The generation of reactive oxygen species (ROS) and other free radicals during metabolism is an essential and normal process that is compensated for by the endogenous antioxidant defence system. Oxidative stress refers to a situation in which a significant imbalance exists between ROS as well as free radicals and the antioxidant defence system; thus, the accumulation of oxidative damage to molecules threatens the normal function of cells and organismsReference Willcox, Ash and Catignani1, Reference Blomhoff2. Oxidative stress has been shown to be related to CVD, cancer, diabetes mellitus and other chronic diseases that account for the leading causes of death in many countries. Hypercholesterolaemia is associated with increased ROS generation and oxidative stressReference Griendling and FitzGerald3, Reference Wassmann, Wassmann and Nickenig4, suggesting that cholesterol may play an important role in the development of several diseases. Numerous studies have indicated that dietary cholesterol exerts an apparent pro-oxidative effect on lipid peroxidation and lipoprotein oxidationReference Tsai, Thie and Lin5–Reference Balkan, Dogru-Abbasoglu, Aykac-Toker and Uysal8. Therefore, it is plausible that antioxidants that inactivate ROS and protect against oxidative damage are thought to prevent cholesterol-induced oxidative stress.
Carotenoids belong to the tetraterpene family and comprise more than 600 known natural structural variants. Carotenoids are divided into two classes: non-polar carotenes (e.g. β-carotene and lycopene) containing only C and H atoms; polar xanthophylls (e.g. canthaxanthin and zeaxanthin) that carry at least one O atomReference Tapiero, Townsend and Tew9. The effects of carotenoids in human disease prevention have been widely reported. One of the mechanisms by which carotenoids act is their ability to modulate cell redox statusReference Palozza, Calviello, Emilia De Leo, Serini and Bartoli10. Someya et al. Reference Someya, Totuska, Murakoshi, Kitano and Miyazawa11 reported that palm fruit carotene prevented UV ray-induced skin lipid peroxidation in mice. Iyama et al. Reference Iyama, Takasuga and Azuma12 reported that β-carotene suppressed carbon tetrachloride-induced lipid peroxidation in mice. Whittaker et al. Reference Whittaker, Wamer, Chanderbhan and Dunkel13 evaluated the effects of dietary antioxidants on lipid peroxidation in rats with dietary Fe overload and found that the animals fed β-carotene plus α-tocopherol had a lower level of lipid peroxidation. Kraus et al. Reference Kraus, Roth and Kirchgessner14 showed that β-carotene decreased oxidative damage in erythrocytes from Zn-deficient rats. Palozza et al. Reference Palozza, Calviello, Emilia De Leo, Serini and Bartoli10 found that canthaxanthin altered the protective ability of tissues against oxidative stress in mice. Riondel et al. Reference Riondel, Wong, Glise, Ducros and Favier15 indicated that β-carotene improved the antioxidant status of mice bearing lymphoid neoplasm. Nicolle et al. Reference Nicolle, Cardinault and Aprikian16 reported that carrot consumption improved the antioxidant status of cholesterol-fed rats.
The afore-mentioned studies suggest that carotenoids, alone or in combination with other nutrients, may function as potent antioxidants against oxidative stress induced by physical, chemical and biological factors. However, most of these carotenoid-related studies examined limited indices of lipid peroxidation or antioxidative enzyme activities. In addition, few studies have investigated the effect of carotenoids on cholesterol-induced oxidative stress. To evaluate the antioxidative potential of carotenoids on cholesterol-induced oxidative stress, the present study examined the effects of β-carotene (a non-polar carotenoid) and canthaxanthin (a polar carotenoid) on lipid peroxidation and antioxidative enzyme activities in rats fed a high-fat, high-cholesterol diet. α-Tocopherol, a known antioxidant, was selected as a referential agent to evaluate the antioxidative potency of carotenoids.
Materials and methods
Materials
β-Carotene and canthaxanthin beadlets purchased from Hoffmann-La Roche (Basel, Switzerland) contained 10 % β-carotene and 10 % canthaxanthin. Crystal β-carotene, α-tocopherol, cholic acid, dl-methionine and choline bitartrate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Cholesterol was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Maize flour was purchased from Roquette Freres (Lille, France). Soyabean oil was purchased from Uni-President Co. (Tainan, Taiwan). Casein, α-cellulose, AIN-76 mineral mixture and AIN-76 vitamin mixture were purchased from ICN Biochemicals Co. (Costa Mesa, CA, USA).
Animals and diets
Seventy-two male Wistar rats were obtained at age 6 weeks from the Laboratory Animal Center of the College of Medicine at National Taiwan University (Taipei, Taiwan). They were housed individually in stainless wire-bottomed cages at a controlled temperature (21 ± 2°C), at a relative humidity of 50–70 % and with a 12-h light–dark cycle. Animals were allowed free access to feed and water. All experimental diets were formulated on the basis of a modified AIN-76 diet17, 18. After 1 week of acclimatization, the animals were assigned to one of the six groups (twelve per group). The negative control group (group NC) received a high-fat (150 g/kg) diet with no cholesterol supplementation and the cholesterol control group (group CC) received a high-fat, high-cholesterol (10 g/kg) diet. The other four groups were fed a high-fat, high-cholesterol diet supplemented with crystal β-carotene (group BC), β-carotene beadlet (group BB), canthaxanthin beadlet (group CX) or α-tocopherol (group AT). Carotenoids and α-tocopherol were both supplemented at a level of 2 g/kg. The composition of the experimental diets is shown in Table 1. The study protocol was approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
Table 1 Composition of the experimental diets (g/kg diet)‡

* Contained 10 % β-carotene.
† Contained 10 % canthaxanthin.
‡ For details of diets and procedures, see Materials and methods.
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol.
Experimental procedures
Animals were fed experimental diets for 6 weeks. Feed intake was recorded daily and body weight was recorded once per week. At the end of the experiment, the animals were anaesthetized with sodium pentobarbital (1 g/kg body weight) after being deprived of food for 12 h. Blood was collected from the abdominal aorta, placed into heparinized tubes and centrifuged at 1800 g for 15 min to separate plasma from erythrocytes. Livers were dissected, rinsed in saline (9 g/l) and weighed. All samples were stored at − 70°C until analysed.
Carotenoids assay
β-Carotene and canthaxanthin were analysed by HPLC according to our established methodReference Shih, Cheng and Shieh19, Reference Cheng, Guo and Shieh20. Aliquots of plasma (1 ml) were mixed with 25 μl trans-β-apo-8′-carotenal solution (0·4 mg/l methanol, internal standard) and 100 μl absolute ethanol and denatured in 200 μl acetonitrile. Samples were extracted with 125 μl n-hexane and centrifuged at 13 000 g under 4°C for 1 min. Supernatants (20 μl) were injected into a HPLC system (Hitachi L-6000 pump and L-4000 UV-VIS detector; Hitachi, Tokyo, Japan). Liver tissues (0·2 g) were homogenized in 2 ml absolute ethanol plus 0·5 μl sodium ascorbate solution (250 g/l) and saponified by adding saturated potassium hydroxide solution (1 ml). After the addition of NaCl solution (50 g/l, 3 ml), samples were extracted with 5 ml n-hexane. The extracts were evaporated to dryness under N2 and dissolved in 1 ml Sudan I solution (0·3 mg/l, internal standard). A 20-μl aliquot was injected into the HPLC system as stated earlier.
Chromatography was carried out with a Cosmosil C18 column (4·6 × 250 mm, 5 μm particle size; Nacalai, Kyoto, Japan). The flow rate of mobile phase (methanol:toluene 3:1, v/v) was 1·5 ml/min and the concentration of carotenoid was measured from a standard curve detected at 470 nm.
Retinol assay
Retinol was analysed by HPLC according to our established methodReference Shih, Cheng and Shieh19, Reference Cheng, Guo and Shieh20. Aliquots of plasma (250 μl) were mixed with 25 μl all-trans-retinyl acetate solution (16 mg/l ethanol, internal standard) and denatured in 100 μl acetonitrile. Samples were extracted with 125 μl n-hexane, mixed with saturated potassium phosphate solution (75 μl) and centrifuged at 13 000 g at 4°C for 1 min. Supernatant fractions (20 μl) were injected into a HPLC system (Hitachi L-6000 pump and L-4000 UV-VIS detector; Hitachi). Liver tissues (0·1 g) were homogenized in 2 ml absolute ethanol containing pyrogallol (30 g/l) and saponified by adding saturated potassium hydroxide solution (1 ml). After the addition of NaCl solution (50 g/l, 3 ml), the samples were extracted with 5 ml n-hexane. The extracts were evaporated to dryness under N2 and dissolved in 0·5 ml all-trans-retinyl acetate solution (40 mg/l ethanol, internal standard) and 5 ml methanol. A 20-μl aliquot was injected into the HPLC system as stated earlier. Chromatography was carried out with a Cosmosil C18 AR column (4·6 × 150 mm, 5 μm particle size; Nacalai). The flow rate of mobile phase (methanol:0·5 % phosphate 97:3, v/v, pH 1·49) was 1·0 ml/min and the concentration of retinol was measured from a standard curve detected at 325 nm.
α-Tocopherol assay
α-Tocopherol was analysed by HPLC according to the method of Liu & HuangReference Liu and Huang21. Aliquots of plasma (0·5 ml) were mixed with 2 ml absolute ethanol containing pyrogallol (10 g/l), 0·1 ml HCl (12 mol/l) and 6 ml n-hexane containing butylated hydroxytoluene (BHT, 1·25 g/l). Extracts were evaporated to dryness under N2 and dissolved in 100 μl methanol. A 20-μl aliquot was injected into a HPLC system (Hitachi L-6000 pump and L-4000 UV-VIS detector; Hitachi). Liver tissues were homogenized in phosphate buffer (10 mmol/l, pH 7·4); the liver homogenate (1 ml) was then deproteinated with 2 ml absolute ethanol containing BHT (10 g/l), saponified with saturated potassium hydroxide and extracted with n-hexane containing BHT (1·25 g/l). The extracts were evaporated to dryness under N2 and dissolved in 100 μl methanol. A 20-μl aliquot was injected into the HPLC system as stated earlier. Chromatography was carried out with a Cosmosil C18 AR column (4·6 × 150 mm, 5 μm particle size; Nacalai). The flow rate of mobile phase (methanol) was 1·0 ml/min and the concentration of α-tocopherol was measured from a standard curve detected at 290 nm.
Lipid peroxidation
Aliquots of liver were mixed with a solution of phosphate buffer (10 mmol/l, pH 7·4) and potassium chloride (11·5 g/l), homogenized and centrifuged at 12 000 g for 20 min. Supernatant fractions (liver homogenates) were collected to determine lipid peroxidation. The assay of thiobarbituric acid reactive substance (TBARS) was based on the method of YagiReference Yagi22. Plasma or liver homogenates were mixed with TCA and centrifuged at 1500 g for 10 min. Supernatant fractions were incubated with thiobarbituric acid and BHT solution at 95°C for 1 h, mixed with butanol, and centrifuged at 1500 g for 10 min. Supernatant fractions were measured with the use of fluorescent method emission at 553 nm with excitation at 515 nm. The assay of conjugated dienes was based on the method of Banni et al. Reference Banni, Salgo, Evans, Corongiu and Lombardi23. Liver homogenates were mixed with an extraction reagent (n-hexane:isopropanol 3:4, v/v) and centrifuged at 10 000 g for 10 min. The absorbance of supernatant fractions was measured at 234 nm.
Antioxidative enzyme activities
Erythrocytes were washed with saline. The haemolysates were then prepared by diluting erythrocytes with double-distillated water and lysed in dry ice/acetone to ensure cell disruptionReference Yuan, Kitts and Godin24. Liver tissues were homogenized with EDTA (1 mmol/l) and Tris (10 mmol/l, pH 7·4) and centrifuged at 20 000 g at 4°C for 5 min. The upper layer was centrifuged at 105 000 g at 4°C for 1 h and supernatant fractions (cytosolic fractions of livers) were collected for analyses of antioxidative enzyme activities. The assay of catalase activity was based on the method of LuckReference Luck and Bergmeyer25. Erythrocyte haemolysates or liver homogenates were diluted with potassium phosphate buffer and mixed with hydrogen peroxide–potassium phosphate buffer. The absorbance was monitored at 240 nm. The assay of superoxide dismutase (SOD) activity was based on the method of BeutlerReference Beutler26 using a commercial kit (RANSOD kit; Randox Laboratories, Crumlin, Antrim, UK). Erythrocyte haemolysates or cytosolic fractions of liver were mixed with phosphate buffer, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium and xanthine oxidase. Absorbance was monitored at 505 nm. The assay of glutathione peroxidase (GPX) activity was based on the method of Prohaska et al. Reference Prohaska, Oh, Hoekstra and Ganther27 and involved the use of a commercial kit (RANSEL kit; Randox Laboratories). Erythrocyte haemolysates or cytosolic fractions of liver were mixed with a reagent containing glutathione, glutathione reductase (GRD) and NADPH followed by the addition of cumene hydroperoxide. Absorbance was monitored at 340 nm. The assay of GRD activity was based on the method of Bellomo et al. Reference Bellomo, Mirabelli, DiMonte, Richelmi, Thor, Orrenius and Orrenius28 and involved the use of a commercial kit (GRD kit; Randox Laboratories). Erythrocyte haemolysates or cytosolic fractions of liver were mixed with oxidized glutathione buffer and NADPH. Absorbance was monitored at 340 nm. Protein concentrations were measured by the method of Lowry et al. Reference Lowry, Rosebrough, Farr and Randall29.
Assays of cholesterol and TAG
Serum cholesterol was measured by the method of RichmondReference Richmond30. Serum TAG was measured by the method of McGowan et al. Reference McGowan, Artiss, Strandbergh and Zak31. Lipids in liver were extracted according to the method of Folch et al. Reference Folch, Lees and Sloane-Stanley32. Liver concentrations of cholesterol and TAG were analysed by the methods of Carlson & GoldfarbReference Carlson and Goldfarb33 and Soloni et al. Reference Soloni34, respectively.
Statistical analysis
Data are expressed as means and standard deviations. Differences in data between the experimental groups were assessed by one-way ANOVA with SAS software (SAS Institute, Cary, NC, USA). Means were considered significantly different at P < 0·05 as determined by Duncan's multiple range test.
Results
The average initial and final body weights of rats were 250 g and 430 g, respectively. During the 6-week experiment, the average weight gain of rats was 4 g/d. No significant differences in these weight-related variables were observed between the groups. Cholesterol feeding led to significantly higher relative liver weight and slightly but not significantly lower daily feed intake and higher feed efficiency; however, β-carotene, canthaxanthin and α-tocopherol intakes did not alter these variables (Table 2).
Table 2 Effects of β-carotene and canthaxanthin supplementation on daily feed intake, feed efficiency and relative liver weight in cholesterol-fed rats‡
(Mean values and standard deviations for twelve rats per group)
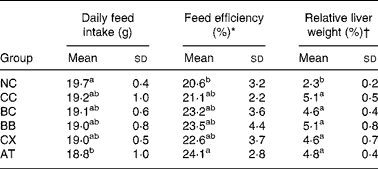
* Body weight gain/feed intake × 100 %.
† Liver weight/body weight × 100 %.
‡ For details of diets and procedures, see Materials and methods.
a,b Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol.
Plasma and liver concentrations of β-carotene and canthaxanthin are shown in Table 3. β-Carotene was detected only in rats fed β-carotene (groups BC and BB); canthaxanthin was detected only in rats fed canthaxanthin (group CX). Both plasma and hepatic β-carotene concentrations were higher (P < 0·05) in group BB than in group BC.
Table 3 Effects of β-carotene and canthaxanthin supplementation on plasma and liver concentrations of β-carotene and canthaxanthin in cholesterol-fed rats†
(Mean values and standard deviations for twelve rats per group)

* Not detectable.
† For details of diets and procedures, see Materials and methods.
a,b Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol.
Plasma and liver concentrations of retinol and α-tocopherol are shown in Table 4. The mean hepatic retinol concentration was significantly lower (P < 0·05) in group CC than in group NC. Groups BC and BB had significantly higher (P < 0·05) plasma and hepatic retinol concentrations than did group CC, and the concentrations were similar to or higher than those of group NC. Groups CX and AT also had significantly higher (P < 0·05) hepatic retinol concentrations than did group CC, but the concentrations were still lower than those of group NC. Plasma retinol concentrations were not affected by canthaxanthin or α-tocopherol supplementation. The mean hepatic α-tocopherol concentration was significantly lower (P < 0·05) in group CC than in group NC. Groups BC, BB, CX and AT had significantly higher (P < 0·05) hepatic α-tocopherol concentrations than did group CC. Furthermore, groups BB, CX and AT had significantly higher (P < 0·05) plasma α-tocopherol concentrations than did group CC.
Table 4 Effects of β-carotene and canthaxanthin supplementation on plasma and liver concentrations of retinol and α-tocopherol in cholesterol-fed rats*
(Mean values and standard deviations for twelve rats per group)

* For details of diets and procedures, see Materials and methods.
a,b,c Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol.
As shown in Table 5, plasma and hepatic TBARS concentrations and hepatic conjugated diene concentrations were significantly higher (P < 0·05) in group CC than in group NC. Groups BC, BB, CX and AT had significantly lower (P < 0·05) hepatic TBARS concentrations than did group CC, whereas groups CX and AT further decreased (P < 0·05) plasma TBARS concentrations. Hepatic conjugated diene concentrations were significantly lower (P < 0·05) in groups BB and AT than in group CC.
Table 5 Effects of β-carotene and canthaxanthin supplementation on plasma and liver TBARS and liver conjugated diene concentrations in cholesterol-fed rats*
(Mean values and standard deviations for twelve rats per group)

* For details of diets and procedures, see Materials and methods.
a,b,c,d Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol; TBARS, thiobarbituric acid reactive substance.
The antioxidative enzyme activities in rat erythrocytes are shown in Table 6. GPX activity was significantly lower (P < 0·05) in group CC than in group NC. However, catalase, SOD and GRD activities were not affected significantly by cholesterol feeding. Groups BC, BB, CX and AT had significantly higher (P < 0·05) GPX activities than did group CC, and the levels were similar to or higher than those of group NC. Groups BB and CX had significantly higher (P < 0·05) catalase activities than did the other groups. In addition, SOD activity was significantly higher (P < 0·05) in group BB than in group CC. GRD activity was not affected significantly by β-carotene, canthaxanthin or α-tocopherol supplementation.
Table 6 Effects of β-carotene and canthaxanthin supplementation on antioxidative enzyme activities in erythrocytes of cholesterol-fed rats*
(Mean values and standard deviations for twelve rats per group)

* For details of diets and procedures, see Materials and methods.
a,b,c Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol; SOD, superoxide dismutase; GPX, glutathione peroxidase; GRD, glutathione reductase.
The antioxidative enzyme activities in rat livers are shown in Table 7. SOD activity was significantly lower (P < 0·05) and GRD activity was significantly higher (P < 0·05) in group CC than in group NC. However, catalase and GPX activities were not affected significantly by cholesterol feeding. Groups BC, BB and CX had significantly higher (P < 0·05) SOD activities than did group CC, and the concentrations were similar to those of group NC. Group AT had a significantly higher (P < 0·05) catalase activity than did the other groups, except for group BB. Hepatic GPX activities were significantly lower (P < 0·05) in groups BB and AT than in group NC; however, the activities were not significantly different from those of group CC. Hepatic GRD activities were significantly higher (P < 0·05) in groups BB, CX and AT than in group CC.
Table 7 Effects of β-carotene and canthaxanthin supplementation on antioxidative enzyme activities in livers of cholesterol-fed rats*
(Mean values and standard deviations for twelve rats per group)

* For details of diets and procedures, see Materials and methods.
a,b,c Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol; SOD, superoxide dismutase; GPX, glutathione peroxidase; GRD, glutathione reductase.
Cholesterol feeding resulted in significantly higher (P < 0·05) plasma and liver cholesterol concentrations and significantly higher (P < 0·05) liver TAG concentrations (Table 8). Groups BC and BB had significantly lower (P < 0·05) plasma cholesterol concentrations than did group CC, whereas group BC further decreased (P < 0·05) the hepatic cholesterol concentration. Group CX had significantly higher (P < 0·05) plasma TAG concentrations than did the other groups, except for group BC; however, hepatic TAG concentrations were not affected significantly by β-carotene, canthaxanthin or α-tocopherol supplementation.
Table 8 Effects of β-carotene and canthaxanthin on plasma and hepatic levels of cholesterol and TAG in cholesterol-fed rats*
(Mean values and standard deviations for twelve rats per group)

* For details of diets and procedures, see Materials and methods.
a,b,c Mean values within a column with unlike superscript letters are significantly different (P < 0·05).
NC, control diet without cholesterol; CC, 1 % cholesterol diet; BC, 1 % cholesterol diet with 0·2 % crystal β-carotene; BB, 1 % cholesterol diet with 0·2 % β-carotene as beadlet; CX, 1 % cholesterol diet with 0·2 % canthaxanthin as beadlet; AT, 1 % cholesterol diet with 0·2 % α-tocopherol.
Discussion
The physiological functions and side effects of cholesterol have been widely documented. Feeding rats a high level of cholesterol commonly results in a fatty liver characterized by greater live tissue weight and an abnormal macroscopic appearanceReference Yuan, Kitts and Godin24, Reference Yuan and Kitts35. The present study showed that administration of cholesterol caused a higher relative liver weight in rats (Table 2) attributed to the accumulation of cholesterol and TAG (Table 8). Furthermore, the nutritional status of vitamins A (retinol) and E (α-tocopherol) was poor in the cholesterol-fed rats (Table 4).
Cholesterol feeding led to an imbalance between oxidation and antioxidation. Plasma and hepatic TBARS and hepatic conjugated diene concentrations were significantly elevated (Table 5), whereas GPX activity in erythrocytes and SOD activity in liver decreased significantly in the cholesterol-fed rats (Tables 6 and 7). The observed effects of dietary cholesterol on antioxidative enzyme activities in the present study were consistent with the findings of other studiesReference Yuan, Kitts and Godin24, Reference Lu and Chiang36, Reference Yuan and Kitts37; however, the findings for lipid peroxidation were not consistent between studies. TsaiReference Tsai38 indicated that cholesterol feeding at levels of 1 % or 1·5 % elevated the rate of lipid oxidation in the liver of rats. Uysal et al. Reference Uysal, Kutalp and Seckin6 reported that cholesterol feeding at a level of 2 % elevated hepatic lipid peroxide concentrations in rats. Aydemir et al. Reference Aydemir, Duman, Celik, Turgan, Uysal, Mutaf, Habif, Ozmen, Nisli and Bayindir7 found that the plasma malondialdehyde concentration in rabbits fed cholesterol at a level of 1 % was higher than that in rabbits fed no cholesterol. Balkan et al. Reference Balkan, Dogru-Abbasoglu, Aykac-Toker and Uysal8 showed that rats fed a diet containing 2 % cholesterol had higher plasma and liver lipid peroxide concentrations than did those fed no cholesterol. On the other hand, a few studies suggested that cholesterol has an antioxidative effect against oxidative stress by stabilizing membranesReference Yuan, Kitts and Godin24, Reference Yuan and Kitts35. In these studies, the antioxidative effect of cholesterol was observed in rats fed a moderate-fat (8 %) diet containing 0·5 % cholesterol compared with those fed a diet containing 0·05 % cholesterol. It is possible that cholesterol has both pro-oxidative and antioxidative effects, depending on the dietary conditions, such as cholesterol dose, the amount of fat and the fatty acid composition. Dietary fat containing high PUFA, such as soyabean oil and fish oil, plays a role in lipid peroxidation of tissues. In the present study, cholesterol exerted a pro-oxidative effect in rats fed a high-cholesterol (1 %), high-fat (15 % soyabean oil) diet.
Malondialdehyde is the principal product of PUFA peroxidation. Most assays used to measure malondialdehyde have been developed on the basis of its derivatization with thiobarbituric acid, so the result is often reported as TBARSReference Del Rio, Stewart and Pellegrini39. TBARS is a common marker of lipid peroxidation. The peroxidation of PUFA is also accompanied by the formation of conjugated diene and it is another referential marker of lipid peroxidationReference Meagher and FitzGerald40. As expected, α-tocopherol was a potent antioxidant; its intake resulted in a reduction in plasma and hepatic TBARS in rats fed cholesterol at levels similar to those of rats fed no cholesterol (Table 5). Furthermore, α-tocopherol also lowered the hepatic conjugated diene concentration of cholesterol-fed rats, although the concentration was still higher than that of rats fed no cholesterol (Table 5).
Carotenoids are believed to act as antioxidants by quenching singlet oxygen and scavenging peroxyl radicalsReference Tapiero, Townsend and Tew9, Reference Palozza and Krinsky41. In the present study, β-carotene (either crystal or beadlet form) and canthaxanthin intakes decreased hepatic TBARS concentrations in rats fed cholesterol and β-carotene beadlet or canthaxanthin intake further decreased hepatic conjugated diene and plasma TBARS concentrations (Table 5). These results suggest that both β-carotene and canthaxanthin have antioxidative activity and that β-carotene beadlet is effective in liver, whereas canthaxanthin is effective in plasma. The antioxidative capacity of carotenoids is dependent on their structure and the environment in which they existReference El-Agamey, Lowe, McGarvey, Mortensen, Phillip, Truscott and Young42. Carotenoids are transported in the plasma of man and animals by lipoproteins. Non-polar carotenoids, such as β-carotene and lycopene, exist exclusively in the hydrophobic core of lipoproteins, which may not allow their transfer between lipoproteins at an appreciable rate, whereas polar carotenoids, such as canthaxanthin and zeaxanthin, exist mainly on the hydrophilic surface, resulting in a rapid surface transferReference Parker43, Reference Yeum and Russell44. The lower plasma TBARS concentration in rats supplemented with canthaxanthin may be attributed to the antioxidative effect of canthaxanthin in the polar region of lipoproteins exposed to the aqueous plasma. Canthaxanthin was a more effective antioxidant than β-carotene in some in vitro studiesReference Terao45, Reference Palozza, Luberto, Ricci, Sgarlata, Calviello and Bartoli46. However, the in vivo antioxidative potency of these two carotenoids has not been well studied due to the complexity of the biological system. In the present study, the antioxidative capacity of canthaxanthin was more potent in plasma than in liver, although the concentration of canthaxanthin was higher in liver than in plasma. It appears that the content (compared with the structure) of carotenoids in tissues plays a minor role in its antioxidative capacity.
Both catalase and GPX destroy H2O2 by converting it to O2 and water. The GPX/glutathione system is thought to be a major defence in low-level oxidative stress, whereas catalase is effective in high-level oxidative stressReference Wassmann, Wassmann and Nickenig4. Despite a possible substitution by catalase in situations of moderate oxidative stress, GPX still appears to be the main antioxidative enzyme of the organismReference Brigelius-Flohe47. Erythrocytes are highly sensitive to oxidative stress because of their high concentrations of Hb and O2Reference Eder, Flader, Hirche and Brandsch48. Even in erythrocytes that have a high content of catalase, H2O2 is essentially degraded by GPXReference Chaudiere and Ferrari-Iliou49. The present study showed that the GPX activity of erythrocytes decreased in cholesterol-fed rats and recovered in rats supplemented with β-carotene, canthaxanthin or α-tocopherol (Table 6). Furthermore, it appeared that β-carotene and canthaxanthin were more potent than was α-tocopherol in enhancing GPX activity in erythrocytes. On the other hand, the catalase activities of erythrocytes increased in rats supplemented with the beadlet form of β-carotene and canthaxanthin. Low GPX activities have been shown to be an independent risk factor for cardiovascular eventsReference Wassmann, Wassmann and Nickenig4. These results suggest that β-carotene and canthaxanthin may overcome the oxidative stress of erythrocytes induced by cholesterol and may prevent CVD.
SOD catalyses the one-electron dismutation of superoxide into H2O2 and O2Reference Chaudiere and Ferrari-Iliou49. When disease is present, an imbalance between the amount of superoxide formed and the ability of SOD to remove it (activity of the enzyme is decreased) exists. The consequence of this imbalance results in superoxide-mediated damage, such as lipid peroxidationReference Muscoli, Cuzzocrea, Riley, Zweier, Thiemermann, Wang and Salvemini50. Several animal models of diseases have shown that GM mice that lack SOD are more sensitive and those that over-express SOD are resistant to diseasesReference Muscoli, Cuzzocrea, Riley, Zweier, Thiemermann, Wang and Salvemini50. In addition, preclinical studies have shown that SOD has a protective effect in animal models of diseases, such as ischaemia-reperfusion injury in the heart, liver and brainReference Muscoli, Cuzzocrea, Riley, Zweier, Thiemermann, Wang and Salvemini50. The present study showed that liver SOD activity decreased in cholesterol-fed rats and recovered in rats supplemented with β-carotene and canthaxanthin (Table 7); these findings suggest that these carotenoids may prevent superoxide-associated injury resulting from cholesterol by reducing oxidative stress in the liver.
Several lines of evidence suggest that carotenoids modify lipid metabolism. Amen & LachanceReference Amen and Lachance51 reported that β-carotene intake decreased serum cholesterol in rats fed a vitamin A-deficient hypercholesterolaemic diet. Erdman & LachanceReference Erdman and Lachance52 reported that β-carotene decreased serum cholesterol in hypercholesterolaemic rats. Lopez & TsaiReference Lopez and Tsai53 indicated that canthaxanthin elevated serum total cholesterol and HDL-cholesterol concentrations and decreased liver cholesterol concentrations in rats fed a cholesterol-enriched (0·2 %) diet. MurilloReference Murillo54 showed that both canthaxanthin and astaxanthin had a hypercholesterolaemic effect in rats. Our previous study found that β-carotene prevented both an increase in serum LDL-cholesterol and a decrease in serum HDL-cholesterol in rats fed 1 % cholesterolReference Shih, Cheng and Shieh19. β-Carotene also showed anti-hyperlipidaemic effects in spontaneously hypertensive ratsReference Tsai and Mazeedi55, Reference Tsai, Mazeedi and Mameesh56. In the present study, dietary cholesterol (1 %) significantly elevated plasma and hepatic cholesterol concentrations, and β-carotene (especially crystal form) supplementation improved the higher plasma and hepatic cholesterol concentrations induced by cholesterol (Table 8). The plasma cholesterol concentration of group BC reached a level similar to that of group NC; the hepatic cholesterol concentration of group BC was significantly lower than that of group CC, although it was still much higher than that of group NC. It was previously shown that both dietary and serum cholesterol are closely related to CVDReference Grundy57, Reference Manninen, Tenkanen, Koskinen, Huttunen, Manttari, Heinonen and Frick58. These results suggest that β-carotene may prevent CVD by modifying cholesterol metabolism. TAG metabolism was not affected by dietary carotenoids, except for canthaxanthin (Table 8). Plasma TAG concentrations increased after canthaxanthin supplementation (group CX). The meaning of this phenomenon remains to be investigated.
In conclusion, the present study showed that β-carotene and canthaxanthin supplementation had modulating effects on lipid peroxidation and antioxidative enzyme activities in rats fed a high-cholesterol, high-fat diet. β-Carotene beadlet significantly decreased hepatic TBARS and conjugated diene concentrations, and canthaxanthin significantly decreased plasma TBARS concentrations. Both GPX activity in erythrocytes and SOD activity in liver decreased with cholesterol feeding and recovered with β-carotene and canthaxanthin supplementation. These findings suggest that β-carotene and canthaxanthin altered the pro-oxidation and antioxidation balance and suppressed cholesterol-induced oxidative stress by modulating endogenously the antioxidant system and cholesterol metabolism.










