Introduction
Hepatitis A is a disease caused by the hepatitis A virus (HAV). HAV can be transmitted from person to person or through the consumption of contaminated food products [Reference Cuthbert1 Reference Acheson and Fiore2]. HAV is prone to foodborne outbreaks as it is able to withstand most processes that are commonly used to control bacterial pathogens in food, such as mild pasteurisation and exposure to high temperatures [Reference Cook3]. HAV is also resistant to desiccation, and can remain infectious for several months on frozen foods [Reference Cook3]. Foodborne outbreaks of HAV have been associated most commonly with fresh and frozen produce, such as semi-dried tomatoes [Reference Carvalho4, Reference Donnan5, Reference Gallot6], frozen berries [Reference Gillesberg7, Reference Severi8] and pomegranate arils [Reference Collier9]. Outbreaks of HAV have also been associated with various shellfish such as raw scallops [Reference Viray10] and oysters [Reference Bialek11, Reference Guillois-Becel12]. Hepatitis A is a nationally notifiable disease in Canada, and is reported at a rate of 0.68 per 100 000 persons [13].
Many investigations into foodborne outbreaks include case-control studies as a means to identify a suspect source. Recruitment strategies for controls in these studies vary, but can include random digit dialling, population registries, nearest-neighbour or friend recruitment and hospital or clinic recruitment [Reference Bernstein14, Reference Kalton and Piesse15]. In a research setting, the critical focus of a case-control study design is often the development of a scientifically sound and cost-effective method for recruiting controls [Reference Bernstein14]. Alternatively, in an outbreak context, the critical focus is often the most time-sensitive means of recruitment, as public health action often depends on quick results. This is particularly the case for outbreaks of HAV, where post exposure prophylaxis is possible within the first 14 days after exposure [Reference Link-Gelles, Hofmeister and Nelson16].
Although the use of random digit dialling or listed phone numbers are common for case-control studies, the number of calls to recruit a single control is often high due to non-answers, invalid numbers and a lack of desire to participate. For example, a cryptosporidium investigation by the United States Centers for Disease Control and Prevention required approximately 11 400 calls to complete 151 cases and 302 control interviews [Reference Fox17]. Response rates for random digit dialling methods have seen a dramatic decline in recent years, due in part to the popularity of caller identification [Reference Kalton and Piesse15, Reference Kempf and Remington18, Reference Wang19]. The use of a pre-established control bank of consenting participants could result in a higher response rate than that of random-digit dialling or calling listed numbers within the general population, potentially reducing time and cost to complete a case-control study [Reference Morton20]. A further limitation for random digit dialling methods is the lack of ability to make targeted calls, adding an additional challenge to recruiting controls in the context of a matched study. A control bank, complete with demographic information for each individual, is one solution to this issue. Several case-control studies have used the control bank approach to recruit controls, including studies in Australia for outbreaks of salmonella, campylobacter and Escherichia coli [Reference McPherson21, Reference Munnoch22, Reference Stafford23], and a Canadian study examining an outbreak of cyclospora [Reference Morton20].
In July 2018, 18 cases of laboratory-confirmed HAV infection with genotype 1a were identified across five geographically dispersed provinces in Canada. All cases had one of two genetically related ribonucleic acid (RNA) fingerprints. Although genotype 1a is common in Canada, the RNA nucleotide sequences specific to this outbreak had not been previously associated with any other outbreak. Onset dates ranged between October 2017 and May 2018. Given the multi-jurisdictional nature of the outbreak, case-to-case transmission was considered unlikely. High risk exposures associated with HAV transmission, such as intravenous drug use and homelessness, were not reported among cases. A national outbreak investigation was initiated to identify the likely source of illnesses and determine appropriate public health action. Initial frequency-based analysis of exposure data did not identify a suspect source. A case-control study was conducted to assess whether the frequency of exposures to various food items was significantly different among cases and controls. A control bank, established via a previous study [24], was chosen as the primary method to recruit controls. The objective of this paper is to describe the results of the case-control study, and examine the advantages and disadvantages of using a control bank to facilitate the recruitment of controls in an outbreak investigation.
Methods
Study design
The study was a retrospective matched case-control design. Three controls were recruited for each case and matched based on the first digit of Forward Sortation Area (FSA), age group (0–19, 20–49, 50–69, 70+) and sex (male, female). The FSA represents a geographical unit in Canada. The first digit of the FSA is a letter that identifies the province or territory. For two provinces, this first digit further specifies a particular part of the province. Cases in this study were distributed across five provinces, suggesting that geography did not appear to be closely associated with exposure. However, geography was included as a matching criteria as dietary habits differ significantly across Canada [24], and the investigators wanted to ensure that the control group would have the same geographic diversity as the cases. Given that the control bank consisted of a higher proportion of women than men, and a higher proportion of older adults than younger adults, additional matching based on age and sex ensured that the controls selected would not be biased based on the sampling frame.
Selection of cases
A confirmed case was defined as a resident of or visitor to Canada with laboratory-confirmed HAV infection with genotype 1A and one of two genetically related outbreak RNA fingerprints; an onset date on or after 1 October 2017; and no close contact with a confirmed case 15 to 50 days prior to illness onset. A secondary case was defined as a resident of or visitor to Canada with laboratory-confirmed HAV infection, close contact with a confirmed case 15 to 50 days prior to illness onset and symptom onset at least 15 days after the laboratory confirmed case. Cases were included in the study if they met the confirmed case definition, had a completed questionnaire, and did not report travel outside of Canada during their exposure period.
Genotyping and RNA fingerprinting of clinical isolates was completed at the National Microbiology Laboratory to compare isolates. A 373 nucleotide fragment in the VP1-2A region was amplified to determine genotype and RNA fingerprint. Isolates were considered to have the same RNA fingerprint when all 373 nucleotides were identical.
Selection of controls
Healthy community controls were recruited by a hired third party contractor using a control bank established as part of a separate Canadian study [24]. The control bank was established in 2014 and 2015 and contained contact and demographic information for Canadians that provided consent to have their information collected and stored for future enteric disease outbreak investigations. As of July 2018, when the case control study was initiated, the control bank included 2113 Canadians from all provinces and territories, of which 122 were age, gender and geographic matches to the cases in the current study. When recruitment from the control bank was exhausted, controls were contacted through the use of random landline and cell phone numbers that were obtained from a sampling company. Individuals were excluded from being controls if they had a previous HAV infection and/or ever had symptoms consistent with HAV infection (i.e. jaundice, dark urine or pale/clay-coloured bowel movements), if they were previously vaccinated against HAV, if they travelled outside of Canada in the 15–50 days prior to interview, and if they travelled outside of their province for >14 days total in the 15–50 days prior to interview.
Exposures
Food items were selected for inclusion in the case-control study based on a review of case exposure information and an examination of potential sources of HAV infection from a literature review. The food items included in the study were: ham deli meat, shrimp/prawns, yogurt, fruit smoothies, dried fruits (such as raisins, figs, dates, cranberries and/or apricots), avocado, semi-dried tomatoes, green onion, bagged or pre-washed lettuce in a salad mix, other bagged salad such as broccoli slaw or coleslaw, spinach, strawberries (fresh or frozen), raspberries (fresh or frozen), blackberries (fresh or frozen), blueberries (fresh or frozen) and other berries (fresh or frozen).
Data collection
Exposure information for cases was collected through initial interview by local public health officials using routine provincial HAV questionnaires, as per routine practice. After a national outbreak investigation was initiated, select cases were re-interviewed by the Public Health Agency of Canada (PHAC) to gather additional detail on food items and to ask about exposures not included in the routine interview. Cases were selected for re-interview based on the level of detail captured for exposures in the initial interview. Controls were interviewed by a hired third party contractor using a control questionnaire script. The exposure period was 15–50 days prior to symptom onset for cases, and 15–50 days prior to interview date for controls.
Analysis
To examine the usefulness of the control bank, the average number of calls required per recruited control was calculated for each recruitment method by gender and age group. The response rate was also calculated for each recruitment method by dividing the number of recruited controls by the total number of individuals contacted. The proportion of potential controls excluded as a result of non-response, not meeting matching criteria, not meeting inclusion criteria, refusals and numbers out of service was also calculated for each recruitment method.
To investigate a potential suspect source for the outbreak, a matched analysis of cases and controls was conducted. McNemar's odds ratios (ORs) for matched pairs were calculated to provide an estimate of risk associated with exposure and contraction of HAV. Exposures identified in univariate analysis with p-value < 0.2 and with OR >1 were included in a multivariate analysis. Models were made using backward stepwise selection, with variables remaining in the model if they changed the significant coefficients by more than 20%. All analyses were conducted in Stata at an α level of 0.05.
Results
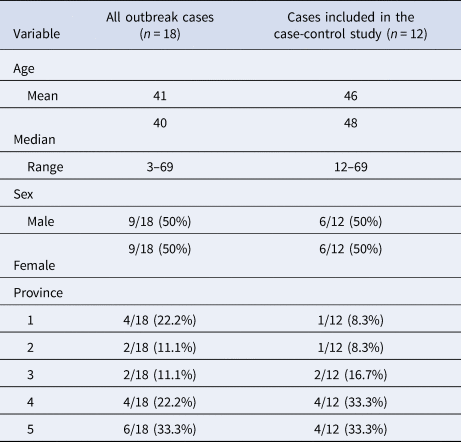
Of the 18 cases in this outbreak investigation, 12 met the inclusion criteria for the study. Six cases were excluded because exposure information was unavailable (n = 3), because they travelled outside of Canada during their exposure period (n = 2), or because they were a secondary case (n = 1). The demographic characteristics of the cases are outlined in Table 1.
Table 1. Demographic characteristics of cases in the outbreak and cases included in the case-control study
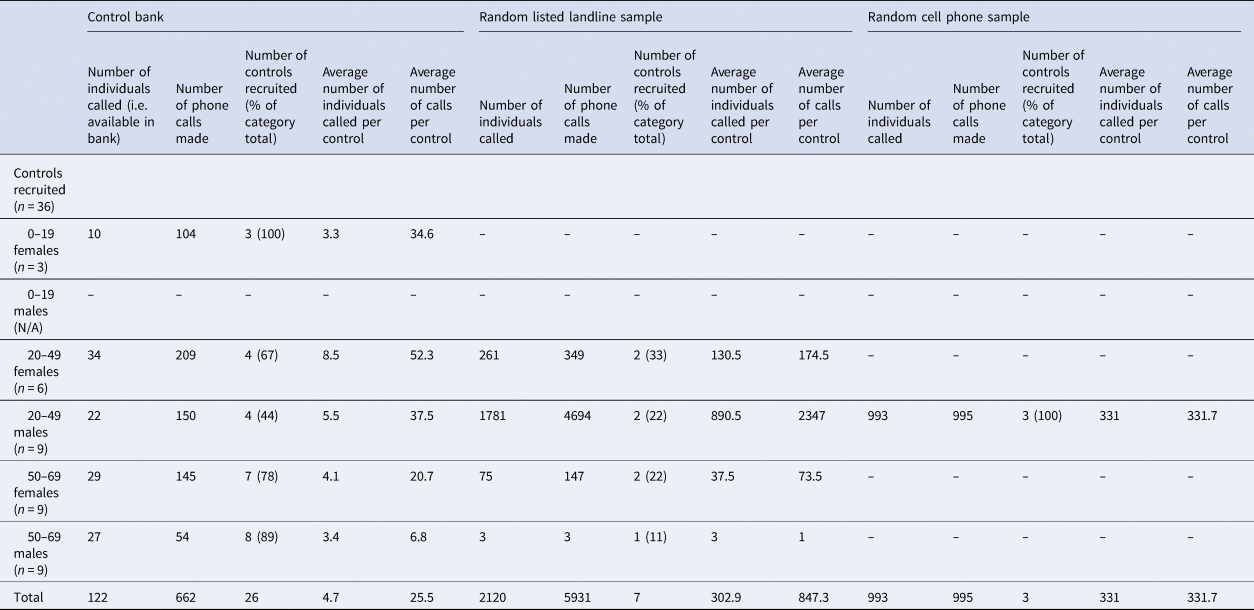
Three controls were matched to each case, for a total of 36 controls. Twenty-six (26/36; 72.2%) of the controls were obtained through the control bank. A total of 662 calls were required to complete interviews for the 26 controls recruited through this method, for an average of 25.5 calls per control (Table 2). The response rate for controls recruited from the control bank was 21%. Females between the ages of 20–49 were the most difficult to recruit using this method, requiring an average of 52.3 calls per control. Seven (7/36; 19.5%) of the controls were obtained through a random dialling from a listed landline sample. A total of 5193 calls were required to complete interviews for the seven controls recruited through this method, for an average of 847.3 calls per control. The response rate for the random listed landline sample was 0.3%. For this recruitment method, males between the ages of 20–49 were the most difficult to recruit, requiring an average of 2347 calls per control. Lastly, three (3/26; 8.3%) of the controls were obtained through a random cell phone sample. A total of 995 calls were required to complete interviews for the three controls recruited through this method, for an average of 331.7 calls per control. The response rate for the random cell phone sample was 0.3%. All three controls recruited with this method were males between the ages of 20–49. Overall, it took 4 weeks to complete interviewing of all 36 controls.
Table 2. Numbers of controls recruited and calls made for each recruitment method. Control bank recruitment was exhausted before proceeding to the landline and cell phone samples
Reasons for exclusion varied by method of recruitment (Fig. 1). When using the control bank to recruit controls, the most common reasons for exclusion were the individual not answering the phone (46% of exclusions) or not meeting the inclusion criteria (29% of exclusions). For the landline sample, the most common reasons for exclusion were the individual not answering the phone (33% of exclusions) and the number not being in service/wrong number (27% of exclusions). For this method of recruitment, exclusions due to refusal or language barrier were also quite high (19% of exclusions). Lastly, for recruitment via the cell phone sample, the most common reasons for exclusion were the number not being in service/wrong number (51% of exclusions) and the individual not answering the phone (40% of exclusions).
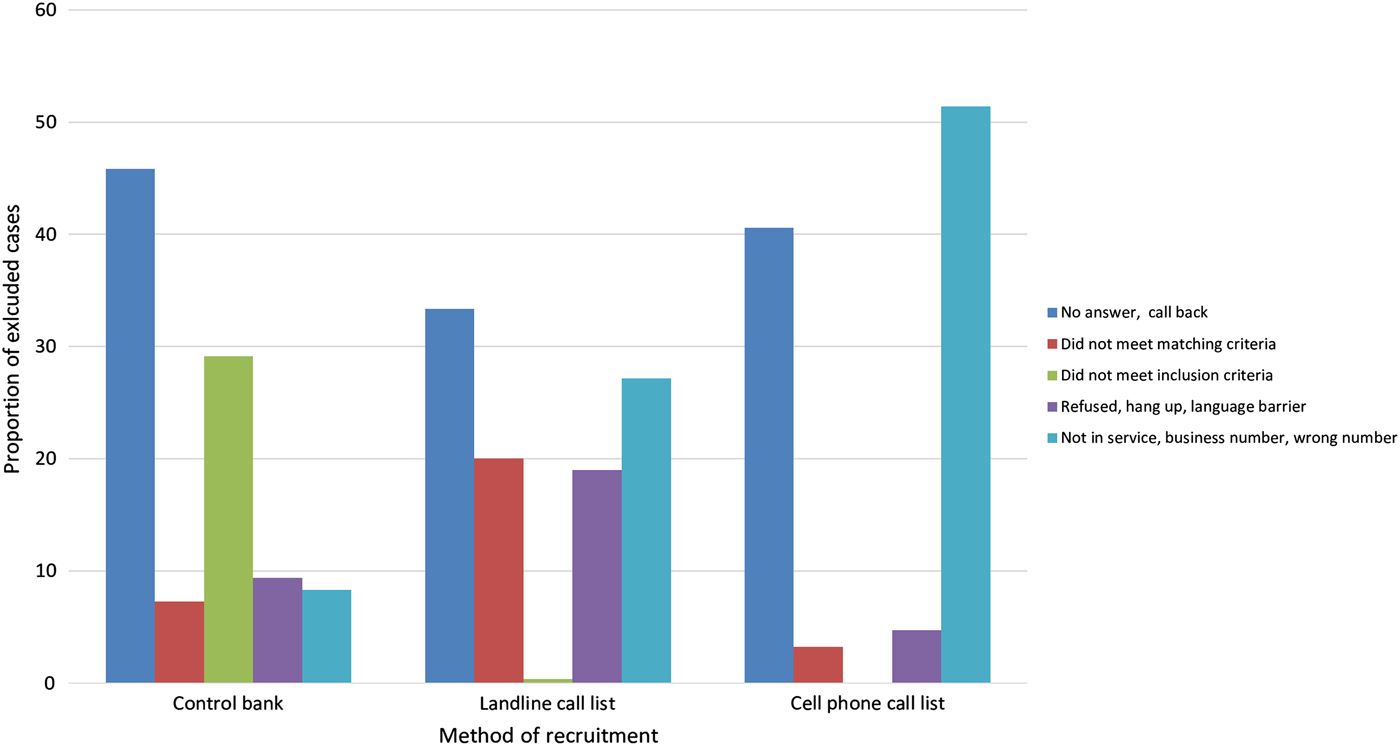
Fig. 1. Reasons for exclusion by method of recruitment.
Food frequencies for cases and controls are summarised in Table 3. Of the 12 cases, five had exposure information based on initial interview alone, while seven cases had exposure information based on initial interview and a re-interview conducted by PHAC. The most frequently reported exposures by cases were: ham deli meat (6/7; 86%), shrimp/prawns (8/10; 80%), yogurt (5/5; 100%), bagged salad (7/8; 88%), fresh strawberries (5/5; 100%), fresh raspberries (6/7; 86%), fresh blackberries (7/8; 88%), any blueberries (10/11; 91%) and fresh blueberries (9/10; 90%). The most frequently reported exposures by controls were ham deli meat (31/36; 86%), any strawberries (33/36; 92%), fresh strawberries (32/36; 89%) and any blueberries (29/36; 81%). Shrimp/prawns had 16 times greater odds of being consumed by cases compared to controls, which was statistically significant at the 0.05 α level (OR 15.75, p = 0.01; Table 3). Blackberries had seven times greater odds of being consumed by cases compared to controls, which was also statistically significant at the 0.05 α level (OR 7.21, p = 0.02). All other food exposures were either not significant at the 0.05 α level, or indicated a protective effect.
Table 3. Food frequencies for cases (n = 12) and controls (n = 36) and matched ORs, confidence intervals and p-values for each food item
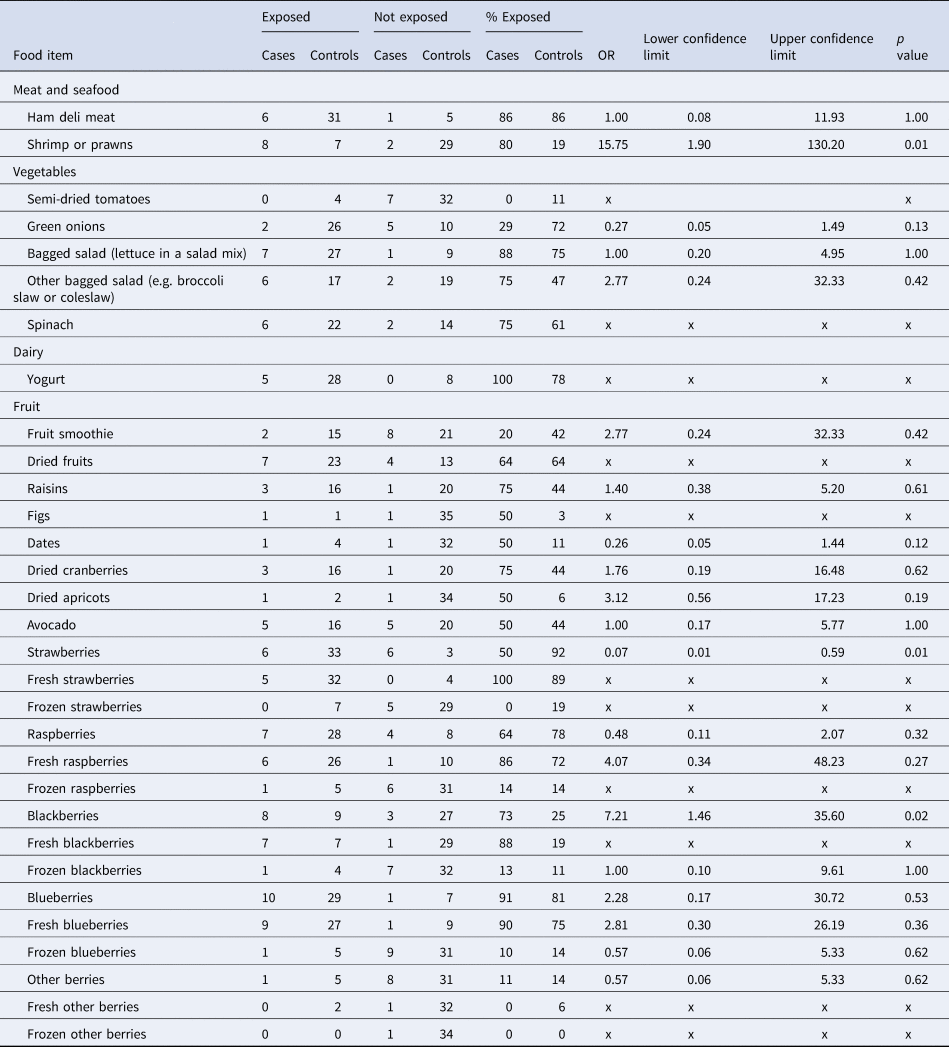
OR, odds ratio; x, odds ratio could not be calculated.
Exposures identified in univariate analyses with a p-value < 0.2 and with an OR >1 were included in a multivariate, conditional logistic regression model. These variables included shrimp/prawns, other bagged salad, and blackberries (Table 3). Due to small cell sizes, the full model did not have sufficient data to produce results. Following the procedure of backward stepwise regression, subsequent models were run, removing variables one at a time, in order of least to most significant based on univariate analyses. Subsequent multivariate models also had insufficient data to produce results.
Discussion
Use of a control bank
Recruitment of neighbourhood or friend controls is one common method of control recruitment in a fast-paced outbreak scenario. In the current outbreak, however, there were several limitations that prevented the use of this method. First, relying on neighbourhood controls assumes that all cases would be able to recruit a minimum of 1–3 controls. However, in our outbreak not all cases were able to be re-contacted after the conclusion of their initial interview; using this method would therefore result in several cases without suitable controls. Second, neighbours or friends may be more likely to share similar food habits as their corresponding case, leading to an underestimate of any effect [Reference Wacholder25]. With a small-sized outbreak, the underestimate of an effect could be detrimental to identifying a potential source. To address these limitations, recruitment of controls via the control bank was chosen as the primary recruitment method.
Overall, the use of the control bank for recruitment in this case-control study was successful. This method had a higher response rate and required the fewest number of calls per control compared to random dialling, likely as a result of several factors. The control bank consists of individuals who have previously agreed to participate in such studies, increasing the response rate when compared to calls to the general population. In this study, the response rate for the control bank was almost 70 times greater than that of the landline or cell phone samples. Similarly, in a recent case-control study related to an outbreak of cyclosporiasis, the response rate for the control bank was 60% compared to only 24% via random digit dialling [Reference Morton20].
The control bank also allows for targeted calls to individuals based on the matching variables of the study. For example, the control bank included information on area of residence for each individual, which was useful for geography-based matching. Given that area codes are not always representative of an individual's home address [Reference Kalton and Piesse15], the use of random digit dialling or call lists likely increases the number of calls needed to obtain a control if geography-based matching is required. The inclusion of age and gender information within the control bank also proved advantageous in comparison with landline or cell phone samples in the context of age and gender matching. Interestingly, the control bank group had the highest proportion of exclusions due to not meeting the inclusion criteria. Compared to landline and cell phone sample recruitment, more of the control bank calls were able to successfully reach a matched individual, and therefore complete the inclusion criteria questions. In addition, compared to the general population (i.e. individuals contacted via the landline or cell phone call lists), it is possible that those individuals who agreed to be included in the control bank are also more likely to be vaccinated for HAV, perhaps a variation of the ‘healthy volunteer effect’ [Reference Delgado-Rodriguez and Llorca26].
Although recruitment via the control bank was the most efficient method for control recruitment, the decision to include three separate matching variables extended the time needed to find eligible controls through all recruitment methods. In this study, it took 4 weeks to recruit 36 controls. Information on the length of time required to recruit controls in other similar case-control studies is limited. Given that case-control studies are often used to collect timely data to inform public health action, reducing the number of matching variables may be an important consideration to expedite the data collection process. A solution may be to control for certain variables in analysis when possible, rather than design.
It's also important to highlight that the initial set up of a control bank may be resource intensive. The current control bank was created through the completion of a separate study, taking place over a period of a year [24]. Although an existing control bank takes a minimal effort to maintain, it's creation requires significant human resources to recruit controls, and may not be feasible for all jurisdictions. Over time, a control bank might become depleted if individuals move, change phone numbers or experience volunteer fatigue, resulting in a need to replenish the bank. In the current study, an expanded control bank would have likely expedited control recruitment. Having more individuals available in each geography, age and gender group may have avoided the need for landline and cell phone samples to supplement recruitment, and in turn hasten the recruitment process. For this reason, expanding the control bank would likely be of value for future case-control studies. Although the control bank was the most efficient method of recruitment in this study, it still required significant human resources to make the 662 calls necessary to obtain the 26 controls from the bank.
The finding that younger age groups were more difficult to recruit is likely indicative of a shift from a preference of phone contact to online contact, a transition that emulates the shift of the previous decade from landlines to cell phones [Reference Kalton and Piesse15]. In this sense future case-control studies may benefit from different avenues for control recruitment, such as online surveys. Online surveys may result in expedited data collection, facilitate recruitment of demographic groups that are more difficult to reach by phone, and lower study costs. However, online surveys may serve to exclude other groups, such as seniors. In addition, online surveys could lead to unrepresentative samples, depending on where the survey is hosted and the population that visits a given website.
Given that this study was designed primarily to identify a suspect source of the outbreak, and not to conduct a comparison of recruitment methods, statistical analyses were not conducted on recruitment numbers. In addition to a very small sample size across all recruitment methods, some demographic subgroups were not represented in landline or cell phone recruitment, as the target number of controls was reached with the control bank alone (e.g. 0–19 year old females). The observations made in this study serve to provide a crude comparison of various methods of recruitment for consideration by other public health professionals when conducting studies for outbreak investigations.
Results of the study
Findings of the case-control study univariate analysis pointed to shrimp/prawns and blackberries as food items of interest. Because there were no leftovers available from case homes for sampling, or product specificity (e.g. brand information) from cases that reported exposures to these foods, it was not possible to conduct traceback or an environmental investigation at the production level.
Although a food safety investigation was not possible, shrimp/prawns and blackberries both present plausible sources for this outbreak. When considering all 17 confirmed cases in this outbreak, shrimp/prawns was one of the most commonly reported exposures. The long shelf-life of frozen shrimp/prawns aligns well with the long duration of the outbreak (8 months), thereby increasing its plausibility as a suspect source. Outbreaks of HAV are commonly associated with shellfish, typically occurring as a result of contamination of harvest beds with human sewage [Reference Bellou, Kokkinos and Vantarakis27, Reference Iwamoto28]. Shellfish implicated in HAV outbreaks have included raw oysters [13, Reference Shieh29, Reference Desenclos30], clams [Reference Sánchez31, Reference Tang32] and less commonly, shrimp [Reference Ashwell33].
Blackberries also produced a significant OR in the univariate analysis, with most cases reporting fresh blackberry exposure. The OR may be underestimated, as controls were interviewed exclusively in the summer, a time when berry consumption is typically higher [24]. One case reported visiting an area of Mexico known for blackberry production during their exposure period. In addition, general import patterns indicate that blackberries in Canada are imported from October to May, with a switch to domestic suppliers in the summer months (unpublished data). This import pattern aligns with the timing of case onsets in this outbreak, which also occurred from October to May. Berries have been identified as the source of numerous HAV outbreaks globally. For example, mixed frozen berries were implicated in outbreaks in Italy [Reference Rizzo34] and Ireland [Reference Fitzgerald35], frozen strawberries were implicated in a multinational Nordic outbreak [Reference Severi8] and fresh blueberries were identified as a suspect source in an outbreak in New Zealand [Reference Calder36].
The high ORs of blackberries and shrimp/prawns may also be an indication of a potential confounding variable, as these exposures are not commonly reported in the Canadian population [24]. Without a multivariate analysis, it is difficult to determine the independent effect of shrimp/prawns or blackberries on the outcome. Limited product specificity from cases and an absence of any product leftovers also prevented a food safety investigation, and therefore, the confirmation of a source.
Interestingly, strawberries appeared to have a protective effect, with an OR less than 1. This again might be a result of the seasonality of control interviewing. While cases had their exposure periods distributed throughout the year, all controls were interviewed in the month of August. This could have increased the frequency of strawberry consumption for controls compared to cases, thereby creating the appearance of a protective effect.
This case control study has several limitations. First, while case exposures were dispersed over an 8 month period, from October 2017 to May 2018, control exposures were collected only in the month of August. As mentioned previously, this difference may affect the comparability of food frequencies between the two groups. Second, accuracy of recall may have differed between cases and controls, as cases were often interviewed and/or re-interviewed weeks or months after their symptom onset; alternatively, controls recalled exposures in the 15–50 days preceding the date of interview. There were only 12 cases included in this case-control study, with some cases having incomplete data on various exposures of interest. This limitation prevented the calculation of some matched ORs and resulted in reduced precision and large confidence intervals. In addition, small cell sizes prevented the completion of a multivariate conditional logistic regression model. Without a multivariate model, it is possible that confounding impacted results.
Conclusion
Findings of the study pointed to shrimp/prawns or blackberries as foods of interest in this outbreak of HAV in multiple provinces across Canada. Although the source could not be confirmed, the methodology of the study allowed for a useful comparison of recruitment strategies. Control banks continue to prove their utility over landline and cell phone samples, but other avenues of recruitment, such as online surveys, may be increasingly useful in the future.
Acknowledgements
The authors would like to acknowledge the contributions of Anton Andov, Eleni Galanis, Marsha Taylor, Victor Mah, Colette Gaulin, Caroline Duchesne, Bev Billard and Sarah Fleming. The authors would also like to thank local public health colleagues and public health laboratories in British Columbia, Alberta, Ontario, Quebec and Nova Scotia as well as the British Columbia Centre for Disease Control, Alberta Health, Alberta Health Services, Public Health Ontario, Ontario Ministry of Health and Long-Term Care, Ministère de la Santé et des Services sociaux du Québec and the Nova Scotia Department of Health and Wellness. Lastly, the authors would like to thank the Canadian Food Inspection Agency, Health Canada and the Public Health Agency of Canada for their support of this investigation.
Financial support
This work was supported by the Public Health Agency of Canada.
Conflict of interest
None.






