Introduction
Medicinal and aromatic plants (MAP) are important sources of secondary metabolites, and therefore they are used in the production of food, dietary supplement products, herbal medicines, drugs, natural health products and plant protection products (Lubbe and Verpoorte, Reference Lubbe and Verpoorte2011).
Global demand for MAP-based products is steadily rising (Chen et al., Reference Chen, Yu, Luo, Wu, Li and Steinmetz2016). A large number of MAPs are collected in the wild, but commercial cultivation in an agricultural setting ensures stable and predictable yields, the possibility to choose accessions with preferable profiles of chemical compounds, and botanical identity (Hassan and Mohammed, Reference Hassan and Mohammed2010). For the abovementioned reasons and in response to the rising demand for organically produced MAPs, large scale cultivation of medicinal plants is increasing (Chen et al., Reference Chen, Yu, Luo, Wu, Li and Steinmetz2016; Jeelani et al., Reference Jeelani, Rather, Sharma and Lattoo2018).
Over 3000 MAP species are being cultivated, including agroforestry, intensive and extensive farming, natural fostering, hydroponics and trial cultivation. Most MAPs have not been the subject of breeding, resulting in a scarcity of registered cultivars. As a result, local wild populations continue to be a valuable source of germplasm, and research of quantitative and qualitative properties as well as selection and breeding is necessary to introduce them to agricultural settings successfully (Jeelani et al., Reference Jeelani, Rather, Sharma and Lattoo2018; Wang et al., Reference Wang, Xu, Fang, Li and Li2020).
Taraxacum is a genus consisting of >2500 species in the Asteracea family (Di Napoli and Bull, Reference Di Napoli and Bull2021). T. campylodes, T. mongolicum, T. laevigatum (syn. T.erythrospermum), T. platycarpum and T. kok-saghyz are the most studied plant species in the genus (Martinez et al., Reference Martinez, Poirrier, Chamy, Prüfer, Schulze-Gronover, Jorquera and Ruiz2015). The common dandelion Taraxacum campylodes G.E. Haglund (syn. Taraxacum officinale (L.) Weber ex F.H. Wigg) (Maggi, Reference Maggi, Nabavi and Silva2019) is a widespread perennial plant with a 1–2 m long taproot. The plant has no stem and develops a rosette of leaves (Stewart-Wade et al., Reference Stewart-Wade, Neumann, Collins and Boland2002, Schütz et al., Reference Schütz, Carle and Schieber2006). Dandelion has high phenotypic plasticity and grows in a variety of environments (Lee et al., Reference Lee, Moyer, Entz, Tovell, Boswall and Kawchuk2010) e.g. as a weed in commercial cropping systems (including annual crops), in lawns, meadows, pastures, overgrazed areas and hay fields (Froese and Van Acker, Reference Froese and Van Acker2003).
Due to its health-promoting effects, dandelion has been used in traditional folk medicine in Greece, Russia, India and China, as well as in North America, Algeria, Latvia and elsewhere (Lis and Olas, Reference Lis and Olas2019; Sile et al., Reference Sile, Romane, Reinsone, Maurina, Tirzite and Dambrova2019, Imene et al., Reference Imene, Abdelkrim, Hayet, Samira, Louiza, Abdelhamid and Abassia2020). Common dandelion is also widely used today and has a wide range of applications; almost all plant parts, including flowers, leaves and roots can be used as food, cosmetics, in the beverage industry (Martinez et al., Reference Martinez, Poirrier, Chamy, Prüfer, Schulze-Gronover, Jorquera and Ruiz2015; Alexopoulos et al., Reference Alexopoulos, Assimakopoulou, Panagopoulos, Bakea, Vidalis, Karapanos and Petropoulos2021a). Root (Taraxaci radix), root with aerial part (Taraxaci radix cum herba), leaves (Taraxaci folium) and flowers (Taraxaci flos) are used in medicine (Lis and Olas, Reference Lis and Olas2019) and are processed into pharmaceutical preparations such as teas, tinctures, capsules and tablets (Williams et al., Reference Williams, Goldstone and Greenham1996; Dias et al., Reference Dias, Barros, Alves, Oliveira, Santos-Buelga and Ferreira2014; Rasool and Sharma, Reference Rasool and Sharma2014). Due to their choleretic, diuretic, antirheumatic, antihyperglycemic, anti-inflammatory, anticarcinogenic, antiangiogenic, antirheumatic, antilaxative and appetite-stimulating properties, herbal dandelion medicines have long been used to treat liver and gallbladder disorders, diabetes, digestive complaints and arthritic and rheumatic diseases (Schütz et al., Reference Schütz, Carle and Schieber2006; Lis and Olas, Reference Lis and Olas2019; Sun et al., Reference Sun, Tan, Wei, Liu, Mai, Liu, Liu, Zhuang, Zou, Zhang, Liu and Ye2022).
The dandelion contains polysaccharides (inulin), amino acids, vitamins (L-ascorbic acid), minerals (potassium, zinc) (Kania-Dobrowolska and Baraniak, Reference Kania-Dobrowolska and Baraniak2022) and a variety of bioactive secondary metabolites, such as terpenes, phenolic acids and flavonoids. Dandelion roots contain hydroxycinnamic acids, derivatives of hydroxyphenylacetic acid (Jedrejek et al., Reference Jedrejek, Lis, Rolnik, Stochmal and Olas2019), sesquiterpene lactones, triterpenes and phytosterols (Kisiel and Barszcz, Reference Kisiel and Barszcz2000) and phenolic compounds (Williams et al., Reference Williams, Goldstone and Greenham1996). Caffeic acid, chicoric acid and chlorogenic (CHA) acid are the most common polyphenolic compounds found in all parts of the plant (Williams et al., Reference Williams, Goldstone and Greenham1996; Lis and Olas, Reference Lis and Olas2019). Chicoric acid (CCA) is effective in preventing the formation and worsening of the atherosclerosis process (Tsai et al., Reference Tsai, Kao, Hung, Cheng, Lin and Chu2017) and it has also antioxidant, anti-adhesive, anti-aggregation and anti-diabetic properties (Wirngo et al., Reference Wirngo, Lambert and Jeppesen2016; Lis and Olas, Reference Lis and Olas2019). Payen (Reference Payen1846) invented the term ‘chlorogenic acid’ to describe components of isolated green coffee beans. Today, it is understood that these substances belong to a widely distributed class of naturally occurring esters formed between caffeic and quinic acid (Kenny et al., Reference Kenny, Smyth, Hewage and Brunton2015) and have been abundant in many plants. CHA has been shown to have scavenging, antioxidant and antiapoptotic properties, as well as anti-inflammatory, antibacterial, antiviral and immunomodulatory properties (Kania-Dobrowolska and Baraniak, Reference Kania-Dobrowolska and Baraniak2022). Due to its anti-inflammatory effect, which is caused by microglial activation and antioxidant brain activity (Gil and Wianowska, Reference Gil and Wianowska2017), the CHA effect on relieving neurodegenerative diseases has been extensively studied.
Despite their widespread use, dandelions are harvested mainly in the wild (Martinez et al., Reference Martinez, Poirrier, Chamy, Prüfer, Schulze-Gronover, Jorquera and Ruiz2015). Commercial cultivation has been reported in Canada, Chile, Germany, Poland, France, India, Mexico, Austria, Hungary, the Netherlands, the United States of America and Italy (Letchamo and Gosselin, Reference Letchamo and Gosselin1995; Brinckmann et al., Reference Brinckmann, Kathe, Berkhoudt, Harter and Schippmann2022). There are neither enough cultivable dandelion varieties, especially ones with medicinal properties (Jambor, Reference Jambor and Hoppe2013), nor enough research on how the farming system and the environment affect the yield and quality of dandelion root (Letchamo and Gosselin, Reference Letchamo and Gosselin1995).
Breeding efficiency can be improved by selecting the best genotypes based on easy-to-measure and correlated traits essential for farming, such as yield and quality. Leaf size is important for plant adaptation because it increases the competitive ability of wild populations in different ecosystems, thus increasing aboveground biomass and seed production (Vellend et al., Reference Vellend, Drummond and Muir2009; Drummond and Vellend, Reference Drummond and Vellend2012). It is influenced by genetic and environmental factors, e.g. nutrient availability (Cox and Ford, Reference Cox and Ford1987), salinity (Alexopoulos et al., Reference Alexopoulos, Assimakopoulou, Panagopoulos, Bakea, Vidalis, Karapanos and Petropoulos2021a), light quality (Brock et al., Reference Brock, Weinig and Galen2005), soil CO2 (Sharma et al., Reference Sharma, Apple, Zhou, Olson, Dorshorst, Dobeck, Cunningham and Spangler2014) and soil contamination with metals (Collier et al., Reference Collier, Keane and Rogstad2010). Previous studies have indicated possible correlation between leaf area and root characters as a trace for evaluating breeding potential. In grasslands, leaf area has been linked to root length and weight in dandelion (Struik, Reference Struik1967), but increasing of salinity decreases both – leaf rosette diameter and root fresh weight (Alexopoulos, et al., Reference Alexopoulos, Assimakopoulou, Panagopoulos, Bakea, Vidalis, Karapanos and Petropoulos2021a). The effect of flower area on root yield in common dandelions is less clear. Potato (Tekalign and Hammes, Reference Tekalign and Hammes2005) and Jerusalem artichoke (Gao et al., Reference Gao, Zhang, Zhu and Coulter2020) have a negative correlation between reproductive growth and root/tuber yield. According to Letchamo and Gosselin (Reference Letchamo and Gosselin1995), removing flower heads increases dandelion root yield.
There are several methods for measuring the parameters of the dandelion leaf and flower area, including manual measurement, calculation based on the number and area of leaves (Vellend et al., Reference Vellend, Drummond and Tomimatsu2010), and use of leaf area metres (Vellend et al., Reference Vellend, Drummond and Muir2009). However, using such methods is time-consuming or destructive and thus hinders further plant growth.
Recently, high-resolution plant phenotyping methods have become very common for precise and fast evaluation of different morphological traits indirectly related to productivity parameters. Among the most popular image platforms are those based on digital red-green-blue (RGB) and single-lens reflex cameras (Zhang and Zhang, Reference Zhang and Zhang2018).
The aim of the study was to evaluate the domestication and breeding potential of wild common dandelion populations. We attempt to characterize variation in productivity, morphological traits and CCA and CHA content in roots. Second, the possibility of indirect detection of root weight was investigated by automated assessment of leaf and flower areas. We hypothesized that there could be a positive relationship between leaf area and root weight and that common dandelion populations with smaller flower areas would have larger roots and therefore the leaf area and flower area traits could be used to select more productive populations or plants.
Materials and methods
Plant material
Thirteen wild populations of T. campylodes G.E. Haglund (Table 1) were collected from different habitats in May and June (2017) in Latvia (Fig. 1 and Supplementary Table S1). In each habitat, approximately 5000 seeds were collected from randomly selected plants with various age (Brown and Marshall, Reference Brown, Marshall, Guarino, Ramanatha and Reid1995). Habitats were crossed by walking in a zig-zag pattern. Only part of the seeds from every inflorescence was picked in order to maintain the genetic diversity of the wild population. Two commercially available accessions namely Nouvelle and TA008 (Germany) were used as a control. Until the experiments, the seeds were stored at 18°C with a humidity of 50%.
Table 1. Weight of fresh and dry roots (g) and yield of fresh and dry roots (kg ha−1) for wild Latvian dandelion populations and dandelion accessions of German origin
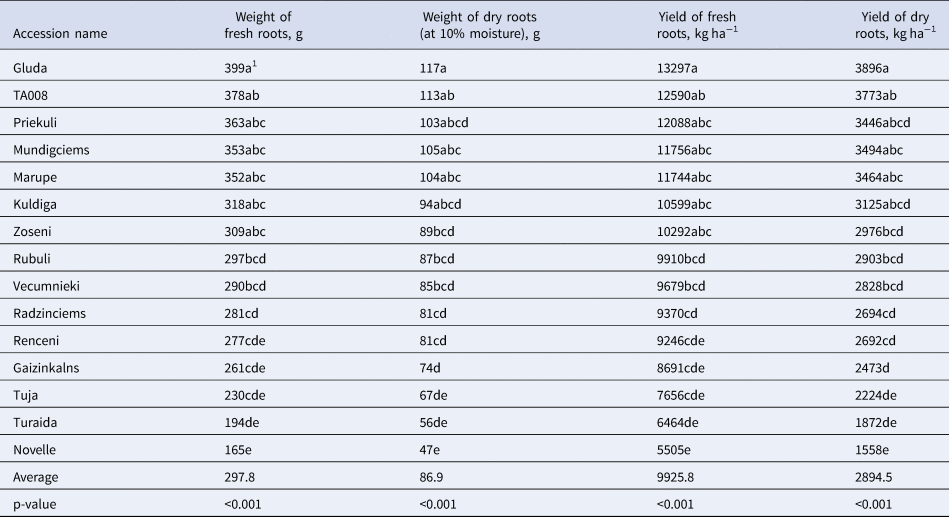
1 Means tracked with different lowercase letters indicate significant differences between accessions according to Tukey's test (P < 0.05).

Figure 1. Locations of the wild dandelion collection in Latvia.
Within the study, the term ‘population’ refers to both wild populations and commercial accessions.
Experimental site and growth conditions
In 2018 and 2019, field trials were conducted on LTD Field and Forest's organically certified fields (57°19’22.7”N 25°10.4”E, 110 m altitude). The trial was designed with a fully randomized block layout. Each population was represented by three replications (plots) of 200 plants per plot, for a total of 600 plants per population. The size of the plot was 60 m2 and the plant spacing was 0.75 × 0.25 m. (3.3 plants per m2). On July 5, 2018, sixty-day-old plantlets were planted. The harvest of the roots occurred on September 30, 2019.
The experiment was carried out under organic farming conditions; thus, no fertilizers or pesticides were applied. Prior to planting, 600 kg ha−1 of granulated lime (CaO 44%) was applied to the fields. The soil properties in the experimental field were the following: soil type: stagnic luvisol, sandy loam, PHKCL 5.7%, organic matter 2.5%, P2O5 93 mg kg−1, K2O 131 mg kg−1. The fields were left at black fallow the previous year. Interrow cultivation and hand weeding were performed on a regular basis during trial maintenance (2–3 weeks).
The climate in Latvia is characterized as continental and cool and corresponds to the Nemoral environmental class NEM2 (Metzger et al., Reference Metzger, Bunce, Jongman, Mücher and Watkins2005). The average annual air temperature in Latvia is 5.9°C, varying from −3.1°C in January and February to 17.8°C in July. The average annual precipitation in Latvia is 685.6 mm, ranging from 35.8 mm in April to 76.8 mm in August (average data from 1961 to 2020, https://videscentrs.lvgmc.lv/lapas/latvijas-klimats, assessed on 1 May, 2023).
The 2018 growing season was generally warmer and drier than the 2019 growing season. The sum of active temperatures (over 5°C) from 1 April to 31 October were 3030 degrees in 2018 and 2742 degrees in 2019, respectively, but total precipitation was 341 mm in 2018 and 528 mm in 2019 (long-term data for the same period – 480 mm) (data from Priekuli meteorological station of the Latvian Environment, Geology and Meteorology Centre, https://videscentrs.lvgmc.lv/).
Measured productivity and morphological parameters
A high-resolution RGB camera and an automated image processing workflow were tested to assess the leaf area of the plant (cm2), the flower area (cm2) and the flower fraction (%) as described by Mežaka et al. (Reference Mežaka, Kronberga, Nakurte, Taškova, Jakovels and Primavera2020). Parameters were measured during flowering (Fig. 2).

Figure 2. An example of image processing of dandelion plants: (a) acquired image, (b) detection of plant, (c) detection of flower heads and (d) masked target plant.
A standard high-resolution RGB camera was used for the documentation of dandelion plants. The target plant was located in the centre of the view (Fig. 2a). A colour statistics-based approach in combination with image thresholding using the Otsu method (Otsu, Reference Otsu1979) was used for the differentiation of plants from the background (Fig. 2b). The largest central object (Fig. 2c) representing the target plant was further extracted. The size of the plant was estimated on the basis of pixel size. For each individual plant, the median value from three image acquisitions was calculated and further used in the study.
The reference dataset was prepared by manually annotating dandelion images which was then used both for the training of NN classifiers as well as validation of the performance. The training and validation subsets did not overlap, and the standard proportion of 20/80 was used. Furthermore, processed images were visually checked to ensure that the automated approach did not produce unexpected results that would affect the overall results and generated statistics.
Image processing consisted of several automated steps:
• Dandelion plant and flower detection by two trained Neural Network classifiers based on pixel colours (plant detection accuracy was ~96% but flower detection accuracy was ~99%);
• Assessment of leaf area (cm2) by counting image pixels from detected target plant mask (Fig. 2b);
• Evaluation of flower area (cm2) by counting image pixels from the flower mask of the detected target plant (2c);
• Evaluation of the flower fraction (%) by dividing the flower area by the leaf area of the plant.
The roots of 20 randomly selected plants from each replication were harvested and washed with clean water. After measuring the fresh weight of each root, they were chopped and dried at 45°C (Jambor, Reference Jambor and Hoppe2013). The dry matter content was determined after drying and the dry root weight was calculated for a sample moisture of 10% (European Pharmacopoeia, 2020). The yield of fresh and dry roots was calculated by multiplying the number of plants per ha (33 333 plants ha−1) by the average weight of fresh and dry roots, respectively.
Determination of the content of chlorogenic acid and chicoric acid
The chlorogenic acid (CHA) and chicoric acid (CCA) content of dried roots from each accession was determined. The loss on drying for T. campylodes samples was performed using thermogravimetric balances at 105°C until the weight of the analysed sample did not change in 90 s (European Pharmacopeia, 2020)
In the EP (European Pharmacopeia, 2020) described method, TLC (thin layer chromatography) is applied, which is used for qualitative rather than quantitative evaluation. According to our initial observations, along with CHA, dandelion roots contain a significant amount of CCA. Based on Stylianou et al. (Reference Stylianou, Gekas, Istudor and Ioniţă2014), we developed a simple and accurate liquid chromatography (LC) method for the separation of CCA and CHA from common dandelion root samples.
Sample preparation and content measurements
70% ethanolic extracts of powdered root samples were prepared by dissolving 0.15 g of the sample in 10 ml of 70% ethanol. Samples were mixed and sonicated in an ultrasonic bath for 30 min. Then the samples were cooled and filtrated through a membrane filter with a nominal pore size of 0.45 μm and injected into a LC system (Stylianou et al., Reference Stylianou, Gekas, Istudor and Ioniţă2014).
Chromatographic analyses were performed on an Agilent 1290 Infinity II series HPLC system (Agilent Technologies, Germany). LC separations were achieved using an Agilent Eclipse XDB-18 column of 3.5 μm, 4.6 × 150 mm (Zorbax) with (35°C) a mobile phase prepared from phosphoric acid in water 99.9/0.1, v/v (solvent A) and acetonitrile (solvent B) at a flow rate of 0.8 ml/min in gradient mode (Supplementary Table S2). The injection volume was 3 μl. Chromatograms were obtained on an Agilent WVD detector (Agilent Technologies, Germany) at a wavelength of 330 nm. The experimental data was handled using ChemStation 32 software (Agilent Technologies). Retention times (tR) in standard solution and analysed samples were compared for peak identification.
The standard solution corresponding to 0.02 mg ml−1 of both phenolic acids was prepared by dissolving 5 mg of reference standards in 10 ml of 70% ethanol. The solution was mixed and sonicated for 15 min. 2 ml of the obtained solution was diluted to 50 ml of 70% ethanol. The quantification of phenolic acids was performed using the calibration procedure. The calibration curve of each standard solution was constructed by plotting the ratio of the average chromatographic peak area and mass concentration (CHA – y = 11,798x-0579; R = 0.9991; CCA – y = 18,682-1385; R = 0.9994).
Data analysis
Rstudio version 4.0.4 is used for statistical analysis of the data. Descriptive statistics, including mean, median and coefficient of variation (CV, %), were applied to quantitative data. One-way analysis of variance followed by Tukey's test (P < 0.05) was used to compare the accession means. The bivariate correlations between traits were evaluated by calculating Pearson's correlation coefficient. The correlation analyses were carried out by the Corrplot package. Hierarchical cluster analysis was done using Ward's clustering method on the basis of average root weight, leaf and flower area, flower fraction, CHA and CCA content in roots.
Results
The wild populations of Latvia were generally characterized by a high variation in leaf area (from 758 to 1329 cm2) (Fig. 3, Supplementary Table S3). The largest leaf area (P < 0.05) was observed in the Kuldiga, Turaida, Gluda, Zoseni and Renceni populations, as well as the commercial population TA008. All wild populations except Tuja had significantly (P < 0.05) higher leaf area than Nouvelle. A large variation of leaf area in-between plants within populations was observed ranging from 27.3% (Marupe) to 44.9% (Tuja).

Figure 3. Leaf area (cm2) (a), flower area (cm2) (b), flower fraction (%) (c) in wild and commercial dandelion populations. According to the Tukey's test, different lowercase letters indicate significant differences between accessions (P < 0.05).
The average flower area ranged from 65.9 cm2 for the Nouvelle to 270 cm2 for the Kuldiga population. The wild populations Kuldiga, Renceni, Gluda, Zoseni, and commercial population TA008 had the highest flower area (>220 cm2). The lowest flower area was observed in Nouvelle and local populations Tuja, Mundigciems, Gaizinkalns and Priekuli (<130 cm2). The average variation in the in-between population (CV 34.5%) of the flower area was higher than that (CV 15.7%) of the leaf area. There was a high degree of variation in flower area between plants in both wild populations (57.5–98.7%) and commercial populations (72.5–79.9%).
The flower fraction ranged from 20.1% in Renceni and TA008 to 8.6% in Nouvelle (Fig. 3c, Supplementary Table S3). The variation in flower fraction between populations was 22.2%, but variation between plants ranged from 37.8% in Renceni to 76.5% in Gluda.
The population had a significant effect on fresh root weight and yield as well as on CHA and CCA content (P < 0.001). The average fresh root weight ranged from 165 g and 399 g, whereas the average dry root weight ranged from 47 g and 117 g in Nouvelle and Gluda respectively (Table 1, Supplementary Table S3). The weight of the roots was significantly (P < 0.05) higher in all wild populations except Gaizinkalns, Tuja and Turaida than in the commercial population Nouvelle.
The average yield of fresh roots varied between 13,297 kg ha−1 (Gluda) and 5505 kg ha−1 (Nouvelle). The dry root yield varied between 3896 kg ha−1 and 1558 kg ha−1 for the same accessions respectively (Table 1, Supplementary Table S3). The highest yield was obtained from the Latvian populations Gluda, Priekuli, Marupe, Mundigciems and TA008, but the lowest yield was obtained from the Nouvelle, Tuja, Turaida and Gaizinkalns.
The average amount of CCA was larger than the average amount of CHA in all accessions. The concentration of CHA ranged from 0.028% (Priekuli) to 0.123% (Turaida), while the concentration of CCA ranged from 0.049% (Priekuli) to 0.153% (Turaida) (Fig. 4a and b, Table S3). CHA was more variable in-between accessions (CV 34.6%) than CCA content (CV 25.1%). When compared to wild populations, the amounts of CHA and CCA in TA008 and Nouvelle were average.

Figure 4. Chlorogenic acid (a) and chicoric acid (b), on dry matter, % for wild Latvian dandelion populations and dandelion accessions of German origin. According to the Tukey's test, different lowercase letters indicate significant differences between accessions (P < 0.05).
The correlations between traits were calculated to determine whether semi-automatically acquired images of aboveground plant size can predict root weight. Dry root weight significantly correlated with leaf area (RPearson = 0.55, P < 0.05) (Fig. S1). There were significant, positive correlations between leaf and flower area (RPearson = 0.87, P < 0.001) and leaf area and flower fraction (RPearson = 0.94, P < 0.001). No significant correlations were observed between the weight of the roots and the content of CCA or CHA, nor between the productivity of the roots and the area of the flowers.
The dendrogram (Fig. 5) categorizes accessions into seven clusters based on principal component (PC) scores calculated from average root weight, leaf and flower area, flower fraction and CHA and CCA content in roots. The clustering did not represent the distance between the geographic collection sites of the sample. Cluster A represented Zoseni and Rubuli, two wild populations with the lowest CHA content (0.039–0.042%). Cluster B included two local populations, Kuldiga and Renceni which had the largest leaf area (1226–1329 cm2), flower area (247–270 cm2) and flower proportion (18.7–20.1%). Cluster C comprised the commercial population TA008 and the population from Gluda which both had a high dry root weight (116–121 g), leaf area (1218–1261 cm2) and flower area (247–270 cm2). Three populations (Turaida, Priekuli, Nouvelle) were assigned to separate clusters. The population from Turaida had the highest amount of CCA (0.123%) and CHA (0.153%) among tested accessions, while the population from Priekuli had a high root weight (106 g) and very low CCA content in roots (0.049%). Nouvelle had the smallest dry root weight (47.8 g), leaf area (758 cm2), flower area (65.9 cm2) and flower proportion (8.6%). The wild populations Vecumnieki, Radzinciems, Gaizinkalns, Tuja, Mundigciems and Marupe comprised Cluster G, with average values across all tested traits.

Figure 5. Hierarchical clustering of common dandelion populations by Ward's clustering method based on average root weight, leaf and flower area, flower fraction, CHA and CCA content in roots.
Discussion
The global demand for medicinal plants is continuously growing. Pharmaceutical and herbal product manufacturers as well as customers prefer cultivated medicinal plants rather than harvest them from the wild, since the supply may be uniform, predictable and certified biodynamic or organic (Hassan and Mohammed, Reference Hassan and Mohammed2010; Alexopoulos et al., Reference Alexopoulos, Marandos, Assimakopoulou, Vidalis, Petropoulos and Karapanos2021b). Before novel MAP accessions can be cultivated on a commercial scale, they must be screened for suitability of cultivation in terms of high yields and sufficient amounts of marker chemical compounds. Although common dandelion has been widely studied, few studies have focused on the morphological traits and chemical composition of wild populations. We measured the leaf and flower area, root weight, CHA and CCA content in the roots of 13 wild populations collected in Latvia and grown organically, as well as two central European (German) populations.
Letchamo and Gosselin (Reference Letchamo and Gosselin1995) tested three populations of T. officinale in a greenhouse and found that the average weight of the dry roots was 21.7 g, with only small differences between populations. In our trials, the average dry root weight varied significantly across the tested wild populations and at best exceeded 100 grams, indicating that some of these populations have high yield potential and could be directly grown commercially. At the same time, a large variation in root weight was found among the plants in each population (CV, ranging from 43.1% to 66.1%), indicating that selection within populations can be used to improve them as the next step of domestication. A large variation in root weight was also observed in commercially available populations (52.4% for TA008 and 66.1% for Nouvelle).
Root yield is influenced by a range of factors, including genotype, growing conditions and plant density. The dry root yield in this study ranged from 1558 to 3896 kg ha−1 (with plant density 33 333 plants ha−1) and was higher for most tested accessions when compared to the results obtained by Michaud et al. (Reference Michaud, Gosselin, Tremblay, Benoit, Belanger and Desroches1993) – 2200 kg ha−1 with higher plant density – 88 888 plants per ha.
The main goal of breeding medicinal plants is to increase the yield and production of bioactive components (Bhandari et al., Reference Bhandari, Nishant Bhanu, Srivastava, Singh and Shreya2017; Jeelani et al., Reference Jeelani, Rather, Sharma and Lattoo2018). All wild dandelion populations tested had a higher root yield than the commercial Nouvelle population, which is recommended by the seed supplier for root production. Five wild populations reached the same dry root yield level (>3000 kg ha−1) as the most productive foreign population included in the trial (TA008).
The content of CHA and CCA in the dry roots of most of the populations was lower than in previously published results. The differences could be caused by genetic variations, different climatic conditions and collection dates. Kenny et al., Reference Kenny, Smyth, Hewage and Brunton2015 (reviewed in Wirngo et al., Reference Wirngo, Lambert and Jeppesen2016) found 0.09–0.51% CHA in extracts of dandelion roots collected in Ireland. Stylianou et al. (Reference Stylianou, Gekas, Istudor and Ioniţă2014) observed 0.13–0.50% CCA in dry dandelion roots harvested in the wild in Cyprus, with a higher accumulation of CCA (0.50%) in roots harvested in December.
The amount of CHA and CCA was average in commercial populations (TA008 and Nouvelle) compared to the range of CHA and CCA content in wild populations. As a result, we can conclude that the best performing local common dandelion populations have yield potential and chemical composition comparable to those of commercially available populations, making them suitable for commercial growing. The wild populations from Marupe and Mundigciems were the ones that combined both a high average root weight and good CHA and CCA content.
CHA and CCA content in roots varied among the wild accessions indicating that populations with the highest content can be selected for breeding. The wild population from Turaida had the most CCA and CHA of the populations tested, but its average root yield was only 47% of that of the most productive accession. This means that this population could be used as a donor for breeding because of its high CCA and CHA content.
The large variation in the morphological parameters is consistent with the previous studies that have observed variation in dandelion biomass, leaf area (Vellend et al., Reference Vellend, Drummond and Tomimatsu2010), leaf length, width and lobe/tooth length and specific leaf area (Vellend et al., Reference Vellend, Drummond and Muir2009), as well as seed coat thickness (Taylor, Reference Taylor1987; Molina-Montenegro et al., Reference Molina-Montenegro, Acuña-Rodríguez, Flores, Hereme, Lafon, Atala and Torres-Díaz2018). Previous studies demonstrated that the variation of morphological traits is more pronounced in field trials than in greenhouses (Vellend et al., Reference Vellend, Drummond and Muir2009).
There was no correlation between CHA and CCA content and morphological traits as in a previous study by Taylor (Reference Taylor1987) thus indicating that the large morphological variation might be largely due to phenotypic plasticity.
The automated screening approach was tested to determine dandelion leaf and flower area. Our tests confirmed the method's suitability for fast screening: the measurement is non-destructive, and relatively quick (a few seconds for each image), and leaf area detection at early plant growth stages can indirectly assess root yield later in the season.
The use of cluster analysis in the evaluation of genetic resources is a very useful tool for identifying diverse accessions with desirable trait combinations (Bhandari et al., Reference Bhandari, Nishant Bhanu, Srivastava, Singh and Shreya2017). This method is widely used on studies of genetic resources of various medicinal plants, such as garlic (Wang et al., Reference Wang, Li, Shen, Oiu and Song2014), ginger (Wicaksana et al., Reference Wicaksana, Gilani, Ahmad, Kikuchi and Watanabe2011) and Asparagus (Mousavizadeh et al., Reference Mousavizadeh, Hassandokht and Kashi2015). In our study, 13 dandelion populations were clustered into seven clusters, and we assisted in selection of two populations from cluster seven that combined both higher root weight and CHA and CCA content. The clustering method did not reveal a link between the characteristics studied and the geographical collection site. This finding goes against what Molina-Montenegro et al. (Reference Molina-Montenegro, Acuña-Rodríguez, Flores, Hereme, Lafon, Atala and Torres-Díaz2018) found. They found a positive relationship between the collection latitude of dandelion populations and traits such as the thickness of the seed coat and the ability to germinate. Although the study revealed morphological diversity and thus the potential of wild populations of dandelions, which will undoubtedly be useful for local medicinal plant growers, for identifying the most valuable breeding material, further research is required, including multilocal tests and evaluation of the inter- and intra-population genetic diversity.
Conclusions
In the current study, high inter- and intra-population diversity of dry root weight, leaf area, flower area, CCA and CHA content in roots was observed in 13 accessions of wild common dandelion collected in different locations in Latvia. The diverse germplasm can be used as a source for breeding activities. We have demonstrated that some wild-sourced dandelion populations have a high potential for domestication and can be grown directly in an organic farming system. The highest average root weight, CHA and CCA content combined the wild populations of Gluda and Mundigciems.
Automated non-destructive detection of leaf area was identified as a marker trait for indirect evaluation of root productivity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262123000436.
Financial support
The research was cofounded by the European Regional Development Fund project Nr.1.1.1.1/16/A/307 ‘Growing Genetic Diversity of MAP’.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.








