Prolonged consumption of a high-fat diet is associated with chronic metabolic disorders including obesity, type 2 diabetes, CVD, hypertension and fatty liver disease(Reference Cani and Delzenne1, Reference Hotamisligil2). These disorders are also closely connected with inflammation as characterised by an increased production of cytokines, such as TNF-α and interleukins(Reference Hotamisligil2–Reference Hotamisligil, Shargill and Spiegelman4), as well as acute-phase proteins such as serum amyloid A (SAA)(Reference Kappelle, Bijzet and Hazenberg5). A high-fat diet has also been shown to induce low-grade intestinal inflammation that precedes and correlates with obesity and insulin resistance in the conventionally raised mouse(Reference Ding, Chi and Scull6) and to change the gut microbiota composition(Reference Cani, Bibiloni and Knauf7).
Mast cells have been traditionally regarded as ‘allergy cells’ involved in allergic disorders(Reference Metz, Grimbaldeston and Nakae8). However, understanding of the multiple functions of mast cells and mast cell-derived mediators in immunomodulation, host defence and the regulation of tissue homeostasis is emerging. Intestinal mast cells have been recognised to be involved in maintaining the function of the intestinal barrier, interaction with epithelial cells, and defence against pathogens and regulation of immune tolerance(Reference Bischoff9). Elevated numbers of mast cells and mast cell-released pro-inflammatory mediators can lead to persistent inflammation and the promotion of inflammatory diseases, e.g. atherosclerosis and metabolic disorders(Reference Kovanen10, Reference Liu, Divoux and Sun11). Of note, some of the mast cell mediators, including IL-10, may elicit anti-inflammatory effects and attenuate inflammation(Reference Galli, Grimbaldeston and Tsai12).
Recently, the role of the gut microbiota in inflammatory diseases has been emerging(Reference Tlaskalova-Hogenova, Stepankova and Kozakova13). Differences in the composition of intestinal bacteria have been detected in animal studies and between patients with inflammatory diseases compared with healthy individuals. Mice fed with a regular chow diet show significant changes in the composition of the gut microbiota after only 1 d of high-fat diet feeding(Reference Turnbaugh, Ridaura and Faith14), demonstrating that dietary factors may have a crucial effect on the gut microbiota. On the other hand, the proportion of beneficial microbes in the gut, and thus the maintenance of homeostasis in the gut microbiota, can be affected by the ingestion of probiotics(Reference Collins and Gibson15, Reference Fooks and Gibson16). Probiotics are defined as live microbes that, when administrated in adequate amounts, have beneficial effects on the host's health(17). Probiotics are recognised by receptors, such as Toll-like receptors, on gut epithelial cells and immune cells, which is followed by a cascade of immunological events(Reference Miettinen, Veckman and Latvala18). The interaction with immune cells may alleviate inflammation, maintain tolerance by preventing any increase in gut permeability and regulate the homeostasis of immunological functions(Reference Parvez, Malik and Ah Kang19–Reference Kekkonen, Lummela and Karjalainen21). In addition, probiotics are suggested to have beneficial effects by eliciting anti-inflammatory actions(Reference Saini, Saini and Sharma22), to prevent high fat-induced insulin resistance(Reference Amar, Chabo and Waget23) and to promote cardiovascular health by reducing cholesterol levels(Reference Lee do, Park and Jang24).
Based on the above-mentioned data, it is hypothesised that the unfavourable alteration of intestinal microbiota induced by a fat-enriched diet is a potential target for probiotic treatment. In our previous studies, probiotics Lactobacillus rhamnosus GG (GG) and Propionibacterium freudenreichii spp. shermanii JS (PJS) were observed to have immunomodulatory or anti-inflammatory capacity both in vitro and in vivo (Reference Kekkonen, Kajasto and Miettinen20, Reference Kekkonen, Lummela and Karjalainen21). Here we wish to further explore and substantiate the putative beneficial effects of these probiotics on metabolic health. To that end, we investigated whether a 4-week oral administration of GG and PJS would have a protective effect on markers of inflammation and lipid parameters in high-fat-fed ApoE*3Leiden mice, a mouse model with a lipoprotein profile which is more human-like than that of wild-type mice(Reference Zadelaar, Kleemann and Verschuren25).
Materials and methods
Bacterial strains
GG and PJS were provided by Valio Limited as a freeze-dried powder. The amount of viable bacteria was estimated by plating several dilutions of peptone-suspended GG and PJS onto de Man, Rogosa and Sharpe plates and buffered propionic agar plates, respectively. The GG plates were incubated at +37°C for 3 d, and the PJS plates in an anaerobic chamber at +30°C for 5–7 d, after which visible colonies were counted and viable count calculations were performed. Using the same protocol, the concentrations of the freeze-dried bacteria were controlled after 2, 4 and 6 weeks of storage at − 20°C. To obtain bacterial suspensions for the experiment, the powder-containing pouches were first brought to room temperature to obtain the ambient temperature. Then, 30 mg of GG or 45 mg of PJS powder were weighed and dissolved in 3 ml of sterile water. The number of bacteria in these suspensions, according to the viable count, was 6·7 × 109 colony-forming units/ml; thus one dose of 150 μl contained approximately 109 colony-forming units bacteria.
Animals and experimental design
A total of thirty-two male heterozygous ApoE*3Leiden mice were used in the study. ApoE*3Leiden mice are transgenic C57B1/6 mice that express a mutated version of the human APOE3 gene. The mutation is associated with familial dysbetalipoproteinaemia in humans. In addition, ApoE*3Leiden mice also carry the human APOC1 transgene and a human-promoter element that regulates the expression of both genes. These mice have impaired apoE metabolism leading to elevated levels of plasma lipoproteins and TAG. ApoE*3Leiden mice are highly responsive to a high-fat diet, which leads to changes in markers of inflammation and to the development of atherosclerosis(Reference Zadelaar, Kleemann and Verschuren25).
Mice were housed in clean conventional animal rooms in macrolon cages at a relative humidity of 50–60 %, a temperature of 21°C and a light cycle of 06.00 to 18.00 hours. Mice were 15–17 weeks of age at the beginning of the experiment. Before the experiment, mice were transferred from the breeding facility to the macrolon cages and fed a chow maintenance diet for 1 week to stabilise and adapt to the new environment. After the adaptation period, mice were divided into four groups (n 8 per group) and started to receive a high-fat diet until the end of the experiment (4 weeks). In addition to high-fat food, group 1 received saline as a vehicle control, group 2 received GG in vehicle and group 3 received PJS in vehicle. Group 4 served as a positive control group and received fenofibrate mixed into the high-fat diet (0·003 %, w/w). Fenofibrate is an activator of PPARα with established anti-inflammatory potency and also lipid-modulating capacity(Reference Kooistra, Verschuren and de Vries-van der Weij26). The animals received either bacteria or vehicle by oral administration. The bacteria were orally administered at a dosage of 109 colony-forming units in 150 μl at a fixed time point per d, five consecutive days per week. All mice also received tap water ad libitum.
Food intake and body weight of mice were monitored weekly. EDTA tail blood samples were collected at the beginning of the experiment (day 0), and at days 3, 14 and 28 after a 4 h fasting period. At day 28, the animals were killed for collection of tissues, including proximal colon and gonadal and visceral adipose tissue.
The rules and regulations set by the Netherlands Law on Animal Experiments were conformed during the experiments. The experiments were approved by the TNO Committee on Animal Experiments.
Diets
During the experimental period of 4 weeks, all mice received a high-fat-containing, Western-type diet (43 % energy of fat derived from bovine lard). The high-fat diet powder was provided by Hope Farms. Pellets were prepared by mixing the powder with 2 % agar and freeze-dried as pellets. To prepare pellets for the group receiving fenofibrate, the compound was mixed stepwise with the powder, followed by mixing with 2 % agar and freeze-drying as pellets. The pellets were prepared freshly before the animal experiments and stored at − 20°C during the experimental period until use.
Immunohistochemistry
To examine whether probiotic bacteria have an effect on the degree of intestinal inflammation after consumption of the high-fat diet, the number of intestinal mast cells and the levels of TNF-α and IL-10 were determined in the intestinal sections of mice. For mast cell detection, frozen sections (10 μm) of mouse proximal colon were fixed in methanol for 10 min followed by washing with PBS. The sections were stained with naphthol AS-D chloroacetate esterase as described previously(Reference Oksaharju, Lappalainen and Tuomainen27). The number of mast cells was calculated in four sections of each mouse. To detect TNF-α or IL-10, frozen sections of mouse proximal colon were fixed in cold acetone for 1–10 min. After fixation, endogenous peroxidase activity was blocked with 2 % H2O2 in methanol for 20 min. To block the binding of non-specific antibodies, the sections were treated with 3 % goat serum in PBS for 30 min. To ensure the blockade of all endogenous biotin or avidin binding sites in intestinal tissue, the Avidin/Biotin Blocking Kit (Vector Laboratories) was used according to the manufacturer's instructions. The sections were incubated overnight with rabbit primary polyclonal anti-mouse TNF-α antibody (final concentration 50 μg/ml; Abcam) or rat primary monoclonal anti-mouse IL-10 antibody (final concentration 20 μg/ml; Abcam). Irrelevant, isotype-matched rabbit or rat IgG antibodies were used as negative controls. The primary antibodies were detected using the avidin–biotin complex system (Vectastain Elite ABC Kit; Vector Laboratories) with 3,3′-diaminobenzidine as a chromogen. The sections were counterstained with haematoxylin (Sigma) and mounted with Aquamount (Dako). The intensity of the stainings was estimated in a blinded fashion by three independent evaluators. The stainings were examined under a Nikon Eclipse E600 microscope and photographed with a Spot RT Color camera (Diagnostic Instrument, Inc.) using Spot Advanced software version 4.6.
Biochemical analyses
For biochemical analysis, EDTA plasma samples from all mice were analysed at each time point. For the analyses requiring a large volume of sample, e.g. for the analysis of the lipoprotein profiles and alanine aminotransferase (ALT) measurements, pooled plasma samples derived from each group were used. Due to the lack of sufficient volume of plasma, ALT analysis of the 3 d time point samples could not be performed, and pooled plasma was used in the ALT measurements of the 14 d time point samples. Levels of plasma SAA (Biosource International), soluble vascular cell adhesion molecule 1 (VCAM-1; R&D Systems), E-selectin (R&D Systems) and adiponectin (R&D Systems) were determined using ELISA, and plasma fibrinogen was measured using an in-house assay(Reference Kooistra, Verschuren and de Vries-van der Weij26). Plasma ALT levels were determined spectrophotometrically using a Reflotron system (Roche Diagnostics). Total plasma cholesterol and TAG were enzymatically measured by using a Chol R1 kit and a Triglycerides GPO-PAP kit (Roche Diagnostics). Lipoprotein profiles were analysed in pooled plasma by fast protein liquid chromatography using gel filtration columns (ÄKTA FPLC system; GE Healthcare). Total cholesterol was measured in the fractions collected from fast protein liquid chromatography.
Statistical analysis
Data were processed by using non-parametric statistical methods. Differences in continuous variables between the control group and the groups administered with the probiotic strains GG or PJS, or with fenofibrate were analysed with the Kruskal–Wallis test and Mann–Whitney U test. Friedman's ANOVA was used to describe differences between repeated measurements, and further paired comparisons were made with Wilcoxon's signed rank test with Bonferroni correction. Correlations between mast cell numbers and immunoreactivities of TNF-α and IL-10 were analysed with the Pearson product moment method. Results are presented as mean values with their standard errors. Statistical significance was considered at P ≤0·05.
Results
Food intake and body weight
No differences in the amount of consumed food were observed for the different groups (data not shown).
Body weight in the control, GG and fenofibrate groups remained constant during the whole experimental period (data not shown). In the PJS group, however, body weight was decreased after 1 week and at the end of the treatment period, a significant decrease was observed in the PJS group compared with the initial body weight at day 0 (initial weight 29·7 (sem 0·9) g, final weight 28·8 (sem 0·8) g, P= 0·003).
Gut immunochemistry
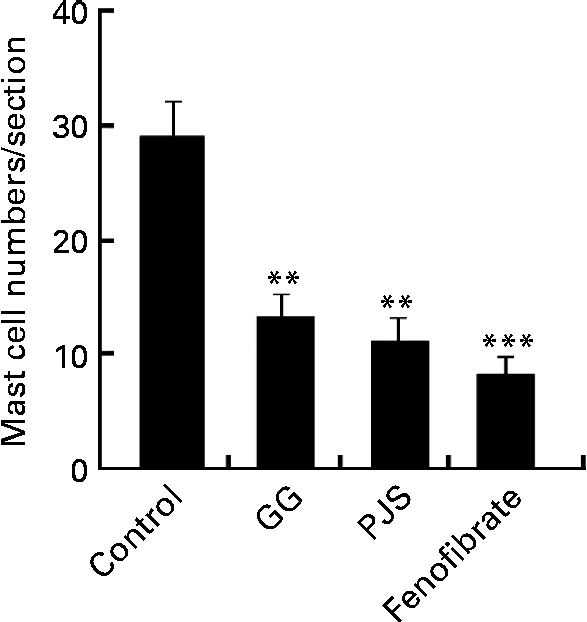
After the experimental period, the mast cell numbers were significantly lower in the GG, PJS and fenofibrate groups compared with the control group (Fig. 1).

Fig. 1 Effect of Lactobacillus rhamnosus GG (GG) and Propionibacterium freudenreichii spp. shermanii JS (PJS) on the intestinal mast cell numbers in ApoE*3Leiden mice during the high-fat diet for 28 d. Intestinal cryosections of mice were stained for mast cells with the naphthol AS-D chloroacetate esterase method. Mast cells in four sections obtained from each mouse were counted. Values are mean mast cell numbers in each group (n 8 per group), with their standard errors represented by vertical bars. Differences between the groups were analysed using the Kruskal–Wallis test and Mann–Whitney U test. Mean values were significantly different from those of the control group: ** P< 0·01, *** P< 0·001.
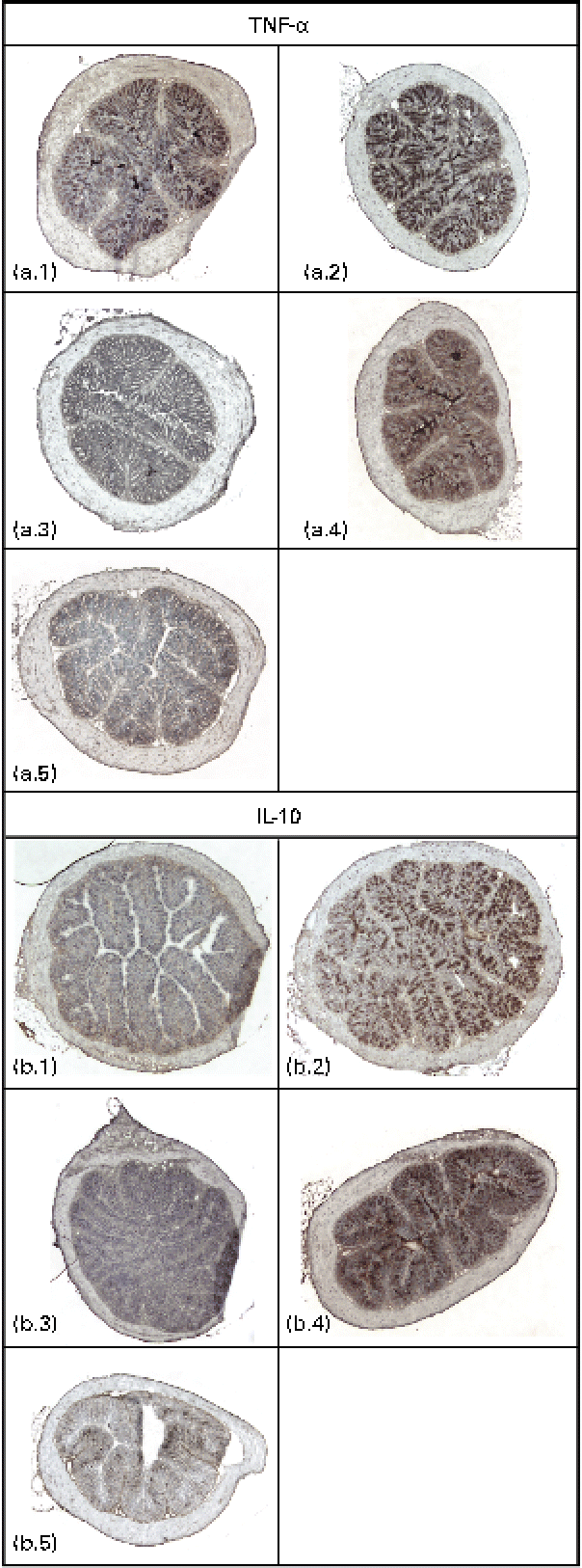
Immunodetectable TNF-α was found in the intestinal wall of the control, GG and fenofibrate groups, and there were no differences in the intensities of TNF-α between these groups (Fig. 2(a)). However, the intensity of TNF-α in the intestines of mice in the PJS group was significantly lower compared with the control group (P< 0·001). There were only low immunodetectable quantities of the anti-inflammatory molecule IL-10 in the intestines of the control group, and no immunodetectable IL-10 in the PJS group (Fig. 2(b)). In contrast, the intestines of the GG group showed strong IL-10 staining compared with the control group (P= 0·008), and the fenofibrate group showed moderate staining of IL-10 compared with the control group (P= 0·038). No correlations were found between the mast cell numbers and the immunoreactivities of TNF-α or IL-10.

Fig. 2 Immunostaining of TNF-α and IL-10 in the intestinal cryosections of ApoE*3Leiden mice after 28 d of high-fat feeding accompanied by the administration of Lactobacillus rhamnosus GG (GG), Propionibacterium freudenreichii spp. shermanii JS (PJS) or fenofibrate. TNF-α stainings in (a) are shown as follows: a.1, control; a.2, GG; a.3, PJS; a.4, fenofibrate; a.5, negative control. IL-10 stainings are shown in (b): b.1, control; b.2, GG; b.3, PJS; b.4, fenofibrate; b.5, negative control. Isotype-matched rabbit or rat IgG antibodies were used as negative controls. Illustrations are shown in 4 × magnification.
Markers of inflammation
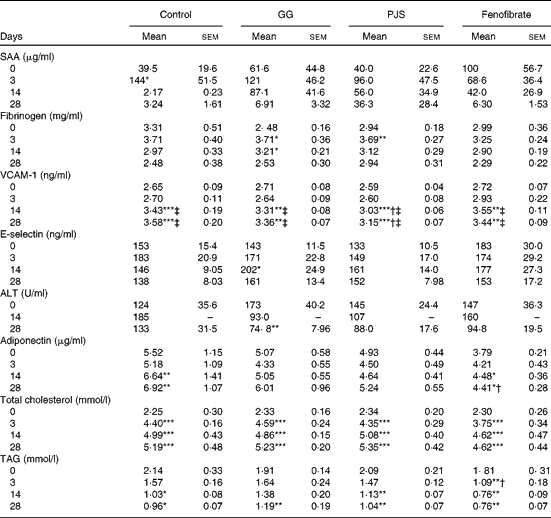
To evaluate whether high-fat food had induced changes in the markers of systemic inflammation, plasma SAA and fibrinogen values were measured. As shown in Table 1, baseline SAA values showed some variation. The high-fat diet had an acute increasing effect on plasma SAA levels in the control group and a statistically significant peak was observed at day 3. There were no statistically significant changes in SAA values in the GG, PJS or fenofibrate groups. Levels of plasma fibrinogen increased at day 3 in all groups in response to the high-fat food, significantly in the GG and PJS groups, but there were no significant differences between the groups (Table 1).
Table 1 Effects of Lactobacillus rhamnosus GG (GG) and Propionibacterium freudenreichii spp. shermanii JS (PJS) on plasma markers of inflammation, liver function, adiponectin and lipids of ApoE*3Leiden mice during 28 d on the high-fat diet§ (Mean values and standard deviations)

SAA, serum amyloid A; VCAM-1, vascular cell adhesion molecule 1; ALT, alanine aminotransferase.
Mean values were significantly different from those of the initial values at day 0 within the group: * P <0·05, ** P< 0·01, *** P< 0·001.
Mean values were significantly different from those of the control group: † P< 0·05.
Mean values were significantly different from those at day 3 within the group: ‡ P< 0·01.
§ The comparison within the groups was made with Friedman's ANOVA and Wilcoxon's signed rank test. To compare values between the different groups, the Kruskal–Wallis test and Mann–Whitney U test were used.
The plasma levels of adhesion molecules, VCAM-1 and E-selectin, were also measured. The plasma VCAM-1 values in all groups increased over time and were elevated significantly at days 14 and 28 compared with the values at days 0 and 3 (Table 1). In the PJS group, however, the increase in the plasma VCAM-1 level was significantly lower compared with the water control group at days 14 and 28.
There was a slight variation in baseline levels of plasma E-selectin between the different groups, as indicated in Table 1. However, a similar trend of an acute increase in E-selectin levels was observed in the control group as the one seen for SAA at day 3. In the PJS and fenofibrate groups, this response was quenched, and delayed in the GG group.
Liver function
Plasma ALT concentration was measured as an indicator of liver function in this experiment. Considering the variation in ALT starting values between the groups (Table 1), the effects of the probiotics were analysed within each group relative to the starting value at day 0. In the control group, plasma ALT levels increased slightly at day 14. In the GG and PJS groups, a decreasing trend in plasma ALT values was observed already at day 14, and the decrease was statistically significant in the GG group at day 28 and a similar trend was observed in the PJS group at day 28 (P= 0·06).
Plasma adiponectin levels and adipose tissue mass
Adiponectin levels of the different groups are shown in Table 1. Plasma adiponectin concentrations significantly increased in response to the high-fat diet in the control and fenofibrate groups at day 14 or 28, but were significantly lower in the fenofibrate group compared with the control group at day 28. Adiponectin values of the GG and PJS groups remained constant during the experiment.
Gonadal and visceral adipose tissue of mice was weighed after killing. The control, GG and fenofibrate groups had similar gonadal adipose tissue mass (control: 0·43 (sem 0·02) g, GG: 0·41 (sem 0·04) g and fenofibrate: 0·49 (sem 0·06)), whereas the PJS group had significantly lower gonadal adipose tissue mass compared with the control group (0·34 (sem 0·03), P= 0·027). There were no differences in the visceral adipose tissue mass between the groups.
Plasma lipids
At the start of the experiment, mice in the different groups were matched on the basis of their baseline cholesterol levels, resulting in the similar cholesterol concentrations in all groups (average 2·31 (sem 0·23) mmol/l). Plasma cholesterol levels increased in all groups in response to the high-fat diet already after 3 d of treatment (Table 1), and remained elevated for the whole experiment. The probiotics did not show the effects on cholesterol levels in this experiment.
The high-fat diet led to an immediate reduction of total plasma TAG levels in all groups at day 28 (Table 1) In the GG and PJS groups, there was no difference in the reduction in TAG levels when compared with the control group. However, the positive control fenofibrate showed a statistically significant reduction in TAG levels during the experiment compared with the control group.
Lipoprotein profiling of pooled plasma of the different groups for VLDL, intermediate density lipoproteins/LDL and HDL-cholesterol after 28 d of experimental treatment revealed no significant effects of GG, PJS or fenofibrate when compared with the control group (data not shown).
Discussion
Low-grade inflammation is typically associated with the consumption of a high-fat diet(Reference Hotamisligil2). Also in ApoE*3Leiden mice, a high-fat diet has been reported to elicit inflammatory changes(Reference Zadelaar, Kleemann and Verschuren25). In the present study, we have explored and demonstrated the effects of probiotics GG and PJS on inflammation and lipid parameters in high fat-induced ApoE*3Leiden mice.
The gastrointestinal tract is the first organ that is subjected to the impact of dietary components, e.g. high levels of saturated fat and probiotics. A high-fat diet modifies the gut microbiota composition, which promotes functional and inflammatory changes in the intestine(Reference Ding, Chi and Scull6, Reference Turnbaugh, Ridaura and Faith14), such as activation of intestinal immune cells, including macrophages(Reference Fujiyama, Hokari and Miura28) and mast cells(Reference Ji, Sakata and Tso29). Activated mast cells in the intestine may cause disruption of the gut function and local inflammation(Reference De Winter, van den Wijngaard and de Jonge30). An important finding of the present study is the ability of probiotic GG or PJS and the positive control fenofibrate to suppress the number of intestinal mast cells when consuming a high-fat diet. This effect of GG and PJS is, to our best knowledge, a novel observation. Importantly, GG and several other probiotic strains have been shown to have a decreasing effect on mast cell numbers in several allergy-related studies in rodents(Reference Sawada, Morita and Tanaka31–Reference Won, Kim and Lim34). Increased intestinal mast cell numbers after probiotic administration have been observed in a rat model of aspirin-induced gastric mucosal injury, where mast cells were implicated to have a protective role(Reference Senol, Isler and Karahan35). In most studies, however, elevated mast cell numbers are associated with pathological conditions, such as metabolic disorders involving atherosclerosis and diabetes-related renal injury(Reference Okon and Stachura36), as well as other inflammatory diseases including irritable bowel syndrome and inflammatory bowel disease(Reference Atkinson, Harlan and Harlan37–Reference Nishida, Murase and Isomoto39). To our knowledge, no reports of the in vivo effects of PJS on mast cells have been published to date. GG and PJS might achieve the mast cell-lowering effect by influencing the gut microbiota or by having an anti-inflammatory impact on the gut immune system, but the exact mechanisms behind this observation need to be further elucidated.
Immunopositivity of TNF-α was detected in the intestines of mice in the control, GG and fenofibrate groups. However, TNF-α was not detectable in the PJS group. On the other hand, the intestines of mice in the GG group showed strong staining for IL-10, which was not observed in the PJS group. The present results suggest that both PJS and GG elicit anti-inflammatory actions in the intestine during a high-fat diet, by either suppressing TNF-α or inducing IL-10 production. PJS has also been observed to have anti-inflammatory potential in a clinical study on healthy individuals, as indicated by decreased serum C-reactive protein levels(Reference Kekkonen, Lummela and Karjalainen21). However, in the same study, no decrease was observed in serum TNF-α levels. In ovalbumin-sensitised allergic rats, GG has been observed to induce elevated levels of intestinal IL-10 and to reduce hypersensitivity(Reference Finamore, Roselli and Britti40). Mast cells are an important source of TNF-α and IL-10, and GG, for example, is known to induce TNF-α and IL-10 expression in human mast cells in vitro (Reference Oksaharju, Kankainen and Kekkonen41). However, in the present study, the number of intestinal mast cells did not correlate with the observed staining intensities of TNF-α and IL-10, suggesting additional cellular source for the production of these mediators in the intestine. Macrophages are possible candidates, since they have been shown to produce TNF-α in the intestines of mice administered with butter(Reference Fujiyama, Hokari and Miura28).
High-fat diet-induced metabolic disorders are associated with low-grade inflammation reflected by increased concentrations of circulating inflammatory markers(Reference Calder, Ahluwalia and Brouns42). In high-fat-fed ApoE*3Leiden mice, plasma concentrations of SAA and VCAM-1 were increased and a similar trend was observed in E-selectin concentrations. PJS showed some anti-inflammatory potential by having a significantly smaller increase in plasma VCAM-1 concentrations compared with the control group. In other studies, a non-pathogenic bacterium, Escherichia coli strain Nissle 1917, decreased plasma levels of SAA(Reference Kamada, Inoue and Hisamatsu43) or Lactobacillus casei suppressed the increased colonic expression of another endothelial adhesion molecule intercellular adhesion molecule-1 (ICAM-1)(Reference Angulo, Llopis and Antolin44) in murine colitis models. Notably, in a recent study, it has been shown that a high-fat diet-induced systemic inflammatory response was suppressed in mast cell-deficient mice(Reference Heikkilä, Trosien and Metso45). Also, fenofibrate has been shown to inhibit leukotriene production in the RBL-2H3 mast cell line(Reference Yamashita46). These results suggest that some of the effects of the probiotics and fenofibrate on inflammation may be mediated by mast cells. In agreement with the fibrinogen result observed in the present study, in a study using a mouse model of induced acute liver injury, a further increase in plasma fibrinogen values was observed in mice receiving probiotic L. casei CRL 431when compared with the control mice, after which the fibrinogen levels returned to near basal levels in both groups(Reference Haro, Zelaya and Lazarte47). In addition to the traditional pro-inflammatory roles of fibrinogen in various inflammatory diseases, molecular mechanisms have also revealed diverse functions of fibrinogen, including immunomodulatory actions and even protection from inflammation(Reference Davalos and Akassoglou48).
SAA values dropped below baseline values at day 14 or 28 in all groups excluding the PJS group. It might be that the first response to the high-fat diet represents a strong acute inflammatory effect which is then followed by anti-inflammatory actions in order to restore homeostasis in the body. However, these reactions might overcompensate the inflammatory effect and lead to diminished concentrations of plasma inflammatory markers. Consuming probiotic bacteria, PJS in particular, might have a balancing effect on the immune system and prevent such dramatic changes concerning this inflammatory marker. A longer experiment would be needed to examine whether the SAA values return to basal values in other groups as well.
In addition to increasing the expression of circulating inflammatory markers, a high dietary fat intake also leads to elevated plasma levels of liver-derived ALT, a marker of hepatic damage and/or activation(Reference Kotronen and Yki-Jarvinen49). GG had a decreasing effect on high-fat diet-elevated plasma ALT concentrations, and a similar trend was also observed in the PJS group, suggesting a beneficial impact of the probiotics on liver function during the high-fat diet. These results are in line with decreased ALT values as observed after administration of a mixture of probiotics bifidobacteria, lactobacilli and Streptococcus thermophilus in ob/ob mice with non-alcoholic fatty liver disease(Reference Li, Yang and Lin50).
The 4-week period of the high-fat diet and probiotic administration did not induce changes in weight in the control, GG or fenofibrate group, or in the visceral adipose tissue mass in any groups. However, in the PJS group, weight decreased significantly during the 4-week experimental period, and also gonadal adipose tissue mass was significantly lower in the PJS group compared with the control group. These results suggest that PJS could affect fat metabolism during a high-fat diet. Gut microbiota is known to influence the energy metabolism of the host possibly by affecting the digestion of nutrients in the gut. It was observed by Ding et al. (Reference Ding, Chi and Scull6) that germ-free mice do not gain weight even after 16 weeks of high-fat dieting, whereas the weight of their conventionally raised littermates increased significantly after 12 weeks of consuming a high-fat diet. In another study, a 4-week period of feeding high doses of Lactobacillus ingluviei, a probiotic strain used in farm animals, induced a significant weight gain in mice without a high-fat diet but did not influence the mass of the adipose tissue(Reference Angelakis, Bastelica and Ben Amara51). Interestingly, wild-type mice that were administered a mast cell stabiliser, disodium cromoglycate, together with a high-fat diet, were observed to gain less body weight and to have an improved glucose tolerance, when compared with their littermates which did not receive the mast cell stabiliser(Reference Liu, Divoux and Sun11). This observation suggests a role for mast cells, along with the gut microbiota, in the regulation of energy metabolism and related glucose intolerance. However, the adiponectin levels in the present study were increased in the control and fenofibrate groups after 4-week administration of the high-fat diet, but remained fairly constant in the GG and PJS groups, i.e. they prevented the increase in the adiponectin level. These results show that the 4-week period of high-fat dieting did not per se unfavourably affect this circulating adipocyte-derived hormone that has anti-inflammatory properties and is normally abundantly present in the plasma(Reference Ouchi, Kihara and Funahashi52). Down-regulated levels of adiponectin are observed in obesity-linked disorders(Reference Flachs, Mohamed-Ali and Horakova53). In contrast to these observations, in a recent study, 11-week administration of a high-fat diet resulted in decreased serum adiponectin levels, and probiotic Lactobacillus plantarum strain no. 14 did not affect the adiponectin levels(Reference Takemura, Okubo and Sonoyama54). In a murine model of colitis, a probiotic mixture VSL#3 was observed to increase the adiponectin levels in mesenteric fat tissue after 10 d(Reference Mencarelli, Distrutti and Renga55). Thus, strain-specific effects of bacteria on the energy and fat metabolism of experimental animals are also observed.
Besides inflammation, dyslipidaemia is a key factor in metabolic disorders. GG and PJS did not have an effect on the elevated cholesterol levels in ApoE*3Leiden mice. Similarly, a cholesterol-lowering effect was not observed in a study where L. rhamnosus Lc705(Reference Hatakka, Mutanen and Holma56) or Lactobacillus fermentum (Reference Simons, Amansec and Conway57) was administered to subjects with elevated serum cholesterol levels. On the contrary, L. plantarum strain has been shown to lower cholesterol levels in hypercholesterolaemic ICR mice(Reference Nguyen, Kang and Lee58), and Lactobacillus acidophilus had the same effect on hypercholesterolaemic male Fischer 344/Jcl rats(Reference Fukushima, Yamada and Endo59) and hypercholesterolaemic human subjects(Reference Anderson and Gilliland60), reflecting species specificity regarding the cholesterol-decreasing ability of probiotics.
Fenofibrate among other fibrates are used in the treatment of hypertriacylglycerolaemia(Reference Watts and Dimmitt61). In the present study, plasma TAG levels were decreased in response to the high-fat diet. Such a reduction in TAG during high-fat feeding is typically observed in ApoE*3Leiden animals(Reference van Vlijmen, van den Maagdenberg and Gijbels62, Reference Wielinga, Yakala and Heeringa63). The positive control fenofibrate lowered plasma TAG levels even further compared with the water control. This result is in accordance with our previous observation(Reference Kooistra, Verschuren and de Vries-van der Weij26).
GG, PJS and fenofibrate failed to affect lipoprotein profiles. However, in a previous study in ApoE*3Leiden mice, fenofibrate treatment of mice tended to decrease the concentrations of the intermediate density lipoproteins/LDL and HDL-sized particles(Reference Kooistra, Verschuren and de Vries-van der Weij26).
In summary, the present study revealed that the probiotic PJS and GG have strain-specific anti-inflammatory effects on high-fat diet-induced inflammation in ApoE*3Leiden mice. PJS had a decreasing effect on intestinal mast cell numbers and showed local intestinal but also some systemic anti-inflammatory potential by decreasing the immunoreactivity of TNF-α, the mass of gonadal adipose tissue and the plasma levels of VCAM-1. On the other hand, in addition to decreasing the intestinal mast cell numbers, GG administration was able to induce strong IL-10 production in the intestines of ApoE*3Leiden mice, but failed to show systemic anti-inflammatory actions. Instead, GG induced decreased levels of plasma ALT levels. Future investigations are needed to further clarify the molecular mechanisms underlying the anti-inflammatory effects of probiotics and a possible therapeutic potential of probiotics in the prevention and treatment of inflammation-related metabolic disorders.
Acknowledgements
This study was supported by the Foundation for Nutrition Research (Helsinki, Finland). Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. The authors acknowledge the excellent technical assistance of Annie Jie, Karin Toet, Suvi Sokolnicki and Mari Jokinen. M. M. and R. A. K. are affiliated at Valio Limited; the other authors declare no conflict of interest. The authors' contributions were as follows: T. K., Ro. K., R. A. K., Ri. K., P. T. K. and A. O. designed the experiments; W. v. D., R. A. K., A. O., Ro. K., T. K., J. L. and P. T. K. analysed the results; A. O. wrote the manuscript; A. O., Ro. K., T. K., P. T. K., K. A. L., J. L. and M. M. edited the manuscript. All authors read and approved the final manuscript.