Introduction
The early-life period is an important window for brain development. During this period, the brain has considerably more plasticity than in adulthood. The external environment can shape the developing brain in ways that could have relevance for risk of later psychiatric illness. Evidence has shown that stressful experiences (such as childhood maltreatment and deprivation) in early life have powerful and lifelong influence on brain functioning and risk of adverse mental health outcomes such as depression and anxiety (Carr, Martins, Stingel, Lemgruber, & Juruena, Reference Carr, Martins, Stingel, Lemgruber and Juruena2013; Herzog & Schmahl, Reference Herzog and Schmahl2018; LeMoult et al., Reference LeMoult, Humphreys, Tracy, Hoffmeister, Ip and Gotlib2020; Lindert et al., Reference Lindert, von Ehrenstein, Grashow, Gal, Braehler and Weisskopf2014; Saleh et al., Reference Saleh, Potter, McQuoid, Boyd, Turner, MacFall and Taylor2017). In contrast, there is limited research on positive childhood experiences (PCEs), defined as experiences in childhood that promote successful health and developmental outcomes (Guo et al., Reference Guo, O'Connor, Mensah, Olsson, Goldfeld, Lacey and Priest2022; Sege & Browne, Reference Sege and Browne2017). Some studies have suggested protective effects of PCEs against depression and anxiety in adolescence and young adulthood (Bethell, Jones, Gombojav, Linkenbach, & Sege, Reference Bethell, Jones, Gombojav, Linkenbach and Sege2019; Narayan, Rivera, Bernstein, Harris, & Lieberman, Reference Narayan, Rivera, Bernstein, Harris and Lieberman2018), but this has rarely been studied in late adulthood. Further, current PCE assessment has primarily focused on interpersonal relations (Bethell et al., Reference Bethell, Jones, Gombojav, Linkenbach and Sege2019; Narayan et al., Reference Narayan, Rivera, Bernstein, Harris and Lieberman2018). Cognitively stimulating activities in early life have potential long-lasting benefits; they have been associated with lower risk of late-life cognitive decline (Wilson et al., Reference Wilson, Barnes, Krueger, Hoganson, Bienias and Bennett2005; Wilson et al., Reference Wilson, Boyle, Yu, Barnes, Schneider and Bennett2013b). Despite the close connection between cognition, anxiety, and depression (Beaudreau & O'Hara, Reference Beaudreau and O'Hara2008; Wang & Blazer, Reference Wang and Blazer2015), cognitively stimulating activities during early life have not been explored in relation to late-life mental health.
Cognitively stimulating activities are mental tasks that require attention, focus, and concentration, and may include reading, writing, visiting libraries, playing games, and attending concerts (Wilson et al., Reference Wilson, Barnes, Krueger, Hoganson, Bienias and Bennett2005). These activities have been associated with positive changes in functional brain network connectivity as well as greater grey matter volumes in areas associated with cognitive functions (Schultz et al., Reference Schultz, Larson, Oh, Koscik, Dowling, Gallagher and Asthana2015; Soldan et al., Reference Soldan, Pettigrew, Zhu, Wang, Bilgel, Hou and Albert2021). Participation in these activities has been associated with reduced β-amyloid (toxic to nerve cells and key factor of Alzheimer's disease pathology) deposition (Landau et al., Reference Landau, Marks, Mormino, Rabinovici, Oh, O'Neil and Jagust2012). Children's participation in these activities may positively affect socio-cognitive functions associated with psychological resilience, such as self-confidence, relationship building, and a sense of belonging (Bungay & Vella-Burrows, Reference Bungay and Vella-Burrows2013; Oberle, Ji, Guhn, Schonert-Reichl, & Gadermann, Reference Oberle, Ji, Guhn, Schonert-Reichl and Gadermann2019; Zarobe & Bungay, Reference Zarobe and Bungay2017), potentially playing a protective role against subsequent development of mental illnesses.
In this early-life exposure–late-life health focused cohort study, we aimed to answer the following research question, ‘Is participation in cognitively stimulating activities in early life associated with lower risk of mental illness in late adulthood?’ We used data from the Saint Louis Baby Tooth – Later Life Health Study to investigate whether engagement in common cognitively stimulating activities in early life is associated with lower risk of depression and anxiety in persons older than 55 years.
Methods
Study population
The St. Louis Baby Teeth – Later Life Health Study is a subsample of the St. Louis Baby Tooth Survey, originated in 1958 (Reiss, Reference Reiss1961). In 2021, we began recontacting participants, asking them to complete questionnaires on participation in cognitively stimulating activities, current mental health symptoms, and other early-life factors. Contact efforts for roughly 35 000 original participants are ongoing, but as of 25 March 2022, 2187 participants had responded.
Cognitively stimulating activities
We used the Rush Alzheimer's Disease Center Lifetime Cognitive Activity Scale, to assess participation in cognitively stimulating activities (hereafter ‘cognitive activities’) (Everson-Rose, Mendes de Leon, Bienias, Wilson, & Evans, Reference Everson-Rose, Mendes de Leon, Bienias, Wilson and Evans2003; Wilson et al., Reference Wilson, Barnes, Krueger, Hoganson, Bienias and Bennett2005, Reference Wilson, Boyle, Yu, Barnes, Schneider and Bennett2013b; Wilson, Barnes, & Bennett, Reference Wilson, Barnes, Bennett and Stern2013a) at ages 6, 12, 18, and 40 years. This measure queries activities such as reading, playing games, attending concerts, and doing homework, etc. The frequency of (or time spent in) each activity was scored on a five-point scale from least to most frequent for each item (online Supplementary eTable 1). Responses were averaged separately for each age, and for main analyses, we further averaged across ages 6, 12, and 18 to create an overall (composite) early-life score. Age-specific scores and the overall score were moderately to highly correlated (0.39–0.87; online Supplementary eFig. 1). Online Supplementary eFig. 2 is a histogram of these scores, which range from 1 to 5.
Outcome assessment
We measured depressive and anxiety symptoms with the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder Screener-7 (GAD-7), respectively (Kroenke & Spitzer, Reference Kroenke and Spitzer2002; Spitzer, Kroenke, Williams, & Löwe, Reference Spitzer, Kroenke, Williams and Löwe2006). For each measure, participants were asked the frequency of being bothered by a list of items over the last 2 weeks (0: not at all, 1: several days, 2: more than half the days, 3: nearly every day). Following scoring manuals, we summed responses to compute continuous scores for participants with complete responses (N PHQ-9 = 2081, 95% of participant; N GAD-7 = 2101, 96%). We dichotomized depression and anxiety scores using the recommended cutoff of 10 or more, which, while not a formal diagnosis, is an indication of probable cases of major depressive disorder (shortened as depression) or generalized anxiety disorder (shortened as anxiety) (Kroenke & Spitzer, Reference Kroenke and Spitzer2002; Kroenke, Spitzer, & Williams, Reference Kroenke, Spitzer and Williams2001; Plummer, Manea, Trepel, & McMillan, Reference Plummer, Manea, Trepel and McMillan2016; Spitzer et al., Reference Spitzer, Kroenke, Williams and Löwe2006).
Covariates
We considered demographics and early-life factors as potential confounders (see directed acyclic graph, online Supplementary eFig. 3). Age, sex, and race were included in the minimally adjusted models. Fully adjusted models further included parental education, childhood family structure at age 12 (lived with both parents, one parent, one parent and a step-parent, or with other relatives/in a foster home), and childhood socioeconomic status (cSES; childhood family financial status: very well-off, well-off, average, poor, very poor) (Bennett et al., Reference Bennett, Buchman, Boyle, Barnes, Wilson and Schneider2018) at age 12. In a subset of 1590 participants for whom we were able to geocode their childhood address (only available at the time participants donated their baby teeth) and link childhood community contextual measures, we conducted secondary analyses additionally adjusted for childhood residential community socioeconomic variables that may relate to childhood stressors, via the National Historical Geographic Information System (NHGIS) at census tract level (Brooks-Gunn, Reference Brooks-Gunn1997; Coulton, Crampton, Irwin, Spilsbury, & Korbin, Reference Coulton, Crampton, Irwin, Spilsbury and Korbin2007; Elliott, Reference Elliott2000; Kohen, Leventhal, Dahinten, & McIntosh, Reference Kohen, Leventhal, Dahinten and McIntosh2008). These included 1950 US-Census-tract median household income, number of occupied units with crowded conditions (>1 person/room), % population with <high school education, % white, % Black, % in single detached home, % in crowded conditions, and % new houses (Manson, Schroeder, Riper, Kugler, & Ruggles, Reference Manson, Schroeder, Riper, Kugler and Ruggles2021). For the remaining participants, geocoding was incomplete because of missing address data, low geocoding certainty, or the location was outside of places for which we have historical census data. We did not include adulthood factors in our analyses, since they could be pathways by which early-life activities affect late-life mental health or consequences of late-life mental health (e.g. late-life socioeconomic status, smoking). In secondary analyses of the age 40 cognitive activities score, we adjusted for the participant's education and job status at age 40.
Statistical methods
We used logistic regression to estimate odds ratios (OR) and 95% confidence intervals (CI) for probable depression and anxiety using dichotomized PHQ-9 and GAD-7 scales (dependent variables), separately, per one-point increase in early-life activity scores (independent variables) as well as by tertile of early-life activity scores adjusting for sets of key covariates described in the covariates section. Our primary analysis was of overall early-life cognitive activities averaged across age 6, 12, and 18. Activity scores were fitted both as linear term and as tertiles. Except for age (fitted linearly), all covariates were fitted as categorical variables with the most common level as the reference group.
In secondary analyses, first, we explored possible effect modification of the associations between positive cognitive activities engagement and mental health risk by cSES. It has been suggested that the association between interpersonal PCEs and mental health is less evident among persons with fewer adverse childhood experiences (ACEs) (Bethell et al., Reference Bethell, Jones, Gombojav, Linkenbach and Sege2019). Although we did not have data on ACEs, we explored whether any associations varied by other factors, including cSES, which have been found to be correlated with ACEs (Walsh, McCartney, Smith, & Armour, Reference Walsh, McCartney, Smith and Armour2019), and sex using stratified models, and tested for heterogeneity with a Q-test in the ‘metafor’ package (Jackson, Reference Jackson2013; Viechtbauer, Reference Viechtbauer2007). Second, we conducted age-specific separate models to examine possible effect heterogeneity by age periods. These age-specific analyses also help inform about possible recall bias resulting from the retrospective reporting of early-life activities, and they help to assess aspects of residual confounding using the negative control exposure approach (Lipsitch, Tchetgen Tchetgen, & Cohen, Reference Lipsitch, Tchetgen Tchetgen and Cohen2010; Weisskopf, Tchetgen Tchetgen, & Raz, Reference Weisskopf, Tchetgen Tchetgen and Raz2016). In addition, the age-specific models were additionally adjusted for cognitive activities at younger ages (e.g. age 12 model adjusted for age 6 activtie score; age 18 model adjusted for age 6 and 12 activity scores), as these earlier activities may confound an association between later activities and the outcomes.
We also conducted supplemental analyses using exploratory factor analysis (EFA) to determine if there were different factors within the early-life activity score questions (Fabrigar & Wegener, Reference Fabrigar and Wegener2011). We created subscales of activity scores by averaging across activity items with ⩾0.4 loadings for each factor, as has been suggested (Howard, Reference Howard2016), for use in logistic regression analyses. To test the linearity assumption between continuous activity scores and outcome risk, a penalized cubic spline term was fitted for early-life activity score in the logistic regression model adjusting for covariates. Curves of activity score – log odds (and probabilities) of outcome risk were plotted. All analyses were conducted in R v3.5.2 software. A two-sided p < 0.05 was considered statistically significant.
Results
The average age of our participants was 63 years (standard deviation, s.d. = 3). Most participants were white (>96%). We identified 84 (4.0%) probable depression cases and 58 (2.8%) probable anxiety cases. Among these, 31 had both probable depression and anxiety (37% and 53% of total depression and anxiety cases, respectively). Participants with probable depression or anxiety v. those without were more likely to be female, less educated, unmarried, have a history of smoking, not consume alcohol, and report low current and cSES (Table 1). Participants with higher v. lower early-life cognitive activity had higher parental education, higher childhood socioeconomic level, and were more likely to live with both parents during their childhood (Table 2).
Table 1. Characteristics of study participants by probable depression and anxietya

a Probable depression defined as Patient Health Questionnaire 9 (PHQ-9) score ⩾10; probable anxiety defined as Generalized Anxiety Disorder Screener 7 (GAD-7) score ⩾10.
Table 2. Characteristics among study participants by tertile of early-life cognitively stimulating activities score
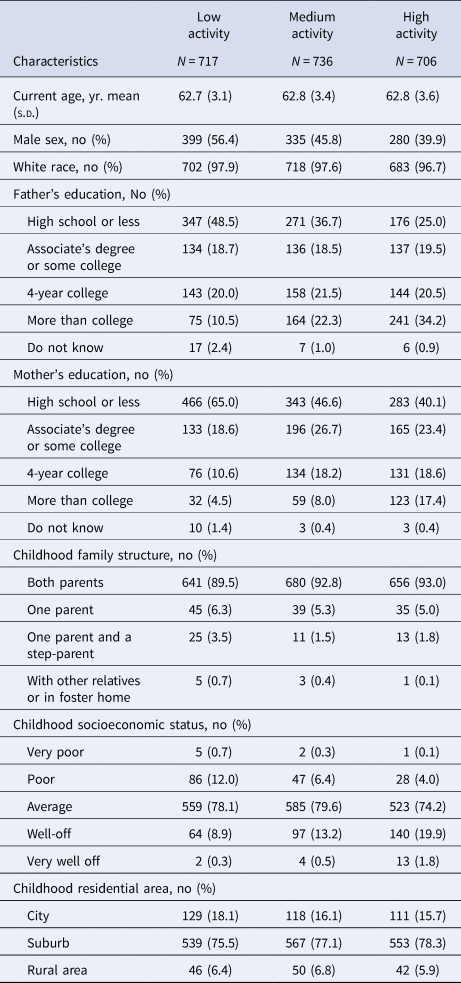
Early-life participation in cognitive activities was associated with reduced risk of late-life depression, but not anxiety (highest v. lowest tertile, depression ORfully-adjusted 0.45, 95% CI 0.24–0.84, Fig. 1). The fully adjusted probabilities of depression by early-life cognitive activities score are also presented in online Supplementary eFig. 4. When activity score was modeled as a continuous linear term, the fully adjusted OR per one-point increase in early-life cognitive activities score was 0.54 (95% CI 0.38–0.77) for depression. No significant associations were observed for anxiety, either by tertile of cognitive activities (Fig. 1) or with the continuous score (online Supplementary eFig. 4).

Figure 1. Odds ratio and 95% confidence intervals of risk of late-life depression and anxiety per tertile increase in early-life activity score (with low activity tertile group as the reference). Minimum (Min) adjustment controlled for age, sex, race; full adjustment controlled for age, sex, race, parental education, childhood family structure, and childhood socioeconomic status.
Results were similar when restricted to participants with geocoded childhood addresses (OR for depression per one-point increase in early-life cognitive activities score: 0.55; 95% CI 0.36–0.84), restricted to participants not reporting a depression diagnosis at age 18 or younger (n = 35 excluded; OR 0.57; 95% CI 0.39–0.81), and when additionally adjusted for community-level economic and demographic indicators (OR 0.54; 95% CI 0.35–0.83). In the fully adjusted model further including cognitive activity, education, and job status at age 40, age 40 activity was not associated with late-life depression (OR 0.84; 95% CI 0.52–1.35) or anxiety (OR 1.06; 95% CI 0.59–1.91). In stratified analyses, the protective association of early-life cognitive activities for depression was stronger in those with lower v. higher cSES, but these differences did not reach statistical significance (Table 3). Associations were similar by sex (Table 3). In analyses separately examining cognitive activities at ages 6, 12, and 18, cognitive activities at ages 6 and 18, but not at age 12, were significantly associated with depression, even after adjusted for cognitive activities at younger ages. There were no associations with anxiety (Table 4).
Table 3. Odds ratiosa (OR) and 95% confidence intervals (CI) for depression and anxiety per one-point increase in early-life cognitively stimulating activity scores by sex and childhood socioeconomic status (cSES)
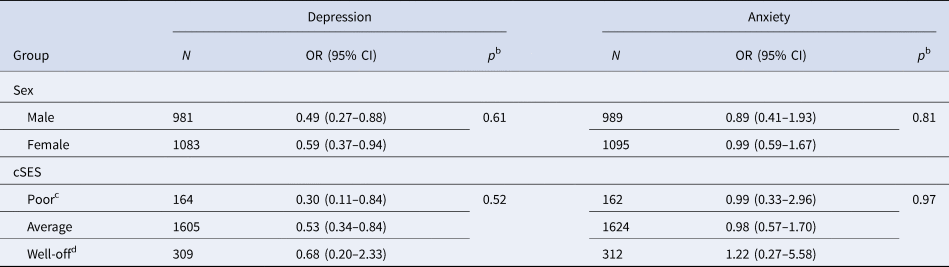
a Adjusted for age, sex, race, parental education, childhood family structure, childhood socioeconomic status.
b p for difference based on heterogeneity test.
c Combining both poor and very poor levels as described in Table 1.
d Combining both well-off and very well-off levels as described in Table 1.
Table 4. Odds ratiosa (OR) and 95% confidence intervals (CI) for depression and anxiety per one-point increase in age-specific cognitively stimulating activity scores at ages 6, 12, and 18
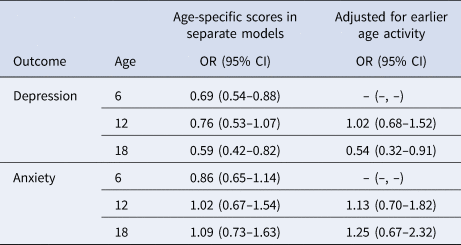
a Adjusted for age, sex, race, parental education, childhood family structure, childhood socioeconomic status.
The Pearson correlation coefficient table and matrix heatmap plot among the 20 early-life cognitive activities items (age 6, 12, 18) are shown in online Supplementary eTable 2 and eFig. 5. EFA that considered activities at ages 6, 12, and 18 separately identified only one factor at each age (online Supplementary eFig. 6). For all early-life activity questions considered together, one dominant factor was identified, although two others had eigenvalues close to 1 (online Supplementary eFig. 7). Summaries of how cognitive activity items at each age were loaded into each factor can be seen in online Supplementary eTable 3. None of these three factors were associated with late-life anxiety, and all three had similar protective associations with late-life depression that were similar to the main analysis (online Supplementary eTable 4).
Discussion
In this cohort study, we observed that higher participation in early-life cognitive activities was associated with lower risk of depression, but not anxiety, in late life. The prevalence of probable depression (4.0%) and anxiety (2.8%) in our study population was similar to reported prevalence of depression and anxiety among older US adults (depression, 5.4%; anxiety, 1–2%) (National Institute of Mental Health. Prevalence of Major Depressive Episode Among Adults, 2022; Pary, Sarai, Micchelli, & Lippmann, Reference Pary, Sarai, Micchelli and Lippmann2019). In age-specific analyses, depression was associated with activities at ages 6 and 18, but not at age 12 or 40, after accounting for activities at earlier ages. The lack of association with the activities at age 12 has important implications. First, our data on early-life cognitive activities was collected retrospectively, likely introducing measurement error. If those with depression reported fewer early-life cognitively stimulating activities because of their depression, then it could account for our findings. However, it seems unlikely that such a differential recall bias would be specific for activities at ages 6 and 18, but not ages 12 or 40. Thus, the age-specific results argue somewhat against this possible bias and any error is likely independent of current mental health, which would bias any findings towards the null. Second, the lack of association with activities at age 12 serves as a negative control exposure. This implies that any factors that affect the cognitive activities score at other ages in the same way as at age 12 (e.g. family history of mental illness, genetics, residual family socioeconomic factors that are consistent across the ages) cannot confound the associations we found with scores at ages 6 and 18 (Lipsitch et al., Reference Lipsitch, Tchetgen Tchetgen and Cohen2010; Weisskopf et al., Reference Weisskopf, Tchetgen Tchetgen and Raz2016).
Recently, there has been increasing attention paid to the relation of PCEs and mental health outcomes, with measures of PCEs focused primarily on interpersonal relationships, such as parental warmth, parental support, sense of belonging, and friend support (Bethell et al., Reference Bethell, Jones, Gombojav, Linkenbach and Sege2019; Slopen, Chen, Guida, Albert, & Williams, Reference Slopen, Chen, Guida, Albert and Williams2017). Extracurricular activities in childhood, including arts and sports, have been linked to better mental health in children and young adults (Boelens, Smit, Raat, Bramer, & Jansen, Reference Boelens, Smit, Raat, Bramer and Jansen2022; Bungay & Vella-Burrows, Reference Bungay and Vella-Burrows2013; Cairns, Yap, Pilkington, & Jorm, Reference Cairns, Yap, Pilkington and Jorm2014; Zarobe & Bungay, Reference Zarobe and Bungay2017). These extracurricular activities have included some that resemble our cognitively stimulating activities. For example, in the longitudinal Northern Finland Birth Cohort 1986, high social leisure time activity in adolescence, including reading, playing, writing, and going to concerts or movies was associated with lower incidence of psychiatric disorders before age 33 years (Timonen, Niemelä, Hakko, Alakokkare, & Räsänen, Reference Timonen, Niemelä, Hakko, Alakokkare and Räsänen2021). This study also included participation in activities like sports and clubs that could also include elements that are cognitively stimulating. While we are not aware of any studies that have assessed the association between cognitive activities in early life and mental health in late adulthood, cognitive activities have been associated with lower risk of cognitive decline in adulthood (Wilson et al., Reference Wilson, Barnes, Krueger, Hoganson, Bienias and Bennett2005; Wilson et al., Reference Wilson, Boyle, Yu, Barnes, Schneider and Bennett2013b), which is itself associated with depression (Wang & Blazer, Reference Wang and Blazer2015).
The association with cognitive activities at age 6 could occur because such activities at this age may have greater effects than those at later ages. It has been found that life stressors and maltreatment in early childhood may matter more than those in later childhood for adulthood depression (Dunn, Nishimi, Gomez, Powers, & Bradley, Reference Dunn, Nishimi, Gomez, Powers and Bradley2018). Among 15 701 participants in the National Longitudinal Study of Adolescent Health, exposure to preschool maltreatment was associated with the highest risk of depression in early adulthood compared with exposure at other times in childhood (Dunn, McLaughlin, Slopen, Rosand, & Smoller, Reference Dunn, McLaughlin, Slopen, Rosand and Smoller2013). If these differences in effect by age in our study are causal, then they suggest important time periods for intervention. Nonetheless, the specific activities queried at age 6 were slightly different from those asked about at age 12, which could have accounted for the different findings. Additionally, all models adjusted for family structure and cSES at age 12, thus may have better captured confounding at age 12 v. 6. However, it seems unlikely that residual confounding would produce a large difference in findings at the two ages, since family structure and cSES at the two ages is likely highly correlated. We cannot completely rule out that the association at age 6 arises from early trauma that is related to late-life depression and also suppresses memory of positively valenced events at early ages. Although, it seems unlikely that this would also account for an association with scores at age 18 and not age 12. The protective independent association seen for activities at age 18 could result from this age being another important time for such activities, possibly reflecting the participant's interest in these activities rather than reflecting parental behavior. The possibility of reverse causation must also be considered: an individual with depression at age 18 may have lower engagement in cognitive activities as a result of depression (de Girolamo, Dagani, Purcell, Cocchi, & McGorry, Reference de Girolamo, Dagani, Purcell, Cocchi and McGorry2012; Desha & Ziviani, Reference Desha and Ziviani2007; Jones, Reference Jones2013; Scult et al., Reference Scult, Paulli, Mazure, Moffitt, Hariri and Strauman2017). However, the lack of association with cognitive activities at age 40 argues against this somewhat, since such an explanation likely would have produced an association with cognitive activities at age 40 as well. The fact that we saw similar results when excluding people who reported a physician diagnosis of depression by age 18 also argues against this.
The protective association we observed between early-life cognitive activities and late-life depression may arise from several mechanisms. Early-life cognitive activities likely correlate with other PCEs, which have been found to be protective of adulthood depression (Bethell et al., Reference Bethell, Jones, Gombojav, Linkenbach and Sege2019; Slopen et al., Reference Slopen, Chen, Guida, Albert and Williams2017). Early-life cognitive activities could be a type of PCE, or correlate with PCEs, to provide positive psychological influences later in life. It is also possible that through frequent participation in early-life cognitive activities, children may enhance their social networks via shared experience with family members, friends, peers, and mentors who also participate in these activities. These connections can build resilience to stress and benefit mental health in later life (Mason, Schmidt, Abraham, Walker, & Tercyak, Reference Mason, Schmidt, Abraham, Walker and Tercyak2009; Oberle et al., Reference Oberle, Ji, Guhn, Schonert-Reichl and Gadermann2019; Ozbay et al., Reference Ozbay, Johnson, Dimoulas, Morgan, Charney and Southwick2007; Timonen et al., Reference Timonen, Niemelä, Hakko, Alakokkare and Räsänen2021). Nonetheless, cognitive activities are somewhat different from other traditional relationship-oriented PCEs, in that they describe activities rather than relationships. Cognitive activities, independent of interpersonal relationships, may enhance feelings of self-efficacy and improve locus of control (Goghari & Lawlor-Savage, Reference Goghari and Lawlor-Savage2018; Wolinsky et al., Reference Wolinsky, Vander Weg, Martin, Unverzagt, Willis, Marsiske and Tennstedt2010), which have been shown to be protective of depressive symptoms and can help build mental resilience (Halse, Bjørkløf, Engedal, Selbæk, & Barca, Reference Halse, Bjørkløf, Engedal, Selbæk and Barca2021; Schwarzer & Warner, Reference Schwarzer, Warner, Prince-Embury and Saklofske2013). It is also possible that the activities of reading, writing, and listening to stories contribute to mentalizing, which is the capacity to reflect on and interpret one's own and others' behaviors based on internal mental states (such as beliefs, thoughts, and emotions) and is associated with better adulthood psychological health (Fonagy, Gergely, & Target, Reference Fonagy, Gergely and Target2007; Gergely, Fonagy, Jurist, & Target, Reference Gergely, Fonagy, Jurist and Target2002; Malda-Castillo, Browne, & Perez-Algorta, Reference Malda-Castillo, Browne and Perez-Algorta2019). Further, participation in early-life cognitive activities has been found to be associated with later-life cognitive health (Wilson et al., Reference Wilson, Barnes, Krueger, Hoganson, Bienias and Bennett2005; Wilson et al., Reference Wilson, Boyle, Yu, Barnes, Schneider and Bennett2013b), which itself is related to depression (Wang & Blazer, Reference Wang and Blazer2015). For example, in adults functional brain network connectivity – thought to be relevant for depression (Li et al., Reference Li, Friston, Mody, Wang, Lu and Hu2018) – was found to be associated with cognitive activities, but not social ones (Soldan et al., Reference Soldan, Pettigrew, Zhu, Wang, Bilgel, Hou and Albert2021). Preventive interventions based on positive parent–child interactions have been proposed to promote attachment and social/emotional development in prior literature (Tereno, Savelon, & Guedeney, Reference Tereno, Savelon and Guedeney2019). Factor analysis of the early-life cognitive activities indicated only one factor underlying the activities at each age, and largely suggested only one for all ages combined. However, for all ages combined, there was some evidence for three factors, but all three had similar (significant) associations (OR 0.67–0.75) with late-life depression that were similar to the main analyses and no association with anxiety. Nevertheless, the factor with the lowest OR seemed to comprise activties that could be considered slightly more social in nature, which could suggest that social interaction may be a component of the associations we found rather than cognitive stimulation alone. Further understanding of the independent contributions of cognitive activities and relationship-based PCEs to late-life mental health could enhance recommendations for early-life activities.
That the association was present for depression, but not anxiety is of note. There are examples of early-life risk factors associated with depression but not anxiety in later-life. Childhood emotional abuse was found to be more strongly related to the risk of depression than anxiety in a study of adult psychiatric outpatients (Gibb, Butler, & Beck, Reference Gibb, Butler and Beck2003). In the longitudinal Netherlands Study of Depression and Anxiety (NESDA), the impact of childhood trauma appeared to be greater on depressive than anxiety disorders in adulthood (Hovens et al., Reference Hovens, Giltay, Wiersma, Spinhoven, Penninx and Zitman2012). There are generally more studies of risk factors for late-life depression than anxiety, but differences in risk factors for the two are clearly present in the literature (Vink, Aartsen, & Schoevers, Reference Vink, Aartsen and Schoevers2008). In contrast, SES has often been found to be more associated with anxiety than depression, e.g. in the Dunedin Multidisciplinary Health and Development Study and the National Comorbidity Study in the USA (Kessler et al., Reference Kessler, McGonagle, Zhao, Nelson, Hughes, Eshleman and Kendler1994; Miech, Caspi, Moffitt, Wright, & Silva, Reference Miech, Caspi, Moffitt, Wright and Silva1999). This could explain why there appeared to be slightly more influence of adjustment for childhood SES factors on our results for anxiety than for depression.
Subgroup analyses hinted at stronger associations among participants with low cSES, but these analyses were underpowered, and differences were not statistically significant in this study. Nonetheless, earlier studies have tried similar subgroup analyses and found that the associations between PCEs and mental health did vary by levels of ACEs, although the direction of modification is not consistent across different outcomes (Kuhar & Zager Kocjan, Reference Kuhar and Zager Kocjan2021; Xu et al., Reference Xu, Zhang, Ding, Zheng, Lee, Yang and Wong2022). Further study of this possibility is warranted since those growing up in poorer family conditions often experience higher adversity and stressors in their early life than those who come from more resourced households, which can lead to higher vulnerability for developing mental illness (Cooper & Stewart, Reference Cooper and Stewart2013, Reference Cooper and Stewart2017; Knifton & Inglis, Reference Knifton and Inglis2020; Macintyre, Ferris, Gonçalves, & Quinn, Reference Macintyre, Ferris, Gonçalves and Quinn2018; Shim et al., Reference Shim, Koplan, Langheim, Manseau, Powers and Compton2014).
Limitations
This study has several limitations. First, residual confounding is always a concern. However, as described above, the lack of association with the cognitively stimulating activities score at age 12 suggests the absence of confounding by factors that are consistent over childhood, which likely represent the largest threats for confounding, e.g. from childhood maltreatment, family history of mental illness, or genetics in general. Such confounding would be expected to affect cognitive activities at any childhood age, yet we did not see an association for all childhood ages. In this case, the activity score at age 12, which was not associated with late-life depression, acts as a negative control exposure to argue against such confounding (Lipsitch et al., Reference Lipsitch, Tchetgen Tchetgen and Cohen2010; Weisskopf et al., Reference Weisskopf, Tchetgen Tchetgen and Raz2016). Further, we controlled for factors that are strongly associated with maltreatment, family dysfunction, and other adversities (e.g. parental education, childhood family structure, and cSES) (Kerig, Reference Kerig1995; McFarlane, Bellissimo, & Norman, Reference McFarlane, Bellissimo and Norman1995), and our sensitivity analysis additionally adjusted for several key neighborhood-level factors did not substantially change the association between early-life cognitive activities and depressive symptoms. Second, broader aspects of PCEs information have not been obtained with which to try and better disentangle the associations of these v. cognitive stimulating activities on depression. Third, we did not have data on many adulthood risk and protective factors for depression and anxiety. However, as factors that occur after childhood, they should not confound an association between early-life cognitive stimulating activities and late-life depression. Such factors may well be on the causal path of that relation, but then as such should not be adjusted for.
Conclusions
We found that more frequent participation in cognitively stimulating activities (such as reading, writing, playing board games, etc.) during early life was associated with reduced risk of late-life depressive symptoms, after adjusting for age, sex, race, parental education, childhood family structure, and cSES. Further elucidating the independent contributions of PCEs and cognitive activities is warranted, but there is the possibility that building community-based access to these cognitive activities and improving parental awareness of the importance of them could be potential early-life interventions for late-life depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002702
Acknowledgements
The authors thank the participants of The Saint. Louis Baby Tooth Survey for agreeing to participate in this follow-up study, as well as the Metals and Metal Mixtures, Cognitive Aging, Remediation and Exposure Sources (MEMCARE) Superfund Research Center at Harvard University for the support.
Funding statement
This work was supported by the National Institute of Health grants (R01-ES031943 and P42-ES030990). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
None.








