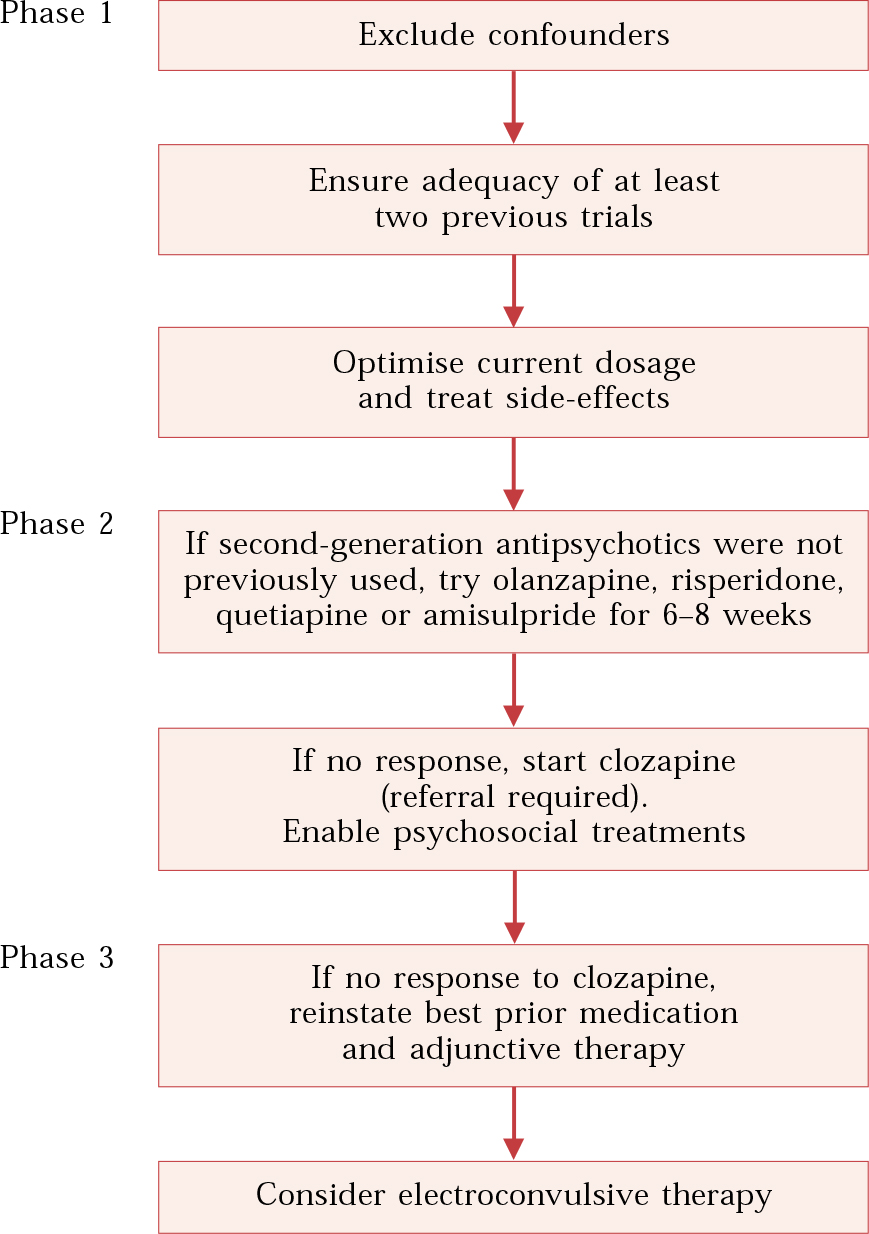
Fig. 1 Pharmacological management of incompletely recovered patients (after Reference Pantelis and LambertPantelis & Lambert, 2003).
Table 1 Symptom domains of schizophrenia targeted by antipsychotics (after Reference Pantelis and LambertPantelis & Lambert, 2003)
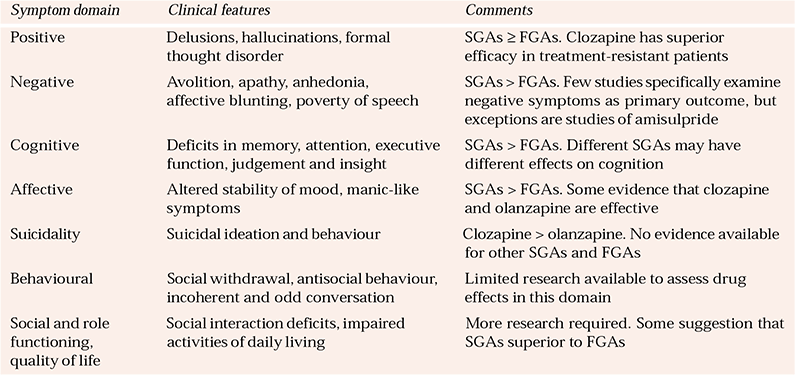
| Symptom domain | Clinical features | Comments |
|---|---|---|
| Positive | Delusions, hallucinations, formal thought disorder | SGAs ≥ FGAs. Clozapine has superior efficacy in treatment-resistant patients |
| Negative | Avolition, apathy, anhedonia, affective blunting, poverty of speech | SGAs > FGAs. Few studies specifically examine negative symptoms as primary outcome, but exceptions are studies of amisulpride |
| Cognitive | Deficits in memory, attention, executive function, judgement and insight | SGAs > FGAs. Different SGAs may have different effects on cognition |
| Affective | Altered stability of mood, manic-like symptoms | SGAs > FGAs. Some evidence that clozapine and olanzapine are effective |
| Suicidality | Suicidal ideation and behaviour | Clozapine > olanzapine. No evidence available for other SGAs and FGAs |
| Behavioural | Social withdrawal, antisocial behaviour, incoherent and odd conversation | Limited research available to assess drug effects in this domain |
| Social and role functioning, quality of life | Social interaction deficits, impaired activities of daily living | More research required. Some suggestion that SGAs superior to FGAs |
Managing clozapine-resistant patients
The most comprehensive review on this topic was published by Reference Barnes, McEvedy and NelsonBarnes et al(1996). Again, basic factors for clozapine-resistance highlighted in this review include comorbid drug misuse, poor adherence, inadequate duration of treatment and/or inadequate dosage. As Barnes et al note, two of these factors warrant further explanation.
The first is inadequate duration of treatment. It is accepted that a proportion of patients have a delayed response to clozapine (Reference MeltzerMeltzer, 1992). Meltzer concluded that 30% would respond by 6 weeks, a further 20% by 3 months and an additional 10–20% by 6 months. Therefore, it seems reasonable to try clozapine monotherapy for 6 months. This leaves a residue of 30% of patients for whom it must be decided whether to persevere with clozapine, consider various augmentation strategies or to cease clozapine therapy.
The second factor is inadequate dosage. Clozapine dosage can be a relatively complicated issue. In particular, there is no meaningful relationship between clozapine plasma levels and clinical response. However, there is a consensus in the literature that a plasma level of about 350–450 ng/ml has to be attained before the patient is considered to be non-respondent to clozapine (Reference Perry, Miller and ArndtPerry et al, 1991; Reference Potkin, Bera and GulaskeramPotkin et al, 1994).
Clozapine is also subject to considerable metabolism by the cytochrome P450 (CYP) enzyme system (Reference Aitchison, Jann and ZhaoAitchison et al, 2000). There are numerous variants of the genes encoding the CYP enzyme family within the general population, resulting in complex individual genetic profiles and a variable response to drugs metabolised by these enzymes (Reference Ma, Nafziger and BertinoMa et al, 2004).† Therefore, in clinical practice, patients can be very susceptible to side-effects at drug dose levels that appear to be below the threshold for clinical efficacy.
Augmentation of clozapine
A frequent treatment strategy for clozapine-resistant patients with schizophrenia is the use of specific augmentors that are suitable for adjunctive therapy. Clozapine is a polyvalent drug but it lacks high-potency dopamine receptor blockade (Reference Kerwin and OsborneKerwin & Osborne, 2000). Therefore, there has been interest in using as augmentors substituted benzamides with highly selective dopamine receptor blocking profiles (Reference KerwinKerwin, 2000). Augmentation strategies incorporating sulpiride are well documented. The authors of one study of sulpiride augmentation in 28 patients partially responsive to clozapine (Reference Shiloh, Zemishlany and AizenbergShiloh et al, 1997) noted a mean reduction of about 40–50% in various clinical response scores (Brief Psychiatric Rating Scale and Scale for the Assessment of Positive Symptoms).
Several groups have been interested in mimicking this study with amisulpride, a relative of sulpiride that is even more selective at the dopamine D2 receptor. A case series by Reference Zink, Knopf and HennZink et al(2004) showed improvement in previously treatment-resistant symptoms following a combined treatment strategy of clozapine and amisulpride. In addition, our group performed an open trial of amisulpride augmentation in a long-term (52 weeks) study. Significant improvement was observed in half of the patients, with no additional side-effects. Moreover, this study monitored plasma levels to determine whether this was a pharmacokinetic interaction. Clozapine levels did not change throughout the duration of the trial, suggesting a pharmacodynamic interaction (Reference Munro, Matthiasson and OsborneMunro et al, 2004).
Augmentation with anti-epileptics
A glutamate hyperfunction hypothesis of schizophrenia has generated interest in the role of glutamate release inhibitors as clozapine augmentors. In a study of 26 treatment-resistant patients receiving lamotrigine (17) or topirimate (9) in addition to their existing antipsychotic treatment (a variety of antipsychotics), a significant improvement was observed when lamotrigine was added to risperidone, haloperidol, olanzapine or flupenthixol. However, no significant effect was observed in patients receiving topirimate augmentation in addition to clozapine, olanzapine, haloperidol or flupenthixol (Reference Dursun and DeakinDursun & Deakin, 2001). The therapeutic effects of lamotrigine augmentation were also assessed in a rigorous randomised placebo-controlled cross-over study of 34 clozapine-resistant patients (Reference Tiihonen, Hallikainen and RyynanenTiihonen et al, 2003). In this 14-week study, lamotrigine treatment significantly improved positive symptoms and general psychopathological symptoms, but had no effect on negative symptoms. The authors suggested that this was the first time a non-dopamine antagonist had proven efficacy in schizophrenia, giving further credence to the hyperglutamate neurotransmission hypothesis for the generation of positive symptoms in the disorder.
Are other atypicals, alone or in combination, of use?
Other atypical antipsychotics were not trialled in the pre-registration phase for their efficacy in treatment resistance. However, there have been a number of phase IV trials that have examined this possibility.
Risperidone
The earliest study examining the possibility that risperidone may be of use in treatment resistance was conducted by Reference Bondolfi, Dufour and PatrisBondolfi et al(1998). This 8-week study of risperidone and clozapine concluded that the two drugs are comparable. However, the study has been criticised for its rapid titration schedule of 1 week, which may have led to clozapine intolerance and therefore to patients receiving suboptimal doses of the drug. Later studies include that by Reference Wahlbeck, Cheine and TuiskuWahlbeck et al(2000), which was a 10-week open-label trial of risperidone v. clozapine using a 20% decrease in Positive and Negative Symptom Scale score as the definition of clinical improvement. Intention-to-treat analysis did not identify significant differences between the two groups, but there was an unusually high dropout rate for clozapine (5 of 10 participants), seriously reducing the power of the study. To date, there has been no large, randomised, pragmatic trial of risperidone v. clozapine that has sufficient power to detect a difference between the treatments.
Olanzapine
The most informative study of olanzapine in treatment resistance is likely to be that by Reference Conley, Tamminga and BartkoConley et al(1998). This reproduced the methodology of the pivotal Reference Kane, Honigfeld and SingerKane et al(1988) study of clozapine but concluded that olanzapine was no better than chlorpromazine in treatment resistance. Additional studies using olanzapine following treatment failures with other antipsychotics (switching studies) have proved disappointing. However, one (Reference Lindenmayer, Czobor and VolavkaLindenmayer et al, 2002), although showing that olanzapine was not a useful switch drug for general psychopathological symptoms, did demonstrate an improvement in symptoms related to cognitive function. In a further switching study, Reference Conley, Tamminga and KellyConley et al(1999) conducted 8-week open and double-blind trials administering olanzapine to 44 treatment-resistant patients and treating subsequent olanzapine-resistant patients with clozapine. Only 5% of patients responded to olanzapine and, of the remaining patients who were switched to clozapine, 41% responded to clozapine. The authors concluded that non-response to olanzapine does not predict failure to respond to clozapine. Some open studies (e.g. Reference Dursun, Gardner and BirdDursun et al, 1999) suggest that olanzapine might be best used at higher doses in treatment-resistant schizophrenia.
Quetiapine
There is relatively little published information on controlled trials of quetiapine v. clozapine in treatment-resistant patients, but a number of small trials and case reports suggest its usefulness in treatment resistance (e.g. Reference Fabre, Arvanitis and PultzFabre et al, 1995; Reference BrooksBrooks, 2001).
Non-clozapine atypicals in combination
At the end of the algorithm shown in Fig. 1, many clinicians try ad hoc combinations of atypical antipsychotics for treatment failures with clozapine. This usually involves a combination of olanzapine and risperidone. There is a limited published evidence base on this strategy. Most publications in this area are case reports and there are likely to be very many unpublished cases where this strategy has been used in clinical practice. However, Reference Lerner, Libov and KotlerLerner et al(2004) have attempted to review this scant literature. They found little evidence other than anecdotal case reports that augmentation of clozapine with other atypicals produces any benefit. Subtracting patients treated with combinations of clozapine and another atypical leaves a very small database on which to draw. However, Lerner et al concluded that there might be some benefit, which necessitates further controlled studies.
Conclusions
Although olanzapine and risperidone are probably the most successful antipsychotics for partially treatment-resistant patients, there is now very little controversy about the role of clozapine in more extreme treatment resistance. Indeed, the NICE schizophrenia guidelines of 2002 strongly recommend broader and earlier use of clozapine, suggesting that only one atypical should be tried before moving onto clozapine.
Most practitioners are now more concerned with managing clozapine-resistant patients, the focus of this short review. There is reasonable evidence to suggest that augmentation strategies with sulpiride, amisulpride and lamotrigine are useful in treatment resistance, but no indication as to which patient will benefit from which strategy. The small proportion of patients who remain densely resistant to any pharmacological treatment strategy should probably be managed by a historical review of their best treatment regime and the reinstatement of this along with psychosocial treatments.
MCQs
-
1 The incidence of treatment-resistant schizophrenia is:
-
a 5%
-
b 10%
-
c 20%
-
d 50%
-
e 75%.
-
-
2 The most commonly used categorical definition of treatment resistance is :
-
a prolonged hospitalisation
-
b less than a 20% improvement on the BPRS
-
c symptoms enduring for more than 2 years
-
d persistent negative symptoms
-
e persistent cognitive symptoms.
-
-
3 Definitions of treatment resistance might also include:
-
a non-adherence
-
b failure of maintenance
-
c comorbidity
-
d aggression
-
e negative symptoms.
-
-
4 The following drugs show evidence that they are specific clozapine augmentors:
-
a aripiprazole
-
b ziprasidone
-
c lamotrigne
-
d zolepine
-
e amisulpride.
-
-
5 The following drugs alone or in combination may be useful for partial treatment resistance:
-
a haloperidol at low dose
-
b risperidone
-
c olanzapine
-
d risperidone and olanzapine
-
e aripiprazole.
-
MCQ answers
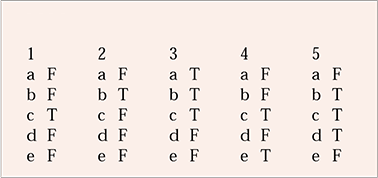
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | T | a | F | a | F |
| b | F | b | T | b | T | b | F | b | T |
| c | T | c | F | c | T | c | T | c | T |
| d | F | d | F | d | F | d | F | d | T |
| e | F | e | F | e | F | e | T | e | F |





eLetters
No eLetters have been published for this article.