Forty to fifty per cent of total skeletal mass at maturity is accumulated during childhood and adolescence. Many factors influence the accumulation of bone mineral during childhood like gender, heredity, endocrine status, nutrition, physical activity and sunlight exposureReference Cadogan, Blumsohn, Barker and Eastell1. Evidence suggests that composition of the diet can play an important role in building and maintaining bone mass throughout life, primarily by providing bone-building nutrients. Diet cannot be isolated from other environmental factors, particularly in the instance of vitamin D, which the body obtains from the action of sunlight on the skin as well as from the diet. The nutrients of importance to bone health are Ca, P and vitamin D. However, other dietary constituents such as protein, oxalates, phytates and fibre may also have an impact on bone health.
Evidence has emerged in recent years that vitamin D can no longer be thought of as a nutrient necessary for the prevention of rickets among children, rather it should be considered essential for overall health and well-being. Sub-optimal levels of vitamin D and decreased exposure to solar UV B radiation are associated with an elevated risk of a number of chronic diseases including malignancies, particularly of colon, breast and prostate gland, of chronic inflammatory and autoimmune diseases (e.g. type 1 diabetes mellitus, inflammatory bowel disease, multiple sclerosis), as well as of metabolic disordersReference Whiting and Calvo2, Reference Peterlik and Cross3.
High prevalence of vitamin D insufficiency in healthy children and adolescents has been reported worldwide in the past few yearsReference Lehtonen-Veromaa, Mottonen, Irjala, Karkkainen, Lamberg-Allardt, Hakola and Vikari4–Reference Rajakumar, Fernsttrom, Janosky and Greenspan9. However, data on vitamin D deficiency among Indian children and adolescents is scarceReference Marwaha, Tandon, Reddy, Aggarwal, Singh, Sawhney, Saluja, Ganie and Singh10. Pubertal age groups are more susceptible to vitamin D deficiency disorders. The onset of puberty often brings about a tremendous change in the lifestyle of young girls with restrictions imposed on their dress, outdoor activities etc. thus resulting in less sun exposure. Moreover, discrimination against the girl child is a well-documented fact in India. In many parts of the country intra-familial distribution of food shows significant male preferences. While there have been scattered epidemiological studiesReference Goswami, Gupta, Goswami, Marwaha, Tandon and Kochupillai11, Reference Tandon, Marwaha, Kalra, Gupta, Dudha and Kochupillai12, few have investigated the contribution of nutritional factors to the prevalence of hypovitaminosis D in the population. This paucity of information has highlighted the need to investigate the association of nutrition and lifestyle with vitamin D status in healthy Indian girls.
The present study was therefore planned to examine the role of lifestyle and diet on vitamin D status in healthy schoolgirls (6–18 years) from two different socioeconomic backgrounds in Delhi, India.
Methods
Subjects
The study design is presented in Fig. 1. The study was conducted in 3127 apparently healthy school girls (6–18 years) from Delhi (28·37°N, 77·13°E), in the month of July. Socioeconomic stratification of the subjects was based on the type of school attended. Of the above 3127 girls, 1477 from government schools representing lower socioeconomic strata (LSES) and 1650 from private schools representing upper socioeconomic strata (USES) were randomly selected. The parents of each participant were informed about the study protocol and gave written informed consent to their children's participation. The study protocol was approved by the institutional ethics committee of the Institute of Nuclear Medicine and Allied Sciences.
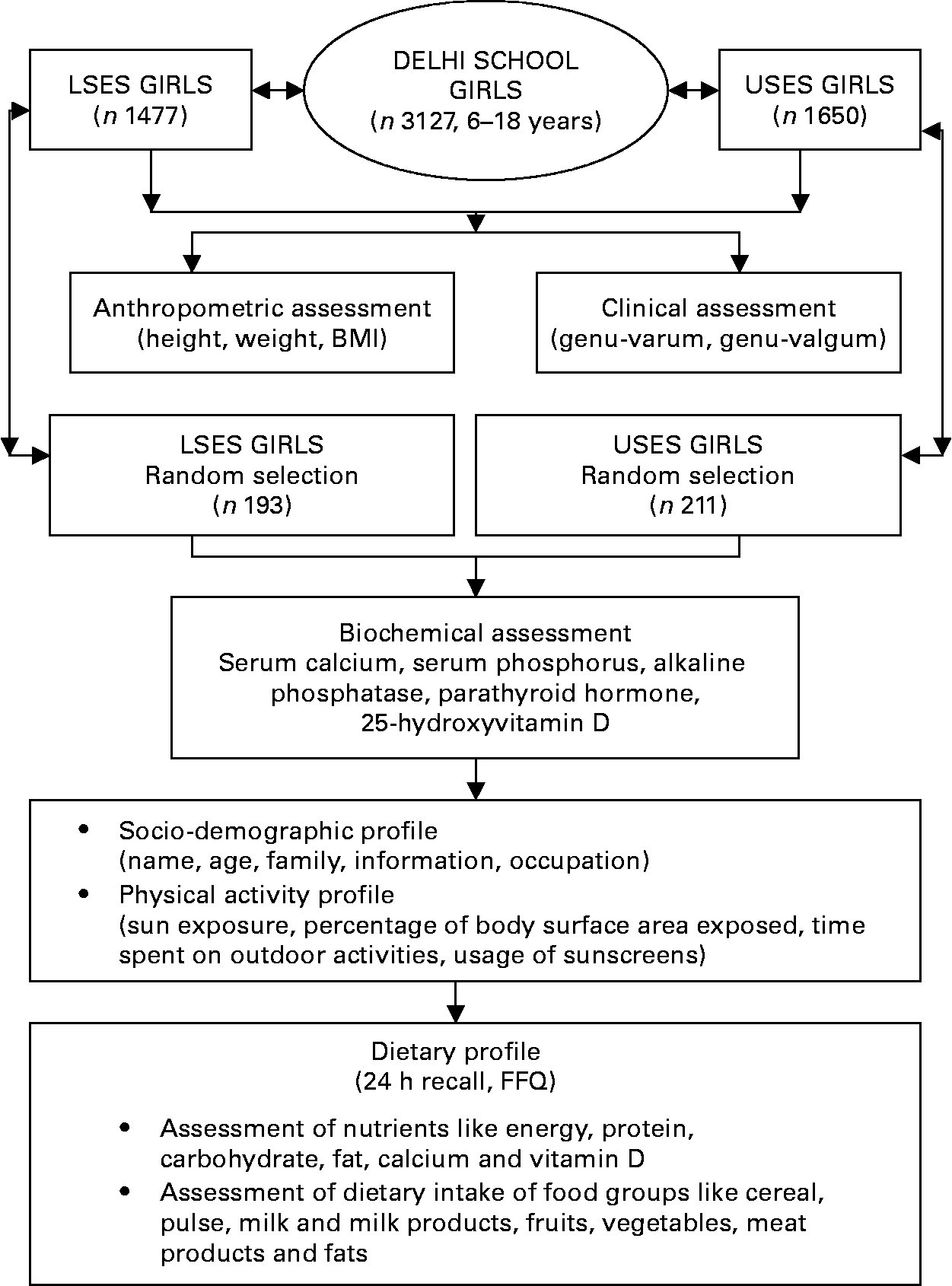
Fig. 1 Study design. LSES, lower socioeconomic strata; USES, upper socioeconomic strata.
Anthropometric and clinical profile
Height was recorded without shoes, using a wall stadiometer to the nearest 1 mm. Subjects were weighed using a clinical balance to the nearest 0·1 kg, wearing light clothing and without shoes. BMI was calculated as weight (in kg)/height (in m2). Every morning, the scale and stadiometer were calibrated with standard weight and height, respectively. The BMI cutoffs provided by Cole et al. Reference Cole, Bellizzi, Flegal and Dietz13 were used to classify children as normal, overweight or obese. Clinical vitamin D deficiency was diagnosed if a subject had either genu-varum or genu-valgum defined by intercondylar and intermalleolar distances >6 and >8 cm, respectivelyReference Solomon, Warwick and Nayagam14.
Of this large cohort, a subgroup of 404 girls (193 LSES, 211 USES) selected by random cluster sampling from each class of the school underwent further laboratory, dietary and lifestyle assessment. Children with systemic illness, endocrine disorders and drugs affecting bone mineral health were excluded from the study.
Biochemical profile
Blood samples were collected from subjects in the fasting state at 08.00 hours without venostasis under basal conditions for estimation of serum Ca, P, alkaline phosphatase, 25-hydroxyvitamin D (25(OH)D) and immunoreactive parathyroid hormone (PTH). Serum was centrifuged at 4°C for 15 min at 1200 g and divided into five aliquots, which were refrigerated. Serum Ca, P and alkaline phosphatase were estimated on the same day, and the remaining aliquots were stored at − 20°C until PTH and 25(OH)D were estimated. Serum Ca (Randox Laboratory Ltd, Crumlin, UK) and P (Clonital; Ampli Medical SPA, Milan, Italy) were measured by a colorimetric method and alkaline phosphatase by a liquid kinetic method (Clonital). The normal laboratory range for serum Ca is 2·02–2·60 mmol/l (8·10–10·04 mg/dl) and for serum P is 0·81–1·55 mmol/l (2·5–4·8 mg/dl), according to the kit manufacturers. The upper limit of serum P in mid-childhood is 1·87 mmol/l (5·8 mg/d1)Reference Portale and Favus15. The normal laboratory range for serum alkaline phosphatase at 37°C is 100–275 IU/l in adults and 180–1200 IU/l in children before epiphyseal closure.
The serum concentration of 25(OH)D (reference range 22·4–93·6 nmol/l (9·0–37·6 ng/ml)) and immunoreactive PTH (reference range 13–66 pg/ml) were measured by RIA and immunoradiometric assay (Diasorin, Stillwater, MN, USA), respectively. The definition of hypovitaminosis D was based on two criteria: firstly, serum concentration of 25(OH)D below 50 nmol/l (20 ng/ml) and, secondly, as recommended by LipsReference Lips16, as mild (25–50 nmol/l), moderate (12·5–25 nmol/l) and severe hypovitaminosis D ( < 12·5 nmol/l).
Dietary profile
The dietary information was collected using well-established tools: 24 h dietary recall, FFQ and using the interview technique.
The 24 h dietary recall provided detailed quantitative information regarding the foods consumed over the previous 24 h in terms of household measures. Using standardized recipes of the food preparationsReference Khanna, Gupta, Seth, Mahana and Rekhi17, the daily intake of various food groups (cereals, pulses, fruits, vegetables, milk, meat products and fat) was determined. Based on the amount of foods consumed in a day, the dietary intake of nutrients (energy, protein, carbohydrate, total fat, dietary fibre, phytate, Ca and P) was calculated using Nutritive Value of Indian Foods Reference Gopalan, Ramasastry and Balasubramaniam18 and was compared with Nutrient Requirements and Recommended Dietary Allowances for Indians 19 for all nutrients except vitamin D. Since no dietary estimates of vitamin D are provided in published Indian food tables, the calculations for vitamin D intake are based on US Department of Agriculture provisional tables on the vitamin D content of foods20.
The FFQ was used to elicit retrospective qualitative information regarding the frequency of consumption of various foods. Over ninety-five food items were listed from all the food groups. These tools have been used in our earlier research workReference Chugh and Puri21. In the present investigation these tools were pre-tested on twenty girls before finalization and administration.
Lifestyle profile
A self-designed structured interview schedule cum questionnaire was prepared by the investigator to elicit information regarding family background, living conditions, style of dress, direct sunlight exposure, parts of the body exposed daily, time spent outdoors during the school day and on weekends, time spent on exercising and play (indoor or outdoor) and sunscreen usage. Direct sunlight exposure was assessed by documenting average duration of exposure and percentage of the surface area of the body exposed dailyReference Tyler, Mann, Russel and Williams22. The ground surface of Delhi received a mean of 2 Minimum Erythemal Dose per hour (MED/h) of UV B radiation (290–320 nm) during the study period (Meteorology Department, Delhi).
In the present investigation these tools were pre-tested on twenty girls before finalization and administration.
Statistical analysis
The data were analysed by SPSS statistical software (version 11.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics are expressed as means and standard deviations. Independent sample t-test (two-tailed) was used to compare differences between the two socioeconomic groups for continuous variables. χ2 test was performed for categorical variables. Pearson's correlations were used to assess the association between variables.
Results
Clinical characteristics
Data were analysed separately for the two socioeconomic groups and appropriate comparisons made. USES subjects had significantly higher mean height (145·7 (sd 15·72) v. 141·3 (sd 13·4) cm, P = 0·013), weight (38·75 (sd 15·37) v. 34·9 (sd 10·8) kg, P = 0·000) and BMI (18·89 (sd 5·02) v. 17·0 (sd 3·1) kg/m2, P = 0·000) than LSES subjects across all ages. High prevalence of overweight (21·5 v. 4·4 %, P = 0·000) and obesity (5·2 v. 0·6 %, P = 0·021) was seen in USES as compared to LSES subjects; as per Cole et al. cutoffsReference Goswami, Gupta, Goswami, Marwaha, Tandon and Kochupillai11. Clinical evidence of vitamin D deficiency was noted in 11·5 % of subjects (12·4 % LSES, 10·7 % USES, P = 0·68, NS). Prevalence of genu-valgum was 5·2 % and genu-varum was 6·4 % in the total cohort.
Physical, biochemical and lifestyle parameters of the subgroup
The age, physical characteristics and biochemical parameters of the subgroup of 404 (193 LSES, 211 USES) randomly selected subjects is shown in Table 1. There is no difference in the mean age between the groups. The height, weight and BMI of USES subjects were significantly higher than LSES. High prevalence of overweight and obesity is seen in USES subjects supported by strong correlations between energy intake and BMI (r 0·368, P = 0·000) and energy and fat intakes (r 0·575, P = 0·000).
Table 1 Baseline characteristics, lifestyle and biochemical parameters of the cohort
(Mean values and standard deviations)
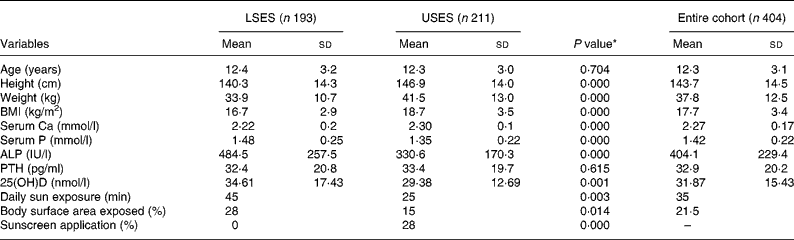
ALP, alkaline phosphatase; LSES, lower socioeconomic strata; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; USES, upper socioeconomic strata.
* As tested by independent sample t-test (two-tailed) of the difference between the means of LSES and USES subjects.
Subjects in USES group had significantly higher mean serum Ca and significantly lower mean serum alkaline phosphatase and 25(OH)D levels. A value of 25(OH)D less than 22·4 nmol/l (9 ng/ml) was noted in 121 out of 404, i.e. 29·9 % of subjects (25·4 % LSES, 34·1 % USES, P = 0·072). However, only 14·8 % had elevated PTH levels (>66 pg/ml). As per Lips classification, hypovitaminosis D ( < 50 nmol/l) was seen in 90·8 % of the population, 89·6 % LSES (5·2 % severe, 25·4 % moderate, 59 % mild) and 91·9 % USES (2·8 % severe, 36·5 % moderate, 52·6 % mild), the difference being non-significant. A significant negative correlation between the mean serum PTH and 25(OH)D (r − 0·14, P = 0·005) and 25(OH)D and BMI (r − 0·170, P = 0·001) was noted. Although mean serum Ca was within the normal range for both the groups, prevalence of sub-clinical Ca deficiency (i.e. serum Ca < 2·02 mmol/l) was significantly higher in the LSES group (15 v. 0·47 %, P = 0·000 as tested by χ2).
The girls in the LSES school had significantly higher sun exposure than did those attending the USES school (Table 1). Girls from both the schools followed a dress code of half-covered arms and fully covered legs. Time spent on outdoor activities which include exercising, play, walking to tuitions and other household chores was higher in LSES subjects (LSES = 41·5 (sd 26·0) min/d, USES = 36·3 (sd 16·2) min/d, P = 0·018). A significantly higher percentage of LSES girls (92·8 v. 64 %, P = 0·000) were involved in various outdoor activities as tested by χ2. A significant correlation was found between serum 25(OH)D concentration and estimated sun exposure (r 0·185, P = 0·001), and between 25(OH)D and percentage of body surface area exposed (r 0·146, P = 0·004).
Dietary profile
Table 2 presents the mean daily food and nutrient intake by the girls from two strata. Both the groups, LSES and USES, consumed predominantly vegetarian diets, i.e. 55·7 % of the subjects were vegetarian (58·9 v. 52·6 %, P = 0·198), 9·7 % ‘eggetarian’ (9·5 v. 10 %, P = 0·88) and only 34·5 % were non-vegetarian (31·6 v. 37·4 %, P = 0·22). Although the USES group had a higher percentage of non-vegetarians, the frequency of consumption of non-vegetarian dishes was only once a week. Fish was a rare diet item in both the groups.
Table 2 Intake of food groups and nutrients of the cohort
(Mean values and standard deviations)
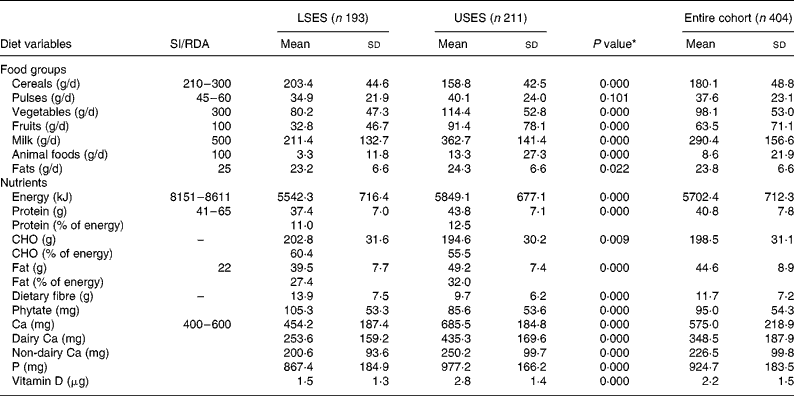
CHO, carbohydrate; LSES, lower socioeconomic strata; SI, suggested intake; USES, upper socioeconomic strata.
* As tested by independent sample t-test (two-tailed) of the difference between the means of LSES and USES subjects.
USES had significantly higher consumption of pulses, milk, animal foods, fruits and fat in their daily diets as compared to LSES girls where cereals formed the major constituent; nevertheless both the groups had daily intakes less than the Indian Council of Medical Research recommendations19. A trend of significantly lower energy, protein, fat and Ca intakes in LSES as compared to USES was seen across all ages. Mean energy intakes were 65 and 70 % lower than the RDA in LSES and USES subjects, respectively. Predictably, the protein intakes showed a linear trend with respect to age of the students. Higher protein intake of USES corroborated with significantly increased intake of milk, milk products (P = 0·000) and animal foods (P = 0·000). A daily fat intake contributing to ≥ 30 % of energy intake was observed in 67 % of USES subjects leading to high prevalence of overweight and obesity.
The mean intake of dietary Ca (dairy Ca + non-dairy sources) was significantly lower in LSES subjects whereas the mean Ca intakes of USES girls was significantly greater than the RDA matched for all age groups (Table 2). The major source of dietary Ca in the USES diets was milk and its products (i.e. dairy foods). It was observed that 85·6 % of USES girls consumed milk daily or frequently in comparison to only 37·4 % of LSES subjects. The consumption of milk products like curd, cheese, cottage cheese and milk-based desserts was influenced by social class with the USES subjects reporting more frequent consumption of milk-based desserts like ice cream, custard, rice pudding and traditional sweets (mithai). Ice creams were a favourite item amongst both the groups but the LSES group consumed mainly ice candies whereas the USES subjects preferred milk-based ice creams. A significant negative correlation was seen between dairy Ca and phytate (r − 0·219, P = 0·000) and between dairy CA and fibre (r − 0·253, P = 0·000), indicating an inverse relationship between dairy foods and cereal consumption.
LSES diets had a significantly higher content of cereals as shown in Table 1, making the diets rich in carbohydrate, phytate and fibre. A negative correlation between serum Ca and phytate (r − 0·067, P = 0·177) and serum Ca and fibre (r − 0·065, P = 0·185) was seen. The daily intake of vitamin D-containing foods like milk, egg yolk, meat and butter was higher in USES as compared to LSES diets but no significant association was found with serum 25(OH)D levels (r 0·074, P = 0·137).
Discussion
The consensus has been that most children and adolescents should be able to synthesize sufficient vitamin D by brief exposure to sunlight and that only children living in northern or southern latitudes may require supplementation with vitamin DReference Oliveri, Ladizesky, Mautalen, Alonso and Martinez23, Reference Weaver, Peacock and Johnston24. Examination of the recent evidence leads to different conclusions. Indeed, 80 % of children and adolescents had insufficient vitamin D levels (25(OH)D < 50 nmol/l) in the winter in four studies conducted in SpainReference Docio, Riancho, Perez, Olmos, Amado and Gonzallez-Macias25, FinlandReference Lehtonen-Veromaa, Mottonen, Irjala, Karkkainen, Lamberg-Allardt, Hakola and Vikari4, FranceReference Guillemant, Taupin, Le, Taright, Allemandou, Peres and Guillemant26 and TurkeyReference Hatun, Islam, Cizmecioglu, Kara, Babaoglu, Berk and Gokalp27. Although it is expected that children of different genders and socioeconomic backgrounds would have different lifestyles (exercise, sunlight exposure and nutrition), the few studies evaluating vitamin D status in children and adolescents have not systematically examined the impact of socioeconomic status and lifestyle on vitamin D levels. However, some studies have suggested that dietary factors possibly play only a minor role in the causation of hypovitaminosis DReference Stephens, Klimiuk, Warrington and Taylor28, Reference Solanki, Hyatt, Kemm, Hughes and Cowan29. In India, similar to other Middle Eastern and some European countries, there are no governmental regulations mandating vitamin D fortification of food products. The main source of vitamin D is, therefore, through skin synthesis in response to sun exposure. Information about vitamin D status in healthy schoolgirls from a sun-drenched country like India is scarce. Furthermore, its association with nutrition and lifestyle has not been objectively studied in detail. Hence, the current study was planned to assess the same in schoolgirls in Delhi.
Endemic prevalence of clinical and biochemical hypovitaminosis was seen in healthy schoolgirls in Delhi. The present finding is in keeping with reports from European countriesReference Outila, Karkkainen and Lamberg-Allardt5, Reference Docio, Riancho, Perez, Olmos, Amado and Gonzallez-Macias25, the USAReference Gordon, De Peter, Feldman, Grace and Emans8, a sun-rich country like LebanonReference El-Hajj Fuleihan, Nabulsi, Choucair, Salamoun, Hajj Shahine, Kizirian and Tannous6 and IndiaReference Marwaha, Tandon, Reddy, Aggarwal, Singh, Sawhney, Saluja, Ganie and Singh10. The present study found that USES subjects had significantly high serum Ca but low 25(OH)D levels. The negative correlation between vitamin D levels and PTH was expected, given the physiologic relation between vitamin D status and PTHReference Guillemant, Taupin, Le, Taright, Allemandou, Peres and Guillemant26.
As expected, a gross difference was found in the nutritional status of the girls from two different socioeconomic backgrounds. USES girls were significantly taller and heavier than LSES counterparts. LSES had significantly lower energy, protein, Ca and higher carbohydrate, dietary fibre and phytate intake with regard to Indian Council of Medical Research recommendations and dietary suggestions19. The energy intake of the entire cohort of girls was significantly lower than the RDA matched for age as also reported by other researchersReference Chugh and Puri21, Reference Chaturvedi, Kapil, Gnanasekaran, Sachdev, Pandey and Bhanti30, Reference Sharma, Shukla and Kannan31. A significant disparity was seen in the consumption pattern of certain food groups with costlier foods like dairy foods, animal foods, fruits and vegetables being predominant in USES diets and cereals constituting the major bulk of the LSES diets. Across all age groups, mean Ca intake of USES was higher than LSES subjects and even higher than the RDA. While Ca intakes of younger LSES children met the RDA, as girls entered puberty (10 years onwards), Ca intakes were significantly lower than the RDA. This could be attributed to decrease in milk intake, which has also been reflected in previous Indian and international studiesReference Fleming and Heimbach32–Reference Khader34, which showed that Ca intakes declined among females at the preadolescent–adolescent periodReference Jasminka, Mario, Thomas and Baoshe35.
We evaluated the possible causative factors for low vitamin D status in the present study population. Obese people have lower basal 25(OH)D and higher serum PTH concentrations than do non-obese persons. Even in individuals with normal BMI, serum 25(OH)D levels decrease with increasing percentage of body fatReference Arunabh, Pollack, Yeh and Aloia36, Reference Parikh, Edelman, Gabriel, Freedman, Janneh, Reynolds and Yanovski37. Wortsman et al. Reference Wortsman, Matsuoka, Chen, Lu and Holick38 reported that vitamin D deficiency is probably due to decreased bioavailability of vitamin D3 from cutaneous and dietary sources because of its deposition in body fat compartments. While we have not measured percentage of body fat, the significant difference in BMI between the LSES and USES subjects suggest that body fat percentage may be higher in USES. This could possibly contribute to the deficiency in serum 25(OH)D levels between the two groups.
Among the dietary variables studied, a negative correlation of serum Ca was seen with phytate and fibre intake. Dietary fibre and phytate are associated with a reduction in the bioavailability of Ca. North Indian diets are traditionally high in fibre and phytate since the staple cereal is whole wheat flour. However, such a correlation has not been consistently reported. Hu et al. Reference Hu, Zhao, Jia, Parpia and Campbell39, Tandon et al. Reference Tandon, Marwaha, Kalra, Gupta, Dudha and Kochupillai12 and Harinarayan et al. Reference Harinarayan, Ramalakshmi and Venkatprasad40 reported findings similar to the present findings, while HeaneyReference Heaney and Peck41 found no such correlation.
It is pertinent to point out that there are no food products with adequate vitamin D fortification in India and none of the cooking mediums used in meal preparations in Indian homes are fortified with vitamin D. Indian diets, being predominantly vegetarian, are not rich in vitamin D. In the present study too, 50 % of the whole cohort was vegetarian and even among the others frequency of consumption of eggs, fish and chicken was at the most one to two times per week. Since no estimates of vitamin D content of Indian foods have been provided by Indian Council of Medical Research19, the dietary vitamin D intakes were based on US Department of Agriculture data20. While dietary vitamin D intakes were higher in USES subjects, no significant association was found between dietary vitamin D and serum 25(OH)D as also reported by other investigatorsReference Gannage-Yared, Chemali, Yaacoub and Halaby42, Reference Das, Crocombe, McGrath, Berry and Mughal43. The contribution from sunlight was not taken into consideration for estimating vitamin D intakes.
Other possible determinants of low vitamin D status could be low sunshine exposure which can be explained by lower body surface area exposed, higher usage of sunscreen, less time spent on outdoor physical activity and greater indulgence in indoor activities like watching television, computer gaming, indoor tuition and other recreational activities among USES girls. This is in keeping with the fact that the main source of vitamin D is that produced by the action of solar UV B radiation acting on 7-dehydrocholesterol in skin; only small amounts are obtained from dietary sources. Avoidance of exposure to sunshine for cosmetic beliefs (usage of sunscreen creams) and style of dress has previously been reported as a risk factor for vitamin D deficiency in other countriesReference Hatun, Islam, Cizmecioglu, Kara, Babaoglu, Berk and Gokalp27, Reference Das, Crocombe, McGrath, Berry and Mughal43. A study by El-Hajj Fuleihan et al. Reference El-Hajj Fuleihan, Nabulsi, Choucair, Salamoun, Hajj Shahine, Kizirian and Tannous6 on healthy schoolchildren also reported low sun exposure, dress code and BMI as significant predictors of vitamin D levels. The observations indicate that clothing that covers from head to toe, application of sunscreen creams and girls from affluent society remaining indoors with low physical activity in spite of better nutrient intake in terms of dietary Ca and vitamin D intake presents a major problem in terms of vitamin D synthesis.
Recent nutritional guidelines targeted to children and adolescents to improve bone health have mainly stressed the importance of Ca and exercise. They have either omitted vitamin D or proposed that vitamin D supplementation is usually not necessaryReference Weaver, Peacock and Johnston24, Reference Bachrach44. Evidence from the presnt study and othersReference Lehtonen-Veromaa, Mottonen, Irjala, Karkkainen, Lamberg-Allardt, Hakola and Vikari4, Reference Docio, Riancho, Perez, Olmos, Amado and Gonzallez-Macias25, Reference Guillemant, Taupin, Le, Taright, Allemandou, Peres and Guillemant26calls for a reconsideration of such a strategy.
Conclusion
Even in a sunny country like India, endemic prevalence of vitamin D insufficiency was seen in the whole cohort of apparently healthy Delhi schoolgirls with the prevalence being significantly higher among the USES girls even though they had better nutritional status. On the contrary, daily sun exposure (LSES 45 min, USES 25 min, P = 0·003) and percentage of body surface area exposed (28 v. 15 %, P = 0·014) was significantly higher in LSES girls. A significant correlation between serum 25(OH)D and estimated sun exposure (r 0·185, P = 0·001) and percentage of body surface area exposed (r 0·146, P = 0·004) was seen. The present observations suggest that lifestyle factors in terms of daily sun exposure, outdoor physical activity and percentage of body surface area exposed may contribute significantly to the optimal vitamin D status of apparently healthy school subjects. Hence, in the absence of availability of vitamin D-fortified food products in India, diet alone appears to have an insignificant role in the causation of vitamin D insufficiency.
Acknowledgements
Seema Puri and Raman K. Marwaha are to be considered as joint first authors. The study was supported in part by research grants from the Institute of Nuclear Medicine and Allied Sciences (INMAS), New Delhi. The contributions of the authors are as follows: Seema Puri and Raman K. Marwaha, design of the study, collection and analysis of data, and writing of the manuscript; Neha Agarwal, collection and analysis of data and writing of the manuscript; Nikhil Tandon, design of the study, analysis of data and writing of the manuscript; Rashmi Agarwal, D. H. K. Reddy and Khushi Grewal, collection of data; and Satveer Singh, laboratory assays.





