Introduction
Oral microenvironment and classification
The oral cavity is composed of diverse microenvironments such as epithelial surfaces and hard non-shedding surfaces that include teeth, buccal mucosa, supra- and sub-gingival plaque, tongue, cheek, lip and soft and hard palate for complex commensal microbial colonisation and each area shows specific microbial communities (Ref. Reference Lu, Xuan and Wang1). Furthermore, the necessity of dental restorations, implants and removal of prostheses make up additional non-shedding surfaces for the growth of microorganisms in the biofilm structure (Refs Reference Lu, Xuan and Wang1, Reference Hao2). Each area is capable of colonising 50–1000 species and the whole oral microenvironment is frequently exposed to saliva, gingival and subgingival crevicular fluid. The diversity of microbes observed in infants is based on the delivery mode and method of feeding. Vaginally born children have more microbes than children born by caesarean section, and breastfeeding infants show a higher presence of microbes than formula-fed infants (Refs Reference Hao2, Reference Lynge Pedersen and Belstrøm3). During the development stage, teeth eruption provides space for microbial colonisation and develops new ecological communities in a child's mouth. Particularly at the toddler stage, the complexity of the oral microbiome increases, and the substitution of primary teeth by adult dentition also significantly changes the oral microbial communities (Refs Reference Hao2, Reference Lynge Pedersen and Belstrøm3, Reference Scannapieco4). The microbial communities that have specific interactions and strong adherence to the local environment offer resistance from washout by fluid flow and mastication (Fig. 1) (Refs Reference Lynge Pedersen and Belstrøm3, Reference Scannapieco4).
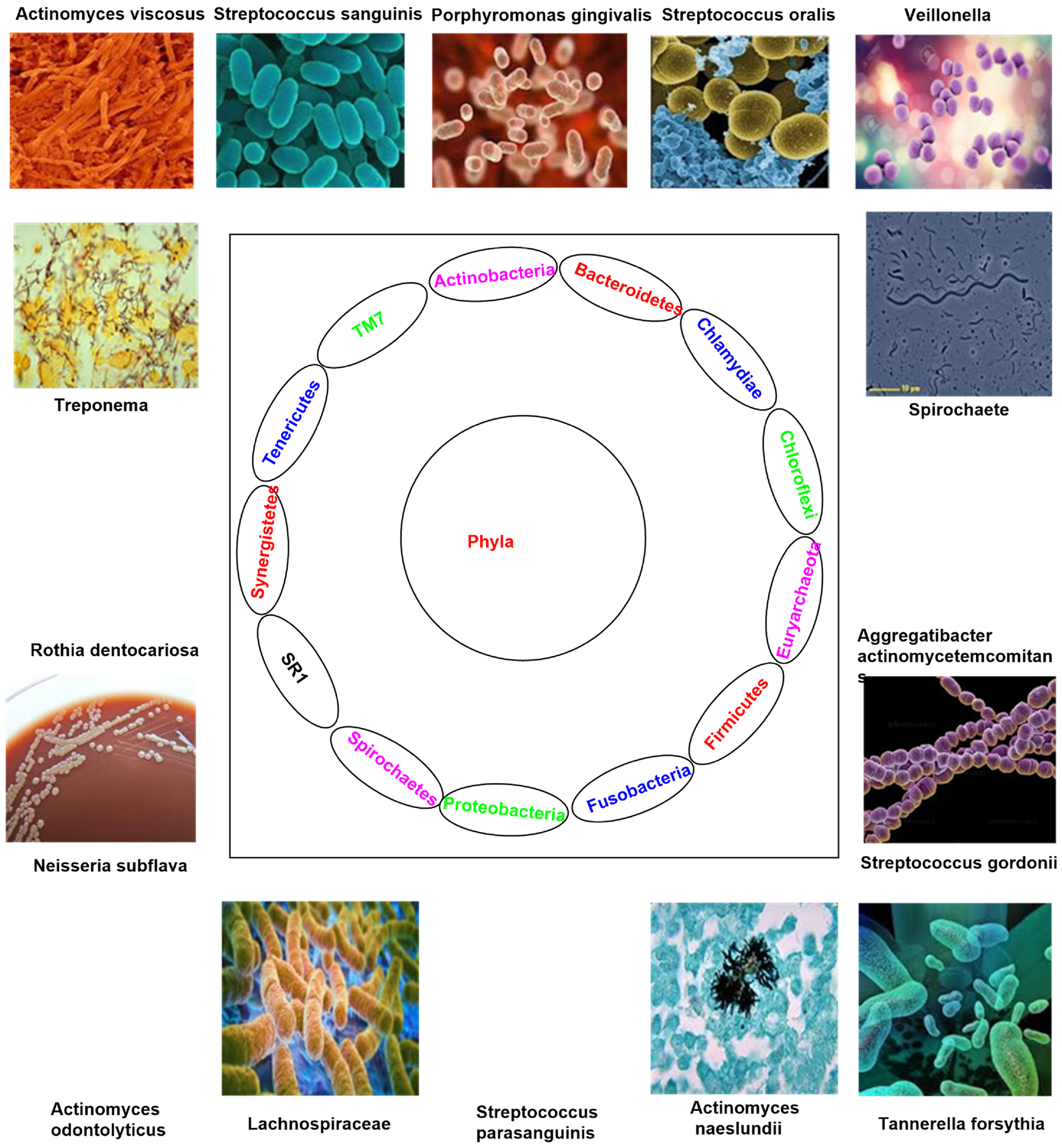
Fig. 1. Types of bacterial species residing in the oral cavity.
The physical and metabolic interspecies interactions of microbial composition are controlled by their spatial and structural orientation that can be beneficial or detrimental. Hard non-shedding surfaces and epithelial surfaces possess different colonisation strategies. In the non-shedding tooth surface, microorganisms form a biofilm, which is fixed with the matrix of extracellular polymeric substances (Refs Reference Scannapieco4, Reference Sultan5). In contrast, the shedding transient epithelial surface microbes develop biofilm that matures more frequently compared to non-shedding surface biofilm maturation subsequently, microbes penetrate and cultivate within epithelial surfaces and reach the intracellular environment (Refs Reference Sultan5, Reference Zhang6). Saliva is a lead source of microbial colonisation which controls the oral microecological system in different niches by maintaining pH, supplying nutrients and transporting antimicrobial substances to protect the residing microbes from exogenous pathogens (Refs Reference Zhang6, Reference Krishnan, Chen and Paster7). In the interest of getting complete information on complex microbes, the National Institute of Health initiated the Human Oral Microbiota Database (HOMD) collection programme to enhance the understanding of the people about the role of oral microbes in health and disease. This is the first online source to get a description of human health and disease-associated microbiome (Refs Reference Krishnan, Chen and Paster7, Reference Palmer8). Researchers strongly believe that acquaintance of human health and disease progression is difficult without getting the complete information of microbes, however, the objective is not fully achieved, the data collection process is being continued to make use of both cultivation dependent and non-dependent molecular technology (16S ribosomal RNA sequencing, new-generation sequencing technology and pyrosequencing) in the identification of microbes (Refs Reference Krishnan, Chen and Paster7, Reference Palmer8). An extended study report of HOMD demonstrated that there are nearly 770 types of microorganisms present in the human oral cavity, which is highly intricate microbial system in our physiological system after the gut. Among the different taxa found by HOMD, 57% are taxa cultivated and named, less than 13% are cultivated but not named and 30% are uncultured taxa. As per the report of HOMD the key resident of mouth is bacteria, which are composed of 13 phyla. The major bacterial phyla in saliva are Bacteroidetes, Actinobacteria, Fusobacteria, Firmicutes and Proteobacteria (Ref. Reference Lynge Pedersen and Belstrøm3). Furthermore, the obtained extensive data from HOMD study revealed the existence of more than 1000 bacterial species in the predicted phyla in the oral system, among which 280 species are cultivated and named, 500 species are cultivated by anaerobic microbiological methods and 600 species are identified by the cultivation-independent molecular method (Refs Reference Krishnan, Chen and Paster7, Reference Palmer8). In the oral cavity, high number of microbiotas has been observed in the gingival plaque and saliva, while lower number of microbes identified in keratinised gingival. In general, bacterial species like Streptococcus, Gemmella, Granulicatella, Fusobacterium and Veillonella are present in nearly all sites of the oral cavities, though the remaining live only at one or two oral sites, e.g., Bacteroides, Corynebacterium, Pasteurella, Prevotella and Neisseria (Refs Reference Krishnan, Chen and Paster7, Reference Palmer8, Reference Willis and Gabaldón9).
Oral microbiome: what are they doing in human health?
Historically microbes were first discovered by Antonie van Leeuwenhoek 200 years back in dental plaque samples using a microscope. He called them tiny living subjects, after that many kinds of research, had been conducted with curiosity to understand the properties of microbes using the microscope and other advanced technologies (Ref. Reference Lane10). Detailed descriptions of oral microbiomes and their mechanism of interactions with distinct environments of the body are essential to assess their physiological functions and contributions to human health. The oral cavity is a major gateway to the human body that allows food to enter through the mouth and processed with saliva on its way to reaching the gastrointestinal tract (Refs Reference Kilian11, Reference Koopman12). Microbes reside in the oral cavity in the physical structure of biofilms, where they get significant advantages such as shield from host defense and antimicrobial agents. For example, hypothiocyanite which has the antimicrobial activity to exogenous microbes by inhibition of bacterial glycolysis process is synthesised from hydrogen peroxide and saliva-secreted thiocyanate by the catalytic action of lactoperoxidase (Refs Reference Koopman12, Reference Doel13, Reference Magacz14). Another anti-microbial component present in the saliva is nitrite that is derived from nitrate present in the food generated through metabolic conversion by oral microbes. Commensally resident microbe which coevolves with the host contributes to derive the necessary level of immune system required to reach the appropriate immune function of the host. Several microbes have a physiological role in the human system such as inhibiting colonisation by exogenous pathogens, digestion of food, supplying enzymes which lack in human and also contribute to appropriate support to the immune system (Refs Reference Koopman12, Reference Doel13, Reference Magacz14, Reference Qu15).
As noted earlier, oral microbes supply some of the important enzymes to the human system that is in short and has significant physiological functions. The human system does not have nitrate reductase enzyme that is used for the conversion of food nitrate to nitrite and nitric oxide. Research reports have predicted that around 14 bacterial species with nitrate reductase activity are there to obtain potent vasodilator and antiatherogenic molecule of nitric oxide (Refs Reference Qu15, Reference Sparacino-Watkins, Stolz and Basu16). One-fourth of nitrate consumed by a human through diet re-enter the oral cavity via an entero-salivary circulation. Bacterial species producing nitrate reductase convert the nitrate to nitrite which then reduces to nitric oxide under acidic environment (Refs Reference Sparacino-Watkins, Stolz and Basu16, Reference Vanhatalo17). The optimum concentration of nitric oxide is important for cardiac system health and supports maintaining the blood vessels in an original state to produce an anti-hypertensive effect. Nutritional intake of nitrate supplements lowers blood pressure by the above-mentioned mechanism; however, the continual use of antimicrobial mouth rinse remarkably reduces the blood pressure-lowering effect of nitrate due to the eradication of health-promoting microbes from the oral system. Hence, excessive follow-up of oral hygiene procedures could have an unwanted effect on what is importantly required for the maintenance of cardiovascular health (Refs Reference Vanhatalo17, Reference Wescombe18).
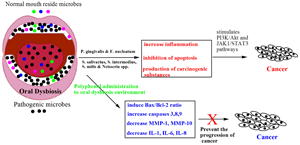
Some health-associated bacteria have been predicted as a retardant to the growth of oral pathogens. Evidence for that is, bacteriocin synthesised by Streptococcus salivarius species can reduce the growth of periodontitis associated Gram-negative species and offer beneficial effects (Refs Reference Wescombe18, Reference Avila, Ojcius and Yilmaz19). Even though several reports claim the health promotional activity of oral microbes, contradictory reports ensure that the commensal microbiota has also been linked with many diseases. Oral microbes play a significant role in oral and non-oral diseases at the dysbiotic state and the detailed information of the disease progression is presented in the next section (Refs Reference Kilian11, Reference Avila, Ojcius and Yilmaz19, Reference Tanner20) Figure 2.

Fig. 2. Symbiotic and dysbiotic environment of oral cavity and their consequence in health and disease.
Association of oral microbiome with dental caries and periodontal diseases
The theory of caries and periodontitis development suggests that the preliminary stages of development of both diseases occur at dental biofilm formation and this process is a normal physiological process rather than pathological (Refs Reference Kianoush21, Reference Zinöcker and Lindseth22). When they are not carefully managed, and when there is a lack of oral health practices for a longer time, it produces the diseases. Caries and periodontitis are the outcomes of healthy microbes' normal metabolic reaction, and the process continues for an entire lifetime (Refs Reference Zinöcker and Lindseth22, Reference Paes Leme23). The interaction between microbes and host and the contributory factors leads to the development of both diseases. From the healthy biofilm formation to the transfer of disease, caries and periodontitis can be caused by changes in the composition of healthy microbes rather than exogenous infections (Refs Reference Ruby24, Reference Forssten, Björklund and Ouwehand25) S. mutans and Lactobacilli is the main component of the oral microbiota, so research has suggested that streptococci are the main components of caries development, however, the outcome of research reports revealed that not a single organism or group of microorganism have a consequence in the development of caries (Ref. Reference Cekici26). Dental caries mainly occurs in the dentin region in the form of tooth demineralisation, which develops due to dietary sugar-based microbial growth and carbohydrate metabolism that influence acidic environment in the dental region and disturb normal tooth mineralisation processes. Specially, S. mutans easily converts sucrose and other sugars into ATP and lactate as reaction products, the higher accumulation of lactate is responsible for acidic environment development (Refs Reference Cekici26, Reference Mombelli27). Recent advanced molecular techniques revealed that along with S. mutans, other acidogenic and aciduric species include Scardovia spp., Bifidobacterium spp., Actinomyces involved in acidic environment development and caries progression. Other than this abundant presence of some non-aciduric species, including Propionibacterium, Corynebacterium and Granulicatella also been detected in caries-associated supragingival plaque. Thus, during caries development, significant changes in microbial communities occur in the supragingival region, which alters the microbial composition from the health-promoting species to the disease-promoting species (Ref. Reference Mombelli27) Figure 3.
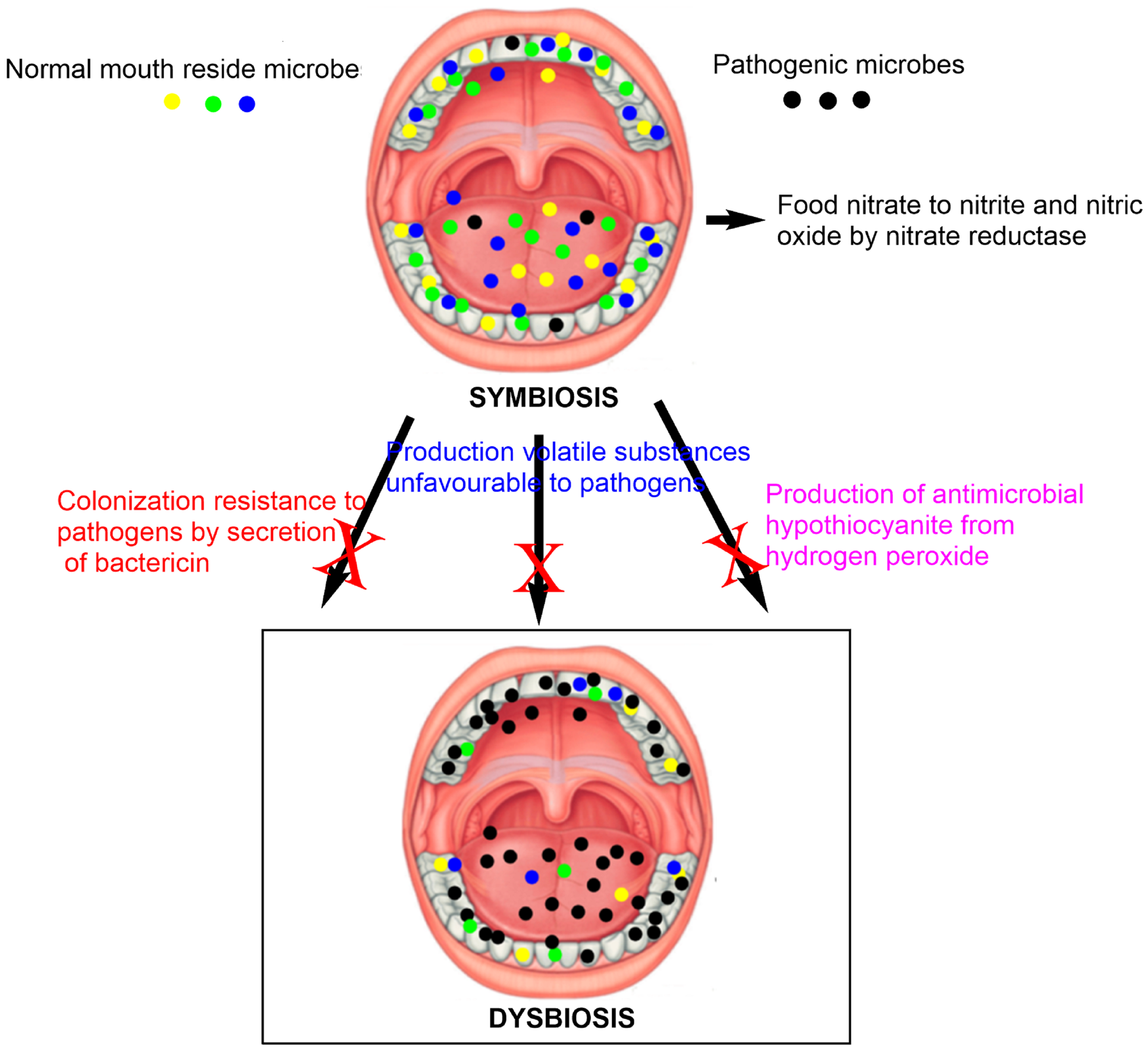
Fig. 3. Schematic representation of health benefits of oral microbes.
Periodontitis is progressed through chronic gingivitis, characterised by extension of the microbial biofilm at the gingival region with development of an inflammatory infiltrate that results in loosen the attachment between connective tissue and tooth, alveolar bone resorption and may result in eventual tooth loss (Ref. Reference Lamont, Koo and Hajishengallis28). As noted from earlier studies, not a single organism is contributed to the transfer of health to periodontal diseases, rather than symbiotic microbial community present in subgingival region shift to the state of dysbiotic, in which community association, diversity, and abundance transfer to disease state. Earlier culture-independent approaches detected three-member of gram-negative microbes as causative factor of periodontitis termed as Red complex includes P. gingivalis, Treponema denticola and Tannerella forsythia. Although these organisms pointed out pathobionts, they are also commonly present in the lower numbers in the sublingual region, and obviously, these organisms can be removed from patients with periodontitis using mechanical debridement (Ref. Reference Lamont, Koo and Hajishengallis28). Apart from these, recent research reports included the Gram positive Filifacter alocis and anaerobes Parvimonas, Fusobacterium and Prevotella as pathobionts of periodontitis.
Emerging roles of the oral microbiota in cancer
Cancer is the major cause of health burden and the second main reason for mortality in the world (Ref. Reference Bhatt, Redinbo and Bultman29). Several causative factors can induce cancer, which include carcinogenic chemicals, age, heredity, diet, tobacco use, inflammation and chronic viral infections. Contradictorily, these reports left some place to associate microbes as one of the causative factors in cancer development. Research on this section is getting more attention and the significance of gut microbes in association with cancer has been proved by several studies (Refs Reference Bhatt, Redinbo and Bultman29, Reference Wroblewski, Peek and Wilson30). In the year of 1994, bacterial species H. pylori was categorised as the causative factor of cancer in humans by the World Health Organization (WHO). Later, there has been an increased amount of data supporting an association between specific microorganisms. Similar to gut microbes, the oral cavity residing microbes also was included as a responsible factor for the development of oral cancer and various types of cancers (Refs Reference Wroblewski, Peek and Wilson30, Reference Chattopadhyay, Verma and Panda31). The progression of cancer with exogenous microbes is not studied well, however, many reports strongly claimed oral communal microbes role in cancer development in vitro and number of in vivo reports inconsistently propose the association with cancer (Ref. Reference Börnigen32). The oral resident microbes are not only reason for the development of oral cancer also contributes to progression of extra-oral cancers at dysbiotic state. A few specific species have been reported as vital contributors to oral cancer development, such as Streptococcus sp., Peptostreptococcus sp., Prevotella sp., Fusobacterium sp., P.gingivalis and C.gingivalis. Also many works have identified that the oral periopathogens F.nucleatum and P. gingivalis play an essential role in developing colorectal and pancreatic cancer (Ref. Reference Börnigen32). According to research reports on the association between oral cancer and resident microbes, it is essential to understand that pathogenesis is useful to early prediction and proper disease control.
Inflammation Based induction of carcinogenesis
The inflammatory process materialises to augment the severity of cancer at various parts of the body. Inflammation that takes place in the epithelium is the causative factor of oral carcinogenesis, and the use of tobacco products induces inflammation in the oral system that is a mechanistic link to increase the risk of cancer development (Refs Reference Chattopadhyay, Verma and Panda31, Reference Börnigen32, Reference Yao33). Inflammatory mediators support the progression of both pre- and pro-malignant processes by activation of important transcription factors such as NF-kB and STAT3 with the consequence of constant tumour-promoting inflammation that persists in the tumour microenvironment (Refs Reference Yao33, Reference Al-Hebshi, Borgnakke and Johnson34). Bacteria mediated induction of chronic inflammation has been reported as one of the probable factors in the development of oral cancer. Most importantly, this report is proved by a strong link with bacterial infectious reaction of periodontitis and increased risk of oral squamous cell carcinoma (OSCC) (Refs Reference Al-Hebshi, Borgnakke and Johnson34, Reference Gaonkar35). P. gingivalis functions in the progression of chronic inflammation by upregulating the inflammatory receptors B7-H1 and B7-DC and also modulating the level of TNF-α and interleukins IL-1, IL-6 and IL-8 reported in several studies (Refs Reference Al-Hebshi, Borgnakke and Johnson34, Reference Gaonkar35).
Influence of microbes on cell signalling mediated cancer development
Microbes present in the oral cavity can support the proliferation of host cells and significantly contribute to oral carcinogenesis. For example, P.gingivalis, an important contributor pathogen in the periodontal disease progression that contains proteins and LPS on its cell wall can increase the proliferation of human fibroblasts (Refs Reference Moffatt and Lamont36, Reference Belkaid and Hand37). Bacterial species inhibit apoptosis by different mechanisms of action which lead to cancer progression that occurs in the human system. P. gingivalis stimulates PI3 K/Akt and JAK1/STAT3 signalling pathways, which manage the mitochondrial apoptosis process (Refs Reference Belkaid and Hand37, Reference Choi38). In another mechanism in the mitochondrial membrane P. gingivalis increases the BCL2 (antiapoptotic): BAX (proapoptotic) ratio, decrease the function of proapoptotic BCL-2-associated death promoter and also reduces the release of the apoptosis effector cytochrome C. Further, P. gingivalis inhibits apoptosis by enhancing the activity of signal transducer and activator of transcription (3STAT3) and downregulating the activity of suppressor of cytokine signalling (Refs Reference Karpiński39, Reference Gallimidi40). The mechanistic action of the executioner caspase-3 and caspase-9 in apoptosis induction by the downstream process is also controlled by this bacterial microbe. Furthermore, P. gingivalis blocks ATP-dependent apoptosis through purinergic receptor P2X7 in gingival epithelial cells by its secreted product nucleoside diphosphate kinase (NDK). NDK also modulates the anti-cancer immune reaction by ATP activation of P2X7 receptor in dentritic cells (Refs Reference Gallimidi40, Reference Schwabe and Jobin41). Another study conducted in the murine model reported that long term coinfection induced by F. nucleatum and P. gingivalis supports the development of oral cancer by chemical induction method especially through establishment of both IL6 and STAT3 (Refs Reference Schwabe and Jobin41, Reference Qu, Tang and Hua42).
Other mechanisms of microbes mediated cancer development
Activation of cell proliferation
Along with anti-apoptotic properties, P. gingivalis also interfere with cell cycle phases especially on S and G2 through phosphorylation of cyclin-dependent kinases, increasing the concentrations of cyclins (A, D and E) and reducing the concentration of tumour suppressor p53 which accelerates the progression of carcinogenesis (Refs Reference Qu, Tang and Hua42, Reference Perera43). All these consequences are related to stimulation of fimbriae of the organisms. P. gingivalis produces trypsin-like cysteine proteinases that activate the β-catenin through its proteolytic activity and contribute to the development of proliferative phenotype in gingival epithelial cells. F. nucleatum also contributes to cell proliferation by its infectious reaction that stimulates a number of kinases, majorly concerned with cell proliferation and survival signalling process, in addition to DNA repair (Refs Reference Perera43, Reference Sudhakara44).
Promoting cellular migration and invasion
Cellular invasion is increased by the microbes F. nucleatum and P. gingivalis mediated infectious reaction infection in OSCC. P. gingivalis infection in the OSCC cell line causes the activation of PAR/NF-kB, ERK1/2-ETS1 and p38/HSP27 pathways and contributes to higher concentration of pro-matrix metalloproteinase-9 (Refs Reference Perera43, Reference Sudhakara44, Reference Takeuchi45). Then the expression of gingipains by the microbes enhances the cellular invasion and converts the proenzyme into active MMP-9. Also, frequent exposure of P. gingivalis is involved in the conversion of epithelial to mesenchymal transition (EMT), enhanced production of MMP-1 and MMP-10 and increased invasiveness of OSCC cells (Refs Reference Sudhakara44, Reference Takeuchi45). Similarly F. nucleatum induced infection in human epithelial cells activates the mitogen activated protein kinase p38, promotes cellular migration, enhances the synthesis of MMP-13 through stimulation of RhoA kinase, Etk/BMX and S6 kinase p70 (Refs Reference Sudhakara44, Reference Takeuchi45, Reference Inaba46).
Production of carcinogenic substances
Long-term consumption of excessive alcohol is considered a significant contributor to oral cancer development. Pure ethanol has not been shown for any carcinogenic property, however, metabolic conversion of ethanol to acetaldehyde, is carcinogenic, which is proven in both in vitro and animal models. Acetaldehyde has been predicted as carcinogenic by following mutagenic effects, such as DNA crosslinking, formation of DNA adducts and chromosomal aberrations (Ref. Reference Fiorentini47). A significant concentration of acetaldehyde cannot be synthesised by alcohol dehydrogenase (ADH) enzymes present in human epithelial cells, however, the oral microflora can contribute to its chemical synthesis by utilising their enzymes, which induces carcinogenic reaction (Ref. Reference Moritani48). A large number of studies confirmed this hypothesis and showed that the gram-positive aerobic bacteria, streptococci and yeasts have the capacity to the production of acetaldehyde. Also, oral cavity nonpathogenic residents like Neisseria species have been reported to possess enhanced ADH activity, which can synthesise more concentration of acetaldehyde (Refs Reference Fiorentini47, Reference Moritani48). Further analysis in the salivary samples reveals that the heavy consumption of tobacco and alcohol augments the microbial ADH activity and aldehyde production. As reported earlier, the accompanied intake of tobacco and alcohol has multiple roles in oral cancer development, and this effect also shows that the combined consumption of tobacco and alcohol produces several times higher carcinogenic induction rather than the intake of alcohol alone (Ref. Reference Stornetta, Guidolin and Balbo49). Besides, oral bacteria may play a role in increased activation of carcinogenic nitrosamines and nitrosodiethylamine (NDEA) from tobacco smoking (Refs Reference Stornetta, Guidolin and Balbo49, Reference Nieminen and Salaspuro50).
Extra-oral cancer
The excessive secretion of cytokines and other inflammatory mediators induced by oral microorganisms may be the reason for the immune-related mechanisms of cancer development (Refs Reference Schwabe and Jobin41, Reference Turesky and Le Marchand51). The pathogen-associated molecular patterns secreted by pathogenic bacteria bind to toll-like receptors of innate immune cells which induce the production of toxins that causes inflammatory reactions subsequently inducing carcinogenesis (Ref. Reference Degoricija52). Periodontal diseases have also been involved in the elevated risk of the cancer occurrence by overexpression of the human telomerase reverse transcription (hTERT) enzyme that was identified in patients with periodontitis. This result strongly suggests that both periodontitis and cancer are linked in disease-oriented pathways to progress to cellular differentiation and immortality (Refs Reference Degoricija52, Reference Katarkar53).
S. anginosus secretes antigen which has been detected to induce nitric oxide synthesis in addition to producing inflammatory cytokines in murine peritoneal exudates cells (Refs Reference Kilian11, Reference Katarkar53). The saliva of periodontitis disease patients is highly present with S. anginosus which is also responsible for elevated inflammatory cell infiltration and oxidative DNA damage due to excessive existence of 8-hydroxy-deoxyguanosine (8-OHdG). Abundant presence of 8-OHdG has already been reported to have an association with early malignant lesions and cancerous tissues (Refs Reference Sasaki54, Reference Sugano55). It has been predicted that S. anginosus infection has a considerable impact on the development of oesophageal cancer by inducing inflammation and supporting the cancer growth process. Suppressing the level of streptococci could reduce the chance of reestablishment of oesophageal cancer (Ref. Reference Sugano55). Another study has proved that immune-suppressed haematological malignancies of patients suffered by high incidence to Enterobacteriaceae lead to septicaemia conditions. This report suggests that abnormal functions of the immune system alter the oral microbes and allows the pathogenic microbes to induce infectious disease like septicaemia (Ref. Reference Mager56). Particularly, haematological malignancy of acute lymphoblastic leukaemia can moderately disturb the immune system, which reduces the numbers and diversity of oral microbes (Ref. Reference Mager56).
Narikiyo et al. observed that the presence of periodontopathic spirochete T. denticola, S. mitis and S. anginosus in saliva samples were associated with oesophageal cancer. Furthermore, the extent of S. mitis and N. elongata were significantly varied between the pancreatic cancer suffered patients and healthy controls (Ref. Reference Narikiyo57). Another report reveals a direct link between P. gingivalis infection and development of pancreatic cancer, whereas Torres et al. examined the excessive presence of P. gingivalis and F. leptotrichia in pancreatic cancer (Ref. Reference Curran, Godfrey and Kline58). There are also data indicating that oral streptococci may be associated with colon cancer (Ref. Reference Thomas59). Oral pathogens A. actinomycetemcomitans and P. gingivalis were identified by genomic studies to have a higher risk factor for pancreatic cancer and the Leptotrichia genus was reported as a lower risk factor in the pancreatic cancer development (Refs Reference Curran, Godfrey and Kline58, Reference Thomas59, Reference Torres60) Figure 4.

Fig. 4. Schematic representation of role of oral microbes in cancer development.
A strong connection between F. nucleatum and CRC (abbrevation) is evidently proved. Fusobacterial adhesion FadA has been reported to bind with E-cadherin on CRC cells and in turn activate the β-catenin signalling pathway which is important to the enhanced activity of oncogenes and proinflammatory cytokines and to support the CRC cell proliferation. It is also found that FadA expression in colon of CRC patients to be 10-times more than those in normal persons (Ref. Reference Rubinstein61).
Polyphenols targeting the oral microbiota
As noted earlier, the number of research reports is increasing in recent days in the interest of getting more detailed information about human oral microbiome, owing to its most close association with the health of humans (Ref. Reference Hao2). The normal state of the oral microbiota is disturbed due to exposure of number of stress factors comprise intake of unhealthy food & drinks, poor oral hygiene, disease, environmental factors and the use of potential antibacterial drugs, which can destabilise the mutual interaction in the oral system and allow the overgrowth of oral pathogens in the dental biofilms (Refs Reference Kilian11, Reference Koopman12, Reference Doel13, Reference Magacz14, Reference Fan62). In the meanwhile, influence of above-said stress factors microbes explicit antibiotic resistance genes (ARGs), to support their existence and genetic persistence. Due to the altered oral microbial environment, the pathogenic microbial populations increase and cause several diseases as mentioned earlier such as gingivitis, caries and periodontitis that may be experienced at least in some parts life of all human beings. Treatment for oral diseases is focused on the prevention of dental plaque formation by antibiotic administration or mechanical approach. But continual or excessive administration of synthetic drugs for the reduction of oral microbial derived diseases can cause unexpected adverse reactions. This might generate resistance to oral cavity residing microbes and ARGs expressing microbial communities leading to a decrease in the effectiveness of therapy and the severity of the disease may be increased and spread to both oral and extra-oral regions by passive movement of microbes to bloodstream and implicate the diseases (Refs Reference Fan62, Reference Reuter63). This complexity in treating oral microbes-associated diseases stresses the need for alternative therapeutic approaches for better disease reduction. In this context, plant-derived natural products are searched for as a better alternative medicine because of their potential therapeutic properties with less side effects in abroad spectrum of human health complications. They may alter the properties of dental biofilms with the notion of targeting pathogenic microbes rather than affecting normal health microbes. The mechanism of action of plant-derived medicines, especially polyphenols for inhibiting microbial growth, is executed by following a number of ways, such as anti-adhesion to buccal surface and inhibition of glucan synthesis, hindrance of biofilm formation, prevention of acid production by bacteria, inhibition of amylases and suppression of hydrophobicity of bacteria. These alternative mechanisms of action of polyphenols may support the prevention of antibiotic-associated resistance development and ensure better reduction of oral microbes associated with complicated diseases (Refs Reference Reuter63, Reference Varoni64, Reference Cardona65, Reference Langdon, Crook and Dantas66).
Polyphenols are plant-derived molecules mainly obtained from higher plants with various functions ranging from protection from environmental factors to controlling the growth mechanism and plant colour. These naturally derived bioactive molecules are abundant in vegetables, fruits, green and black tea, coffee, red wine, extra virgin olive oil and chocolate (Refs Reference Reuter63, Reference Varoni64, Reference Cardona65, Reference Langdon, Crook and Dantas66, Reference Eckert, Sullivan and Shi67). The polyphenol compounds constitute a diverse group of molecules that are segregated according to their chemical structure, such as flavones, isoflavones, flavonols, flavanols, catechins and phenolic acids. In addition, the dietary-derived secondary metabolites have significant implications for human health (Refs Reference Zhang and Tsao68, Reference Singh69, Reference Ferrazzano70). Due to their considerable diversity in structures, polyphenols possess extensive biological activities such as improvement of the endothelial function, antioxidant, anti-inflammation, anti-atherosclerosis, anti-apoptosis, anti-aging, anticarcinogen, prevention of neurological diseases, cardiovascular protection also inhibition of cell proliferation activity and angiogenesis (Refs Reference Veloz71, Reference Tresserra-Rimbau, Lamuela-Raventos and Moreno72). The attention of polyphenols has increased in health applications due to its unique antioxidant properties. In our physiological system when the regulatory mechanism fails in the production and removal of reactive oxygen or nitrogen species, it causes oxidative stress associated inflammatory reaction that leads to development of several diseases (Refs Reference Esteban-Fernández73, Reference An74). As it is already known, oxidative stress mediated inflammation is one of the major contributors to oral disease progression along with microbial related factors, hence polyphenol molecules may serve as a suitable substituent in the reduction and elimination of oral inflammatory diseases (Ref. Reference Chinsembu75). It has been observed that the consumption of polyphenol-rich products helps to protect against microbial associated diseases especially gut microbes mediated inflammatory diseases (Refs Reference An74, Reference Chinsembu75, Reference Panche, Diwan and Chandra76). However, the awareness of the effects of polyphenols in association with the prevention of dental diseases and their application in health practice is in a preliminary stage. This section is intended to deliver the therapeutic actions of polyphenols in major oral microbial diseases caries, periodontitis and oral cancer. Furthermore, details of in vitro, in vivo and clinical studies of polyphenols in oral microbial diseases are also explained.
Effect of polyphenols in the management of microbiome induced dental caries and periodontitis
According to the WHO, almost all adult population presents with caries, also approximately 80% of children have suffered from dental caries. Overgrowth of S. mutans is a main causative factor in the advancement of caries, hence the control of the levels of S. mutans may help to reduce the risk of caries (Refs Reference Zinöcker and Lindseth22, Reference Paes Leme23). The first line of therapy given for caries is fluoride, which is considered the gold standard among different anticaries agents. However, it has narrow antimicrobial actions and it is not suitable to manage complex cariogenic challenge exposed individuals (Ref. Reference Henao-Mejia77). The other treatment option is focused on commonly used antimicrobials; however, they lack in differentiating healthy and disease-causing microbes and completely eradicate oral microflora, including the microbes that provide health benefits (Refs Reference Henao-Mejia77, Reference Li and Webster78). Another complication in this current treatment approach is, after withdrawing from medication, the affected tooth surface can reproduce the eradicated microbes as a consequence of reestablishing the caries (Ref. Reference Shekarchizadeh79). Due to these many complexities with the current day medicine, alternative approaches are essentially required to reduce the dental dysbiosis with similar effects of fluoride on the de-/remineralisation balance in the treatment of dental caries (Refs Reference Sweeny80, Reference Takagi, Liao and Chow81, Reference Koo82). Multiple types of research support the use of polyphenols as a possible caries risk reduction agent. Polyphenols generally interact with salivary proteins and produce several health-promoting effects. The presence of sufficient concentrations of polyphenol is expected to reduce the dental caries formation along with anti-bacterial effects by inhibiting the enzyme glucosyltransferase B and C. This enzyme supports the production of insoluble polysaccharides which help for attachment of oral microbes to the dental surface (Refs Reference Takagi, Liao and Chow81, Reference Koo82, Reference Philip, Suneja and Walsh83).
In this article we look at some specific mechanisms of action of polyphenol compounds against caries development. Cranberry polyphenol, particularly proanthocyanidins have several cariostatic actions including reducing the bacterial adhesion, aciduricity, acidogenicity, glucan synthesis and biofilm physical structure without harming the microbial viability (Ref. Reference Philip and Walsh84). Polyphenols present in the cocoa, coffee and tea exert anti-cariogenic activity due to their antibacterial properties. Cocoa polyphenol reduces the activity of S. mutans and S. sanguinis biofilm formation, acid production and control dental caries. Green and roasted coffee containing trigonelline, caffeine and chlorogenic acid disturbs the adsorption of S. mutans to saliva-coated hydroxyapatite beads and reduces the caries environment development (Ref. Reference Daglia85). Polyphenol compounds like epicatechin, epigallocatechin and gallocatechin demonstrate anti-caries effect through anti-microbial mechanism of action. Furthermore, due to the structural diversity of polyphenols, they produce less microbial resistance and have different mechanisms of antimicrobial action (Ref. Reference Fialho86). All these results support polyphenol rich diet/medicine as an ideal agent in the reduction of biofilm virulence, alterations of the microbial community in dental plaque microbial community, and prevention of the progress of pathogenesis of dental caries.
According to a statement by the WHO, periodontitis affected 5–15% of populations worldwide (Ref. Reference Nazir87). Clinical manifestation of periodontitis is explained by swelling and bleeding of the gums. The major causative microbes P. gingivalis and P. intermedia produce various toxic materials that crosstalk with cellular components and activate the host immune response. This unwanted interaction between the microbes secreting toxins and the immune system can induce multiple inflammatory mediators such as cytokines, chemokines and metalloproteinases (MMPs) in the host cells and produce periodontitis (Refs Reference Zinöcker and Lindseth22, Reference Paes Leme23, Reference Ruby24, Reference Forssten, Björklund and Ouwehand25, Reference Cekici26). Conventionally, machine-driven or manual instrumentation approach is practiced to reduce the microbial load and periodontitis. Additionally, antimicrobial treatment is followed to improve the antimicrobial effects of mechanical debridement (Ref. Reference Nazir87). Since numerous unwanted side effects surge with the current day treatment in periodontitis like the significant development of bacterial resistance against antibiotics, it emphasises the requirement of alternative efficient treatment strategies for the treatment and avoidance of this widespread disease (Ref. Reference Tunkel, Heinecke and Flemmig88). Recent research reports have pointed out the potential use of polyphenols as supportive approach to managing oral inflammatory conditions. Natural polyphenols display anti-inflammatory and anti-bacterial activities and they can be used as alternative therapy in the control of periodontitis (Ref. Reference Tunkel, Heinecke and Flemmig88).
Supportive in vitro data for the beneficial effect of polyphenols against dental caries and periodontitis
Several in vitro study reports have supported the role of polyphenols in the reduction of inflammatory reactions and oral microbial pathogenesis linked to caries and periodontitis. Mezoneuron benthamianum root extract polyphenols such as trans-resveratrol, piceatannol and gallic acid were studied for anticaries activity against oral pathogens (S. mutans, S. aureus, P. aeruginosa and E. coli). It was observed that the root extract of the polyphenol showed significant antioxidant, anticaries and cytotoxic activities. Additionally, it was suggested that M. benthamianum can be used as a suitable agent in preservation of the oral hygiene (Ref. Reference Osamudiamen89). The polyphenols procyanidins and catechin/epicatechin found in plant sorghum episperm remarkably reduced the acid production from the cariogenic bacteria S. mutants and S. sobrinus and prevented dental caries through scavenging the superoxide anion free radicals (Ref. Reference Xu90). Zhao et al. examined the property of grape seed extract (GSE) in the inhibition of caries lesion formation in an in vitro S. mutans biofilm model. They used bovine incisors to separate enamel fragments for the growth of caries microbe S. mutans and observed the relative optical density and lesion depth (LD) and through microscopy methods. It was found that treatment with GSE produced dose-dependent inhibition of enamel caries formation due to the reduction of the growth of S. mutans and inhibition of the biofilm formation (Ref. Reference Zhao91).
Polyphenols present in hops were tested in vitro to demonstrate its effect in reduction of lactic acid secretion of S. mutans. Outcome of the study revealed that the bakuchiol and macelignan polyphenols produced anti-biofilm activity by reducing the growth of Actinomyces viscosus, S. mutans and S. sanguinis (Ref. Reference Katsura92). Another in vitro study was performed to check the cranberries polyphenol function in caries reduction. Results of the study demonstrated that the polyphenol effectively reduced the biofilm formation, organic acid production by cariogenic bacteria and prevented the attachment and coaggregation of streptococcus species (Ref. Reference Gregoire93). Further study was executed to examine the utilisation of orange peel extract as caries preventive material. Research of the study explained that the orange peel reduced the growth of S. mutans and is recommended for detailed study to inclusion of this kind of polyphenol as caries reducing agents in dentifrices and mouth rinses (Ref. Reference Shetty94). Red propolis chemical constituents p-coumaric acid and luteolin were studied for the reduction of growth of S. mutans and inhibition of dental demineralisation. Oral fibroblast cell and bovine dental enamel blocks were used in this study to examine the cytotoxicity and dental demineralisation properties. The results revealed that polyphenolic compounds effectively reduced the S. mutans colonisation which inhibited the concentration of extracellular polysaccharides and dental enamel demineralisation (Ref. Reference Martins95).
Natural polyphenols present in the cranberry (proanthocyanidins) and green tea epigallocatechin-3-gallate (EGCG) synergistically reduced the secretion of inflammatory substances such as cytokines and chemokines in LPS-stimulated oral mucosal cells. This combination approach demonstrates promising utilisation of polyphenol compounds as potential adjunctive therapies for the treatment of inflammatory periodontitis (Ref. Reference Lombardo Bedran, Palomari Spolidorio and Grenier96). The rhizome of Limonium brasiliense was identified to contain the polyphenol compounds such as gallic acid, EGCG and samarangenins. The effect of these polyphenol compounds against P. gingivalis was tested under in vitro conditions in human KB cells to evaluate the potential inhibition of virulence factors. The mechanism of action of these polyphenols against P. gingivalis was studied using human KB cells by estimation of the potency in reduction of periodontitis associated virulence factors. The results revealed a dose-dependent reduction of the adhesive and proteolytic activity of polyphenol for better control of periodontitis (Ref. Reference de Oliveira Caleare97). Lagha et al. studied the effect of tea extracts and their bioactive compounds against periodontal pathogen F. nucleatum. They reported that polyphenols theaflavins and EGCG present in the tea extracts effectively reduced the microbe adhesion to oral epithelial cells. Further, they observed that components present in the tea reduced F. nucleatum stimulated haemolysis, hydrogen sulphide production and virulence to prevent the occurrence of periodontitis (Ref. Reference Ben Lagha, Haas and Grenier98). Another study was conducted to investigate the role of R. acetosa extract possessing proanthocyanidin against periodontal pathogen, which is strongly involved in chronic and aggressive periodontitis. They found that proanthocyanidin-enriched extract prevents the KB cells from P. gingivalis infection by ensuring anti-adhesive properties (Ref. Reference Schmuch99). In another study blueberry extract containing phenolic acids, flavonoids and procyanidins were tested for their reducing effect against periodontitis causative factors. The blueberry extract showed dose-dependent antibacterial activity to reduce the F. nucleatum biofilm formation property. Further, the extract also reduced the NF-κB and other inflammatory cytokines and produced dual antibacterial and anti-inflammatory action to significantly control the periodontal disease (Ref. Reference Ben Lagha100). These complimentary in vitro mechanistic actions of the polyphenols in changing multiple inflammatory processes that could support the disease reduction in periodontitis justify the broad examination of polyphenols using preclinical and clinical studies.
Supportive in vivo data for the beneficial effect of polyphenols against dental caries and periodontitis
Few in vivo studies using animal models have demonstrated the function of dietary polyphenols in reducing features of inflammatory markers and macroscopic damage associated with oral inflammatory diseases. Bioflavonoids naringenin, rutin, quercetin and naringin supplementation effect on cariogenic bacteria prevalence and dental caries were studied in male albino rats. Rats were examined for dental plaque accumulation and dental caries and subsequently, it was found that naringenin significantly reduced the dental caries and plaque accumulation (Ref. Reference Wood101). The Oolong tea polyphenolic compounds when tested in streptococci mutans infected Sprague-Dawley rats revealed that the oral intake of the oolong tea extract which contains the polymeric polyphenol compounds resulted in significant reductions in plaque accumulation and caries development (Ref. Reference Ooshima102).
Effect of cacoa bean husk extract (CBH) on caries inhibitory activity was studied in both in vitro and in vivo models. In vitro study revealed that CBH effectively reduced the growth of streptococci, synthesis of glucosyltransferases and reduced the cell adherence of the microbe. In vivo evaluation reported that CBH administration to S. mutans and S. sobrinus infected Sprague-Dawley rats inhibits the caries development and dental plaque accumulation (Ref. Reference Ooshima103). Resveratol which is a stilbenoid polyphenol was tested in an animal model induced with periodontitis by ligature and LPS. Resveratrol administration most probably reversed the inflammatory reaction induced by LPS. Micro-CT and histological study results explained that resveratrol treatment prevented the alveolar bone loss induced by LPS in rats. Overall, their finding proposed that resveratrol protects the rats from periodontitic tissue destruction by reducing inflammatory reactions (Ref. Reference Bhattarai104). Another research team evaluated the effect of resveratrol on antioxidant activity mediated reduction of the periodontitis. They particularly focused on the role of resveratrol in specific oxidative stress reduction pathways through nuclear factor E2-related factor 2 and sirtuin 1/AMP-activated protein kinase in male Wistar rats. It was observed that treatment with resveratrol reduced the alveolar bone resorption and reduced the oxidative stress by induction of the specific pathway. Finally, it was concluded that ingestion of resveratrol controls the development of periodontitis and oxidative stress (Ref. Reference Tamaki105).
Green tea extract polyphenol EGCG has plenty of pharmacological function. Cai et al. studied the effect of EGCG on P. gingivalis stimulated periodontitis in BALB/c mice. They have observed in the periodontitis induced mice markedly increased alveolar bone resorption and inflammatory mediators. After treatment with EGCG they found improvement in the alveolar bone and marked reduction in inflammatory substances. Based on the obtained results it was suggested that the EGCG could reduce P. gingivalis-induced periodontitis by its anti-inflammatory effect (Ref. Reference Cai106). Tominari et al. observed the role of EGCG in reduction of inflammatory reaction mediated bone resorption. They used calvarial organ cultures in the study and observed EGCG bone resorption reduction capacity after bone resorption induction by LPS. Furthermore, EGCG decreased the LPS-stimulated elevation of inflammatory mediators in osteoblasts, as well as prostaglandin E2 production, which is required for osteoclast development. Thus, EGCG functions in both in vitro and in vivo model systems for reduction of mandibular alveolar bone resorption and prevention of loss of mouse alveolar bone mass (Ref. Reference Tominari107). Almeida et al. performed the study in Wistar rats to evaluate the property of green tea extract as adjuvant therapy in scaling and root planning to reduce the inflammatory reaction. They found that green tea extract act as an adjuvant to dental hygiene process by preventing the damage effects to periodontal tissues and alveolar bone loss. Further, they observed that the green tea extract reduces the inflammatory mediators and osteoclasts (Ref. Reference de Almeida108).
Supportive clinical data for the beneficial effect of polyphenols against dental caries and periodontitis
Green tea extract polyphenols (catechin) possess different pharmacological activities including antimicrobial, anticariogenic and anti-collagenolytic properties. They also have significant anti-inflammatory and antioxidant properties. Randomised clinical study was performed to predict the effects of polyphenols on reduction of chronic periodontitis suffered patients. A comparative study was performed between standard fluoride/triclosan dentifrice and green tea dentifrice containing epigallocatechin. Result of the study indicated that green tea administration effectively reduced pathological conditions of periodontitis compared to standard treatment. And the study concluded that green tea dentifrice may be suitable for adjunct periodontal therapy (Ref. Reference Hrishi109). Catechin loaded thermo-reversible sustained-release gel was studied by 4-week randomised clinical trials in control and chronic periodontitis patients. The reports revealed an advancement in the site-specific release of catechin which significantly reduced pockets and inflammation in chronic periodontitis affected patients (Ref. Reference Chava and Vedula110). Single-blind randomised clinical trial was performed to predict the effect of cocoa administration in the inhibition of periodontitis. The role of cocoa compounds in dark and white chocolate on lipid peroxidation of saliva and total antioxidant capacity (TAC) were evaluated. The results concluded that consuming dark chocolate increase the TAC, decrease lipid peroxidation and moderate periodontal inflammation (Ref. Reference Roodgaryan111). Clinical study was performed in a group of 25 people diagnosed with chronic periodontitis to estimate the curcumin gel utilisation as additive to scaling and root planning during the treatment of periodontitis. The outcome of the study explained that curcumin gel effectively improved all the clinical parameters of periodontitits, significantly and reduced the growth of periodontal pathogens confirming its suitability for adjunct to periodontitis treatment procedure (Ref. Reference Bhatia112). A randomised clinical trial was carried out to compare turmeric and chlorhexidine gluconate mouthwash in inhibition of dental plaque formation and gingivitis. The observed results suggested that both turmeric and chlorhexidine gluconate were effective in reduction of plaque index, gingival index and total microbial count. This study concluded that turmeric may be used as low-cost adjunct therapy in the mechanical removal of dental plaque (Ref. Reference Waghmare113). A double-blind, clinical study was tested to evaluate the effect of hop bract polyphenols (HBP) mouth rinse in dental plaque expansion. The obtained results showed that plaque sample taken from HBP mouth rinse received people presented with a smaller number of streptococci mutants compared to placebo received. Further, the report concluded that HBP could be used to reduce the expansion of dental plaque in humans (Ref. Reference Shinada114). To investigate the effect of polyphenol rich drinks in control of dental plaque formation, clinical study was conducted in 75 adult subjects for two years. The microbial populations checked using advanced techniques indicated that the supra and subgingival dental plaque microbial growth in coffee and wine drinkers considerably reduced and this report suggested that polyphenol containing drinks can influence the oral microbial composition (Ref. Reference Signoretto115).
Progress on the applications of polyphenols against oral microbiota induced cancer
Polyphenol metabolism mainly occurs in the gut environment; however, oral cavity also involves polyphenol processing by mechanical and chemical methods. Salivary microbes possessing β-glucosidases metabolise the flavonoid glycoside to glycone free flavonoids and epithelial cells involve in hydrolytic activity of polyphenols (Ref. Reference Walle116). However, the oral metabolic activity of polyphenols depends on it is chemical structure and inter individual variations in microbial compositions. Details of function of oral microbiome in polyphenol metabolism are less studied and further studies are required to find polyphenol structure based higher metabolic compounds acquired in the oral cavity to investigate its health beneficial action in microbial-mediated diseases (Refs Reference Ding117, Reference Stevens and Maier118). Oral cancer is a deleterious and increasing health burden all over the world. The annual incidence is rising, importantly a greater number of cases occur in developing countries. The mortality rate is higher in men than women (Refs Reference Zinöcker and Lindseth22, Reference Olsen119). Long term experience of inflammatory reactions in the oral environments caused by dysbiotic microbes' capacity to produce carcinogenic reactions leads to development of lethal cancers. Periodontal pathogens alter the concentration of inflammatory mediators such as tumour necrosis factor-α (TNF-α), interleukins and matrix metalloproteinases (MMPs) that transforms the premalignant cells to malignant form (Refs Reference Cekici26, Reference Mombelli27, Reference Zhou120).The present-day medicine for the control of cancer follows chemotherapy, surgery and radiotherapy. The treatment approaches are suitable for short term administration and not for chronic administration since they produce severe side effects. Hence, it is important to design novel treatment approaches for the effective treatment of lethal oral cancer. Recent approaches of plant derived polyphenols as cancer preventives and treatment agents are getting more attention due to their anti-inflammatory, antioxidant and anti-carcinogenic activities. Polyphenols may have the property to inhibit carcinogenesis stages of initiation, promotion and progression. Very specifically, dietary polyphenols reduce cancer occurrence and produce defence against oral cancer by induction of apoptosis and inhibition of tumour growth, invasion and metastasis. Number of studies was conducted with the objective of treatment benefits of polyphenols in the oral microbes mediated cancer progression (Refs Reference Pandey and Rizvi121, Reference Anantharaju122). The following section describes the in vitro, in vivo and clinical studies of polyphenols in reduction of microbes associated cancer.
Polyphenols against oral microbiota induced cancer: supportive in vitro data
Preventive effect of the polyphenol curcumin on fibroblasts to tumour cell conversion was studied. In this study SCC-25 oral squamous carcinoma cells (OSCC) and periodontal ligament fibroblasts (PDLs) were co-cultured which transformed PDLs into carcinoma-related fibroblasts and provoked EMT of OSCC tumour cells. Curcumin treatment reduced the early response kinase (ERK) and nuclear factor kB (NFkB) in co-cultured and tumour cells and decreases the expression of integrin αv protein in fibroblasts. Also, curcumin suppressed the mediators of EMT and reduced the cancer invasion (Ref. Reference Dudás123). The proliferation and motility inhibitory activity of curcumin was studied in human YD-10B OSCC cells. The study found that curcumin reduced the expression of MMP and mRNA of urinary plasminogen activator (uPA). Further curcumin reduced the activation of MAP kinases (especially ERK) and altered the transcriptional pathways. The cumulative effect of curcumin in reducing the activities of MMP and mRNA of uPA and activation of MAP would serve as potent treatment option for oral cancer (Ref. Reference Shin124). The oral cancer prevention effect of EGCG was studied by Ho et al. in oral cancer cell line OC2. They reported that EGCG suppressed the invasion and migration of human oral cancer cells by decreasing the levels of urokinase plasminogen activator, MMP-2 and MMP-9 (Ref. Reference Ho125). In another study, the effect of quercetin on cell cycle regulation, cell growth and necrosis/apoptosis in human oral squamous carcinoma was observed. The results indicated that quercetin inhibits the thymidylate synthase and induces apoptosis (Ref. Reference Haghiac and Walle126). Black tea polyphenol extracts on suppression of proteases activity and cancer invasion were observed in human oral cancer cells. Tumour invasion was reduced by the black tea polyphenol extracts by reversing the EMT in human oral cancer cells (Ref. Reference Chang127). The plant polyphenol ethyl gallate produced dose dependent toxicity to oral squamous carcinoma cell line KB and reduced the cancer development by reduction of apoptosis (Ref. Reference Mohan, Thiagarajan and Chandrasekaran128). Inaba et al. studied about apple- and hop-polyphenols as reducing agents of cellular invasion induced by P. gingivalis gingipains in oral squamous cell carcinoma cell lines. They observed that selected polyphenols significantly reduced the expression of Proteinase activated receptor 2 (PAR2), precursor form of proMMP9, and induced the activation of nuclear translocation of NF-kB and HSP27. Finally, they concluded that plant polyphenols potently inhibit the P. gingivalis medicated OSCC progression by causing reduction of proMMP9 expression and cellular invasion (Ref. Reference Inaba129). Role of resveratrol in OSCC cell line in the prevention of adhesion, migration and invasion was studied by MTT and Transwell assay. Results of the study demonstrated that 100 μmol resveratrol decreased the adhesion, migration and invasion properties of OSCC, suggesting that resveratrol could be used as effective molecule in the prevention of cancer (Ref. Reference Shan130). Another research team used resveratrol obtained from peanuts, grapes and red wine to study cancer prevention on OSCC cell lines. The outcome of the study explained that resveratrol showed time and dose dependent reduction of proliferation of cells. Further, it enhanced the expression of cyclin A2, cyclin B1 and phospho-cdc2 (Tyr 15) and modulated the cell cycle phase in the OSCC cells. Hence, resveratrol demonstrates preventive effect on the progression of OSCC oral cancer cells (Ref. Reference Yu131). Flavonoids and phenolic acids rich propolis ethanolic extract was examined for it is anti-cancer properties in human tongue squamous cell carcinoma cell line. The polyphenolic extract produced dose-dependent toxicity to CAL-27 cells. Also, the extract enhanced the activity of caspases −3, −8, −9 and induced apoptosis by both intrinsic and extrinsic pathways. The results concluded that the extract which possesses multiple polyphenolic compounds could synergistically act to reduce the cancer progression (Ref. Reference Czyzewska132). Han et al. isolated the extractable polyphenols (EPs) and non-extractable polyphenols (NEPs) from Cranberries (Vaccinium macrocarpon), which contain anthocyanine and phenolic acids. Then they studied the effects of the extracts on colon cancer cell line HCT116. The study's results indicate NEPs showed better anti-cancer activity, reduction of cell viability, colony formation, cell cycle arrest and induction of apoptosis (Ref. Reference Han133). Blueberries' resveratrol derivative Pterostilbene (PTE) properties in colon cancer prevention were studied using HCT116 and HT29 cell lines. The study explains that PTE metabolite of Pinostilbene significantly reduced the growth of cancer cells, arrested the cell cycle at the S phase, and induced apoptosis (Ref. Reference Sun134).
Polyphenols against oral microbiota induced cancer: supportive in vivo data
Caceres et al. examined the phenolic compounds catechins, potassium apigenin, cocoa, rosmarinic acid and eriocitrin relation in cancer reduction in oral cancer stimulated hamster's animal model. They observed that in the potassium apigenin and rosmarinic acid treated animals, the tumour volume significantly reduced, and both the molecules effectively reduced the severity of the cancers (Ref. Reference Baldasquin-Caceres135). The effect of green tea EGCG activity against the oral tumour prognostic factors Hepatocyte growth factor (HGF) and c-Met were studied in the C3H/HeJ syngeneic mouse model. Overexpression of these two proteins activates the signalling mechanisms involved in the invasion and metastasis of cancer. When treated at a concentration of 25 mg/kg, EGCG was able to reduce the phosphorylation of the cMet, expression of HGF proteins and apoptosis (revealed through tissue caspase 3) in the animals. Interference with the HGF/c-Met signalling by EGCG reveals the inhibitory effect of the polyphenol against oral cancer growth and invasion (Ref. Reference Koh136). Yoshimura et al. evaluated the therapeutic potential of EGCG in controlling OSCC. The study conducted in in vivo xenograft mice reported that EGCG treatment significantly reduced the tumour size. Apoptosis profile of EGCG treated animals greatly modified compared to control. The study concluded that EGCG reduced cancer progression by modulating cell cycle and apoptosis processes (Ref. Reference Yoshimura137). EGCG effect on reduction of squamous cell carcinoma-9 (SSC-9) was studied in vivo in xenografted nude mice. EGCG showed maximum inhibition of MMP-2, uPA, cell migration, motility and adhesion in SSC-9. Further EGCG reduced the phorbol-12-myristate-13-acetate-induced MMP-9 expression and cell invasion in mice model. Overall results revealed EGCG significantly reduced cell growth, invasion and could be used as potent agent in treatment of cancer (Ref. Reference Chen138). Letchoumy et al. studied the chemopreventive action of black tea polyphenols BTF-35 and Polyphenon-B [P-B] on DMBA stimulated hamster buccal pouch carcinogenesis. Results of the study revealed that the polyphenol compounds modulate the several molecular pathways of cancer development and alter the cancer cell proliferation, survival, infiltration and angiogenesis (Ref. Reference Letchoumy139). Another research work reported about combination approach of polyphenol resveratrol and curcumin in suppression of head and neck squamous cell carcinomas (HNSCC). The combined administration increased the potency of treatment and induces the Bax/Bcl-2 ratio, poly (ADP-ribose) polymerase 1 cleavage and decreases the phosphorylation of ERK1 and ERK2 compared to single administration. Overall reports revealed enhanced oral cancer preventive activity of combined polyphenols (Ref. Reference Masuelli140). The preventive effects of the phenolic compounds carnosic acid and apigenin in opposition to 7,12-dimethyl benzanthracene (DMBA) induced cancer progression was studied in hamster animal model. The results of the study explained that both the bioactive molecules possess significant capacity for chemopreventive activities (Ref. Reference Gómez-García141). Role of theaflavin rich mangrove tea extracts on support of salivary bacterial flora and its effect on DMBA stimulated hamster buccal pouch carcinoma was studied. After induction of cancer by chemical method, reduction in beneficial bacteria and abundance in harmful bacteria were observed. Reports of the study suggested that treatment with mangrove tea extracts reversed the cancer progression and produced health benefits (Ref. Reference Sithranga Boopathy142). Methanolic extracts of Veronica urticifolia Jacq. was identified for the presence of flavones and hydroxycinnamic acid and investigated for anti-bacterial, antimutagenic and anti-tumour activity. The report of the study showed that the polyphenolic content produced better antibacterial activity and anti-mutagenic activity against nitroquinoline-N-oxide (4NQO) in Salmonella typhimurium. Further, the extract demonstrated reduction in tumour cell viability, tumour cell count and ascites volume in Ehrlich ascites carcinoma mice model (Ref. Reference Živković143). The function of the polyphenol derivative of flavonoid present in green propolis on reduction of oral dysplasia was investigated in rat tongues. The results of the study demonstrated that chemically induced dysplasia was significantly reduced in green propolis administered animals (Ref. Reference Cavalcante144). Polyphenol rich lyophilised strawberries were studied for their property in alteration of tumour specific genes and potent tumour prevention in the hamster cheek pouch model of oral cancer. Histopathological examination of the study revealed that treatment with lyophilised strawberries significantly reduced the dysplasia and molecular studies reported the modulation of tumour related genes after treatment (Ref. Reference Casto145). Coca polyphenols inflammation prevention activity was examined in the inflammation induced Caco-2 cells and rat model. The result is indicated that coca polyphenol reduced the NF-κB, pro-inflammatory enzymes and JNK phosphorylation. From the obtained results, they concluded that coca polyphenols decrease inflammation-mediated colon cancer progression (Ref. Reference Rodríguez-Ramiro146). Wu et al. studied whole cranberry powder (WCP) protective effects of colitis-associated mouse colon tumorigenesis. The result of the experiment displayed that WCP treated mice tumour incidence, burden and tumour size reduced compared to control mice. Gene and protein analysis revealed inflammatory cytokines TNF-α, IL-1β and IL-6, levels significantly reduced by WCP treatment. Moreover, WCP treatment control proliferation, apoptosis, angiogenesis and metastasis (Ref. Reference Wu147) Figure 5.

Fig. 5. Role of polyphenols in reduction of oral dysbiosis associated diseases.
Polyphenols against oral microbiota induced cancer: supportive clinical data
Zhou et al. conducted a case control study to confirm the association between consumption of green tea and decrease the exposure of HNSCC. The effect of green tea on oral cancer prevention was measured based on the quantity of consumption. Population with more consumption of green tea experienced less HNSCC compared to non-consumption population. The results also suggested performing cohort or clinical studies to get more information about cancer prevention effect of green tea (Ref. Reference Zhou148). To observe the link between green tea consumption and oral cancer development, a prospective study was conducted in Japan. Totally 20 550 men and 29 671 women subjects were involved in this study without previous experience of cancer. Results of the study suggested that more consumption of green tea tends to reduce the progression of oral cancer compared to less consumption population particularly in female (Ref. Reference Ide149). Phase II randomised clinical trial on Green tea extract was conducted to check the effect on oral premalignant lesions. The results of the study revealed that the extract decreases in the clinically responding patients, the expression of cyclin D1 and vascular endothelial growth factor than non-responsive people. The study concluded that a higher dose of green tea extract is suitable for short-term prevention of cancer and long-term administration is required to reduce oral cancer (Ref. Reference Tsao150). Black raspberries showed potent aerodigestive tract carcinogenesis reduction property in animal models. However clinical data lacks in to prove the cancer reduction functionality of Black raspberries. Hence phase 0 clinical study was performed to extent the usage of Black raspberries polyphenols in reduction of oral cancer. The study's results confirmed that administration of black raspberries reduces the oral carcinogenesis factors of antiapoptotic and proinflammatory molecular biomarkers (Ref. Reference Knobloch151). A prospective cohort study was conducted to investigate the efficiency of flavonoids in the risk reduction of head and neck cancer (HNC) in US men and women. Results of the study demonstrated that high intake of flavonoids comparatively has better reduction of HNC than low intake. The outcome the study that concluded flavonoids administration would be a better treatment approach in reduction of HNC (Ref. Reference Sun152). A clinical study was carried out in Uruguay to estimate the association between tomato polyphenol lycopene for reduction of upper aerodigestive tract cancer. Result of the study revealed that raw tomato and lycopene rich tomato intake reduces the risk of cancer. The conclusion of the study explained that combined consumption of lycopene and tomato decreases the upper aerodigestive tract cancer (Ref. Reference De Stefani153). Dietary flavonoid and lignan intake in cancer risk reduction was performed by case-control and prospective studies. Prospective study showed that isoflavones administration significantly reduces the risk of lung and stomach cancer (Ref. Reference Grosso154). Case-control study revealed that flavonoids consumption decreases the upper aerodigestive tract, colorectal, breast and lung cancers. Pooled analysis of case-control study was carried out to examine the connection between caffeinated coffee intake and HNC. Results of the study explained that caffeinated coffee consumption is inversely associated with oral cavity and pharynx cancer development (Ref. Reference Galeone155). Epidemiologic studies were performed with both case-control and prospective cohort studies to examine the link between consumption of flavan-3-ols intake and reduction of cancer progression. The study reports demonstrated that flavan-3-ols ingestion is inversely associated with threat of laryngeal and oropharyngeal cancer (Ref. Reference Lei156). Curcumin inhibitory effects on cancer in patients with colorectal cancer was examined. After treatment with curcumin, the samples from cancer patients depict increased body weight, lower TNF-alpha, enhanced apoptotic tumour cells and increased levels of p53 in tumour tissue (Ref. Reference He157). The study report concluded that curcumin administration improves the cancer patient's health profile by altering the expression of p53 molecule. Lazarevic et al. (Ref. Reference Lazarevic158) conducted block randomised double-blinded, placebo-controlled phase 2 study to examine the role of genistein in prostate cancer prevention. The clinical report mentioned that genistein treatment reduced the prostate-specific antigen (PSA) levels compared to the control group. The study mentioned that a soy diet contains genistein able to control the PSA levels and blood cholesterol without altering the hormones.
Conclusions and future prospects
The present review outlines the knowledge regarding oral microbes and their role in both health and diseases. Along with that, the role of polyphenols in controlling oral microbes mediated oral diseases and systemic diseases including IBD, diabetes, neurological, cardiac has been explained with high emphasis being given to cancer. The diverse microbial population present in the oral cavity maintains balanced interaction between microbes and host that render them symbiotic to host and it is very essential to maintain its natural diversity to prevent the development of diseases. Lack of healthy practices and consumption of modernised food can perturb and trouble the natural diversity of human oral microbiome responsible for the development of oral and systemic diseases. Treatment of microbial associated diseases focuses to restore the symbiotic equilibrium by application of synthetic drugs and natural plant medicines. Among them, natural derived polyphenols get more attention in the treatment of diseases due to their significant therapeutic activity and fewer side effects compared to synthetic medicaments. Particularly oral inflammatory diseases like caries, periodontitis and others are very effectively cured by diverse class of polyphenol compounds as proven by in vitro, in vivo and clinical studies. In a similar way treatment of microbes associated cancer with polyphenol is studied widely. Similarly, polyphenols are being widely studied to examine their function in reduction of oral microbes associated cancer progression. Polyphenol derivatives interfere with the three stages of cancer development including initiation, propagation and progression and all the causative factors of cancer development which include inflammatory reactions, production of carcinogenic materials and disturbance of signalling pathways. Several in vitro studies in oral cancer cell lines demonstrated the significant oral cancer reduction capacity of polyphenols and in vivo models confirm their efficacy. However, the results of the in vivo data need to be forwarded to perform clinical studies, so that the positive outcomes obtained will support the role of polyphenols in controlling the microbes associated cancer. Overall, this extensive review article suggests that symbiotic residence of oral microbes is important to health and dysbiotic environment may act as an indicator of disease development. This can act as an early diagnostic factor for microbes' specific diseases and aid for rushing up to avail suitable medicaments to be free from diseases and make life comfortable.
Data
Not applicable.
Acknowledgement
DST-SERB-NPDF scheme (PDF/2018/002779) is acknowledged by the author MGA for the post-doctoral fellowship. The authors wish to acknowledge the (i) Alagappa University Bioinformatics Infrastructure Facility (DBT, BT/BI/25/012/2012, BIF) (ii) DST-FIST (SR/FST/LSI-639/2015(C)) (iii) UGC-SAP (F.5-1/2018/DRS-II(SAP-II)), (iv) DST-PURSE (SR/PURSE Phase 2/38 (G)), (v) RUSA 2.0 [F. 24-51/2014-U, Policy (TN Multi-Gen), Dept of Edn, GoI] and (vi) the University Science Instrumentation Centre (USIC), Alagappa University.
Authors' contributions
All the contributing authors have participated in the manuscript. All authors contributed to the interpretation of the data and writing of the manuscript.
Financial support
This research received no external funding.
Conflict of interest
The authors declare that they have no competing interest.
Ethical standards
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.