Long-term sequelae of the metabolic syndrome (MetS) include the clinical presentation of both macro- and microvascular disease(Reference Russell, Graham and Richardson1). The obese, insulin-resistant JCR:LA-corpulent (cp) rat, homozygous for the corpulent trait (cp/cp), is a unique model of the MetS(Reference Russell, Graham and Richardson1–Reference Mangat, Su and Scott4). This model displays characteristic macro- and microvascular complications of the MetS, including effects on the kidney such as glomerulosclerosis, proteinuria, decline in renal function and ultimately end-stage renal disease, consistent with human pathological complications(Reference Proctor, Kelly and Stanhope5). Indeed, all of the diagnostic criteria of the MetS are associated with reduced renal function and elevated proteinuria and albuminuria(Reference Thomas, Sehgal and Kashyap6). Further, circulating insulin and lipids are contributing factors to the detrimental effects of the MetS on renal function, affecting the glomerulus, causing damage during obesity and diabetes, resulting in glomerulosclerosis and proteinuria even in the early stages of these disorders(Reference Abrass7–Reference Schaffer11).
We recently reported that MetS JCR:LA-cp rats treated with dietary fish oil (rich in n-3 PUFA) showed improvements in fasting and postprandial dyslipidaemia, hyperinsulinaemia and the frequency of late-stage myocardial lesions(Reference Lu, Borthwick and Hassanali12). Consequently, we hypothesised that improvements to hyperlipidaemia and hyperinsulinaemia by dietary fish oil may also have beneficial renal effects in the JCR:LA-cp rat, potentially via alterations to eicosanoid metabolism.
Eicosanoids are primarily produced from arachidonic acid (ARA) by cyclo-oxygenase (COX) enzymes, yielding prostanoids, and by lipoxygenase (LOX) enzymes to produce leukotrienes and hydroxy fatty acids (hydroxyeicosatetraenoic acid, HETE). Prostanoids, such as PGE2 and thromboxane A2, derived via the COX pathway, regulate glomerular filtration rate, water and salt homeostasis, as well as inflammatory and fibrotic processes in response to kidney damage(Reference Klahr13–Reference Hao and Breyer15). In diabetic kidneys, increased COX or prostanoid levels are associated with development of the disease, and inhibition of this enzyme results in nephroprotection(Reference Schambelan, Blake and Sraer16–Reference Hao and Breyer20).
Renal 12-HETE is a product of the 12/15 LOX enzyme and is a vasoconstrictor in the kidney(Reference Hao and Breyer20), which also has been shown to be associated with experimental diabetic nephropathy(Reference Antonipillai, Nadler and Vu21–Reference Xu, Miao and Cui23). Pharmacological inhibition or gene knockout of 12/15 LOX reduces glomerular volume, mesangial cell hypertrophy, extracellular matrix formation and proteinuria(Reference Kim, Reddy and Lanting24–Reference Guo, Mia and Li26). Such effects of select eicosanoids in diabetic nephropathy suggest a role in renal injury during the MetS. Due to the multiple interactions and roles of select eicosanoids in the kidney(Reference Hao and Breyer20, Reference Câmara, Martins and Landgraf27), understanding changes in both prostanoids and HETE may reveal mechanisms underlying renal injury during the MetS.
Fish oil (n-3 PUFA) can both compete with ARA for eicosanoid synthetic enzymes as well as inhibit these enzymes. Furthermore, eicosanoids derived from n-3 PUFA are generally less potent than those derived from ARA(Reference Grimminger, Mayer and Kramer28–Reference Darlametsos and Varnos30). Supplementation with n-3 PUFA provides nephroprotection in a variety of models of renal disease(Reference Donadio and Grande31–Reference Shapiro, Theilla and Attal-Singer37). Hence, the objectives of the present study were to determine the long-term renal effects of fish oil feeding in a model of the MetS, with a particular focus on glomerular injury and renal eicosanoids.
Materials and methods
Animal model and experimental procedures
Male JCR:LA-cp rats, both with the MetS (cp/cp) and lean (+/?), were raised in an established breeding colony at the University of Alberta, as previously described(Reference Russell, Amy and Graham3). Rats were weaned at 6 weeks, and allowed to age until week 8. MetS rats were randomly allocated to one of three diets (Table 1); a control hypercholesterolaemic isoenergetic lipid-balanced diet (LBD; n 8); a LBD supplemented with 5% (n 8) or 10 % (n 8) fish oil (Table 1) for 16 weeks. Lean rats (n 8), of the same age, were fed the control LBD diet (Table 1) for 16 weeks. Food consumption and body weight were recorded throughout the study. At 24 weeks of age, rats were fasted overnight and killed under isoflurane anaesthesia. Liver, kidney and peri-renal fat pads were weighed and snap-frozen in liquid N2 at − 80°C, for subsequent analysis where appropriate. Animal care and experimental procedures were conducted in accordance with the Canadian Council on Animal Care and approved by the University of Alberta Animal Care and Use Committee (ACUC-Livestock).
Table 1 Nutrient and lipid summaries for each dietary group*

LBD, lipid-balanced diet; FO, fish oil.
* The composition of the LBD and diets supplemented with FO containing EPA and DHA; 5 and 10 % FO diet, as determined by GC.
† 5 % FO diet contained approximately 5·1 g EPA/kg diet and 2·6 g DHA/kg diet.
‡ 10 % FO diet contained approximately 9·5 g EPA/kg diet and 4·8 g DHA/kg diet.
§ Product code no. XO4825TG (Ocean Nutrition Canada Limited).
Urine samples
Following 16 weeks of feeding, rats were euthanised in the fasted state and urine samples were collected. Urine albumin and creatinine concentrations were measured using immunoturbidimetric and Jaffé methods, respectively(Reference Proctor, Kelly and Stanhope5, Reference Carfray, Patel and Whitaker38). The laboratory CV for albuminuria test was 8·0 (level 1) and 3·2 % (level 2).
Renal histology
After killing, the left kidney from all animals was excised and fixed in formalin. After conventional processing, kidney sections were examined to quantify glomerulosclerosis, as per the method of Schäfer(Reference Heidbreder, Schafferhans and Schramm39). In brief, each kidney was divided along the long axis, fixed, sectioned and stained with haematoxylin and eosin(Reference Proctor, Kelly and Stanhope5). For each kidney, ten random fields were viewed ( × 4 objective) and recorded digitally. A total of eight complete glomeruli in each field of view were blindly scored as sclerotic (mild-to-severe glomerular sclerosis) or normal (minimal sclerosis or normal). The fraction of sclerotic glomeruli was calculated for each kidney and data represented as the average incidence of sclerotic glomeruli in each kidney.
Eicosanoid analysis
Lyophilised tissue (45 mg) from the right kidney was homogenised on ice in 1·25 ml of freshly prepared Tyrodes buffer(Reference Warford-Woolgar, Peng and Shuhyta40). After homogenisation, Triton X-100 (Fisher Scientific) was added and mixed to achieve a final concentration of 0·01 %. Endogenous eicosanoid levels and in vitro production were determined in aliquots (200 μl) under the following conditions: (1) 0 min for determination of endogenous levels and (2) 10 min at 37°C for determination of in vitro production, as described(Reference Warford-Woolgar, Peng and Shuhyta40). Reactions were stopped with 1 % formic acid in methanol and eicosanoids extracted in acidified methanol–water–ethanol (5:10:1, by vol.) containing 10 μl of antioxidant solution (0·2 mg/ml butylated hydroxytoluene, 0·2 mg/ml EDTA, 2 mg/ml triphenylphosphine, 2 mg/ml indomethacin in a solution of 2:1:1 methanol–ethanol-water). An internal standard mix consisting of 7·5–25 ng of 2H-labelled standards (mentioned later) was added. After centrifugation, supernatants (pH < 3·0) were applied to Strata-X SPE columns (Phenomenex) pre-conditioned with methanol and water (pH 3). After loading, columns were washed in 10 % methanol in water (pH 3) and samples eluted with 100 % methanol(Reference Deems, Buczynski and Bowers-Gentry41). Liquid chromatography tandem MS (liquid chromatography/MS/MS) was performed based on the method described by Deems et al. (Reference Deems, Buczynski and Bowers-Gentry41). Dried down samples were re-suspended in water–acetonitrile–formic acid (70:30:0·02, by vol., solvent A) and eicosanoids separated by reverse-phase HPLC using a C18 column (Luna, 250 × 2·0 mm, Phenomenex) at a flow rate of 300 μl/min. The column was equilibrated in solvent A and samples were eluted with a linear gradient from 0 to 20 % solvent B (acetonitrile–isopropyl alcohol, 50:50; v/v) for 11 min, then increased to 100 % by 13 min and held until 16 min, then dropped to 0 % by 16 min and held until 19 min. The HPLC was coupled to a triple quadrupole tandem mass spectrometer (API 2000) with electrospray ionisation source (Applied Biosystems). Eicosanoids were analysed via multiple-reaction monitoring in negative ionisation mode. Mass transitions of 2H-labelled standards and eicosanoids were as follows: 5-HETE-d8 (m/z 327 → 116) for 5-HETE (m/z 319 → 115), 8-HETE (m/z 319 → 155), 9-HETE (m/z 319 → 151) and 11-HETE (m/z 319 → 167); 15-HETE-d8 (m/z 327 → 226) for 12-HETE (m/z 319 → 179) and 15-HETE (m/z 319 → 219); 6-keto PGF1α-d4 (m/z 373 → 211) for 6-keto PGF1α (m/z 369 → 163); thromboxane B2 (TxB2)-d4 (m/z 373 → 173) for TxB2 (m/z 369 → 169); PGF2α-d4 (m/z 357 → 197) for PGF2α (m/z 353 → 193); PGE2-d4 (m/z 355 → 275) for PGE2 (m/z 351 → 271) and PGE3 (m/z 349 → 269); PGD2-d4 (m/z 355 → 193) for PGD2 (m/z 351 → 189) and PGD3 (m/z 349 → 269). Quantification of eicosanoids was determined using the stable isotope dilution method(Reference Hall and Murphy42).
Statistical analysis
Data were tested for normal distribution and differences between the MetS control (cp/cp LBD) compared with the lean control (+/? LBD) group were analysed using the unpaired t test (P< 0·05). Statistical differences between the MetS fish oil treatment (5 and 10 %) groups compared with the MetS control (cp/cp LBD) group were analysed by one-way ANOVA followed by Tukey's post hoc tests, with significance set at P <0·05 (Sigma Stat, Jandel Scientific and GraphPad Prism, GraphPad Software Inc.). For data that could not be normalised by logarithmic transformation, the Kruskal–Wallis test was used. All results are shown as means with their standard errors.
Results
Liver, kidney and peri-renal fat pad weight
Untreated MetS rats had significantly greater liver weight (2-fold; P <0·05), compared with the lean control rats (Table 2). MetS rats fed a 5 % fish oil diet for 16 weeks had significantly lower liver weight ( − 29 %; P <0·05), compared with the untreated MetS rats (Table 2). Relative to lean control rats, untreated MetS rats had significantly increased kidney weight (27 %; P <0·05) (Table 2). There was no significant difference in kidney weight of either 5 or 10 % fish oil groups (Table 2), when compared with the untreated MetS rats. Compared with the lean group, all MetS groups had a higher peri-renal fat pad weight and ratio of peri-renal fat pad weight to body weight (5- to 11-fold increase) (Table 2). However, relative to untreated MetS rats, both 5 and 10 % fish oil-treated rats had significantly lower peri-renal fat pad weight ( − 34 to 57 %; P <0·001) (Table 2) and ratio of peri-renal fat pad weight to body weight ( − 27 to 47 %; P <0·01) (Table 2).
Table 2 Body and organ weights of lean and metabolic syndrome (MetS) male JCR:LA-cp rats fed a lipid-balanced control diet (LBD), or a 5 or 10 % fish oil (FO) diet (Mean values with their standard errors, n 8 per group)

Mean values were significantly different from that of the lean control: * P <0·05, ** P <0·01.
Mean values were significantly different from that of the MetS control: †P <0·05, ††P <0·01, †††P <0·001.
Urinary biochemical profile
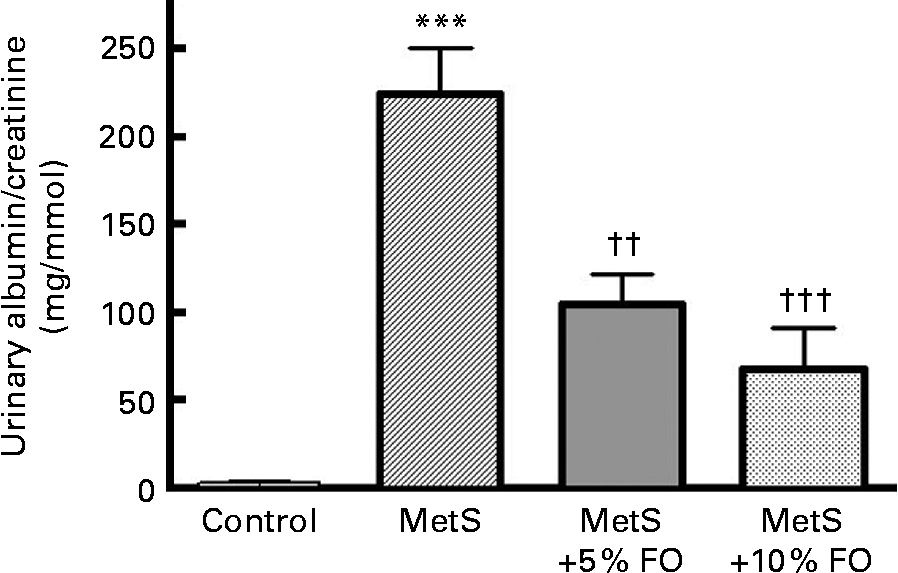
Urinary albumin/creatinine concentrations were markedly higher (78·5-fold; P <0·001) in untreated MetS rats compared with the lean control rats (Fig. 1), consistent with previously published work by our group(Reference Proctor, Kelly and Stanhope5). After 16 weeks of feeding, rats fed the fish oil diet (5 or 10 %) had lower urine albumin excretion compared with the untreated MetS rats ( − 53 and − 70 %, respectively; P <0·01 and P <0·001) (Fig. 1).

Fig. 1 The ratio of urinary albumin to creatinine levels of JCR:LA-cp rats in each of the treatment groups. Values are means, with standard errors represented by vertical bars (n 8). Mean value was significantly different from that of the lean control: *** P <0·001. Mean value was significantly different from that of the metabolic syndrome (MetS) control: †† P <0·01, ††† P <0·001. FO, fish oil.
Glomerulosclerosis
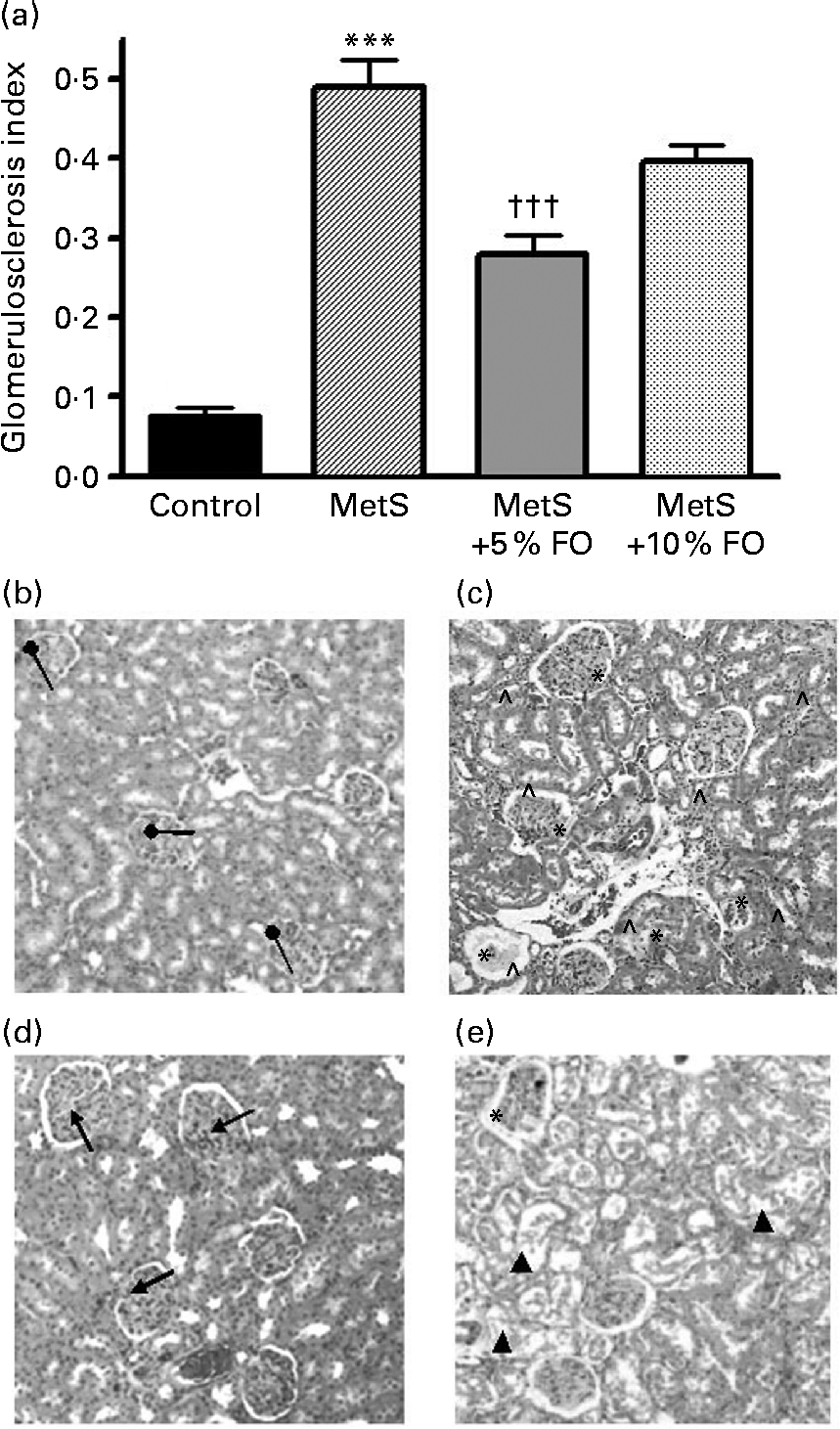
Untreated MetS rats exhibited a substantial increase in the frequency of glomerulosclerosis compared with the lean control rats (6·4-fold; P <0·001) (Fig. 2) and is consistent with previous studies using this rat model(Reference Proctor, Kelly and Stanhope5). Further, untreated MetS rats exhibited a greater severity of glomerulosclerosis and interstitial inflammation compared with their lean counterparts (Fig. 2). MetS rats treated with 5 % fish oil had a significant reduction in the fraction of sclerotic glomeruli ( − 43 %; P <0·001), relative to the untreated MetS group (Fig. 2). A similar significance was not achieved in the 10 % fish oil group. Intriguingly, sporadic tubular damage was observed in kidneys of MetS rats fed the 10 % fish oil diet (Fig. 2(b–e)).

Fig. 2 (a) Proportion of sclerotic glomeruli in JCR:LA-cp rats in each of the treatment groups. Values are means, with standard errors represented by vertical bars (n 8). Mean value was significantly different from that of the lean control: *** P <0·001. Mean value was significantly different from that of the metabolic syndrome (MetS) control: ††† P <0·001. (b–e) Kidney sections stained by haematoxylin and eosin. Representative micrographs of kidneys from (b) normal glomeruli (●) of lean control rats. (c) Kidneys of MetS control rats illustrating some sclerotic glomeruli including interstitial inflammation and malformed glomeruli ( ∧ ). (d) Glomeruli from MetS rats treated with 5 % fish oil (FO) showing relatively normal glomeruli ( ↑ ). (e) Glomeruli from MetS rats treated with 10 % FO showing malformed tubular structure (▲) and glomerulosclerosis. The tubule structures were malformed by fat vacuoles.
Renal eicosanoid levels and in vitro production
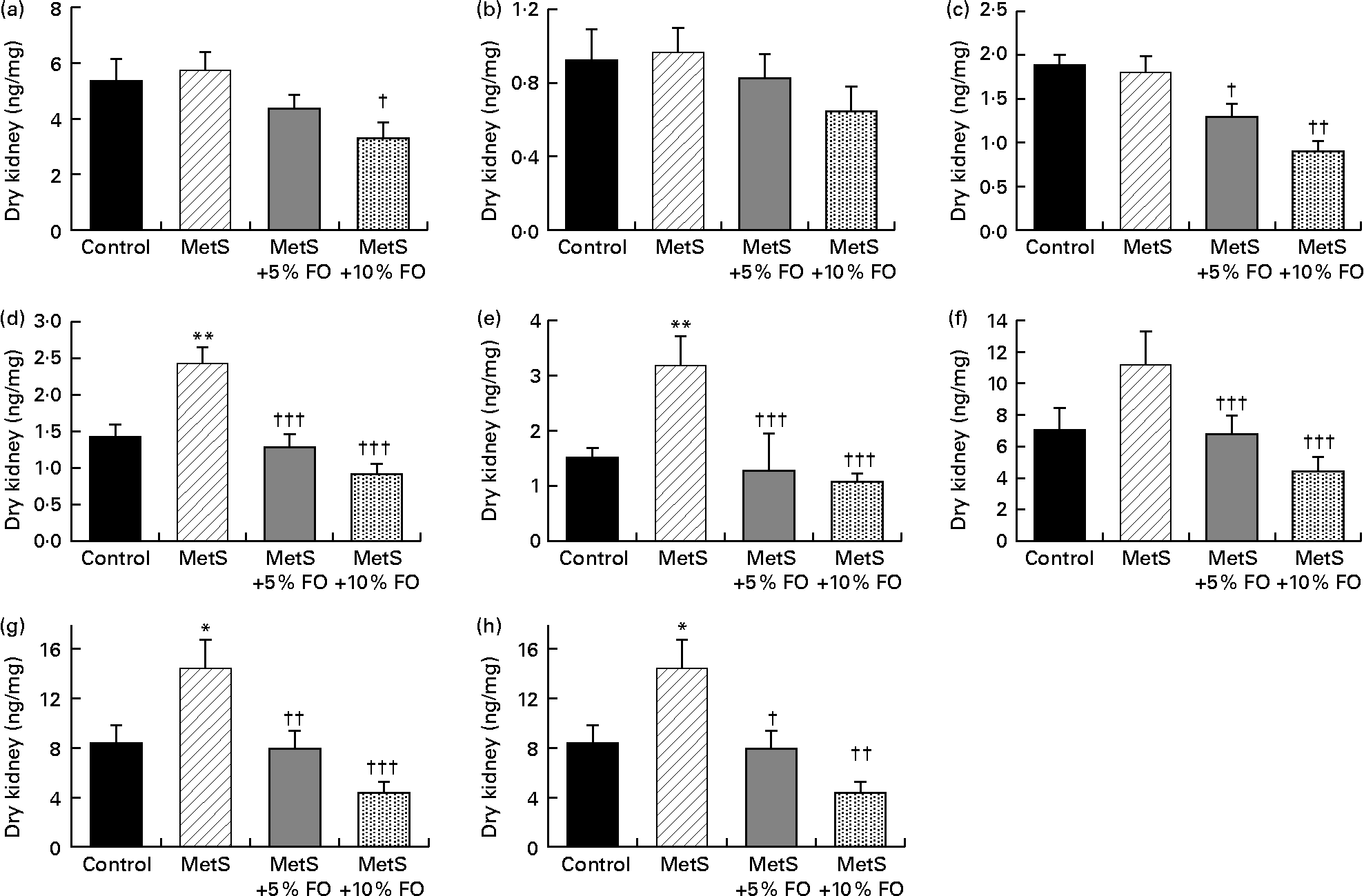
The levels of 11-HETE and 12-HETE were 71 and 109 % higher, respectively, in untreated MetS compared with the lean rat kidneys (Fig. 3(d) and (e)). These levels were reduced by fish oil feeding to similar values observed in lean rats fed the control diet. Renal 15-HETE levels followed the same pattern (Fig. 3(f)), with the exception that the difference between MetS and lean rats was not significant (P= 0·0885). As 15-HETE is produced by both COX activity (along with 11-HETE) and by 12/15 LOX activity (along with 12-HETE)(Reference Dobrian, Lieb and Cole43–Reference Calviello, Di Nicuolo and Gragnoli46), the levels of these combined HETE were also analysed. Both 11- and 15-HETE, as well as 12- and 15-HETE, were higher by 73 and 99 %, respectively, in MetS compared with lean rat kidneys (Fig. 3(g) and (h)). These combined levels were also reduced by the fish oil diet in MetS rats to levels similar to those in lean control rats. While the levels of other detectable HETE (5-, 8- and 9-HETE, Fig. 3(a)–(c)) in the kidney were not altered by genotype, fish oil treatment did reduce 5- and 9-HETE levels in MetS rats. Corresponding HEPE derived from EPA were not detected, nor was there significant production of HETE detected in vitro.

Fig. 3 Endogenous hydroxyeicosatetraenoic acid (HETE; (a) 5-HETE; (b) 8-HETE; (c) 9-HETE; (d) 11-HETE; (e) 12-HETE; (f) 15-HETE; (g) 11-HETE+15-HETE; (h) 12-HETE+15-HETE) levels in the kidneys of JCR:LA-cp rats in each of the treatment groups. Values are means, with standard errors represented by vertical bars (n 8). Mean values were significantly different from that of the lean control: * P <0·05, ** P <0·01. Mean values were significantly different from that of the metabolic syndrome (MetS) control: † P <0·05, †† P< 0·01, ††† P <0·001. FO, fish oil.
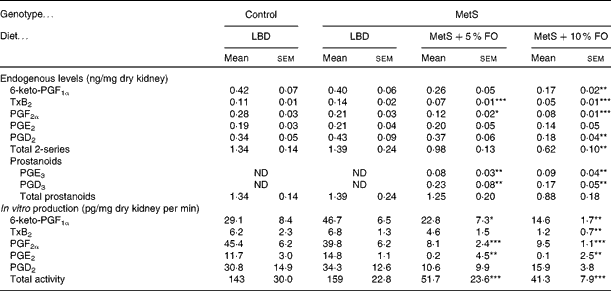
Endogenous prostanoids in kidneys were not different between untreated MetS and lean control rats (Table 3). However, fish oil feeding resulted in marked reductions of the 2-series prostanoids. Of these prostanoids, 6-keto PGF1a, TxB2, PGF2α and PGD2 were reduced by approximately 60 % in the MetS rats fed the 10 % fish oil diet compared with the untreated MetS rats. Kidneys from rats fed the 5 % fish oil had 40–50 % lower levels of TxB2 and PGF2α (Table 3) compared with the untreated MetS group. Interestingly, both fish oil diets resulted in the appearance of the 3-series prostanoids PGE3 and PGD3, which were not detectable in kidneys from untreated MetS rats.
Table 3 Endogenous prostanoid levels in the kidneys of lean and metabolic syndrome (MetS) JCR:LA-cp rats fed a lipid-balanced control diet (LBD), or a 5 or 10 % fish oil (FO) diet (Mean values with their standard errors, n 8 per group)

TxB2, thromboxane B2; ND, not determined.
Mean values were significantly different from that of the MetS control: * P <0·05, ** P< 0·01, *** P <0·001.
When in vitro production of eicosanoids was examined, only prostanoids were produced and subsequently used to assess COX activity. Consistent with endogenous levels, in vitro production of renal prostanoids was not affected by genotype, but dietary fish oil markedly reduced COX activity in MetS rats in both the 5 and 10 % fish oil groups, compared with the LBD group (Table 3). COX activity, as measured by four out of five prostanoids, was reduced by 63–99 % in the 10 % fish oil group, with lesser effects in the 5 % fish oil group. Overall, COX activity was reduced in kidneys by 72 % in the 5 % fish oil group and by 78 % in the 10 % fish oil group, compared with the kidneys from untreated MetS rats (Table 3).
Discussion
The purpose of the present study was to examine whether a long-term fish oil-supplemented diet would improve renal function and glomerulosclerosis in the MetS JCR:LA-cp rat model. A secondary objective was to investigate the corresponding renal eicosanoid profile and the effects of dietary fish oil on eicosanoid levels. The present findings show that chronic fish oil feeding can improve renal pathology and normalise the elevated HETE levels associated with the MetS in the JCR:LA-cp rat.
Urinary albumin is an indicator of renal microvascular damage, and often reflects elevated glomerular permeability and inability to retain albumin(Reference Hamano, Nitta and Ohtake47). Glomerulosclerosis is a major cause of end-stage renal failure in diabetic and obese individuals, impairing the filtering process and allowing protein to leak into the urine(Reference Eknoyan48). In the present study, JCR:LA-cp rats fed fish oil had significantly less albuminuria, glomerulosclerosis and obesity. These results are consistent with previous studies of dietary fish oil in other models of renal disease. Diabetic KKAy/Ta mice injected intraperitoneally with EPA ethyl ester (1 g/kg per d) had attenuated glomerulosclerosis, mesangial matrix accumulation, tubulointerstitial inflammation and albuminuria(Reference Hagiwara, Makita and Gu49). In a model of focal glomerulosclerosis, a fish oil-enriched diet substantially lowered urine albumin excretion, probably reflecting a lower severity of kidney disease(Reference Goldstein, Wheeler and Sandstorm50). In human studies of diabetic nephropathy, reduced albuminuria is the most common, although not consistent, finding with fish oil supplementation(Reference Shapiro, Theilla and Attal-Singer37). The beneficial effects of fish oil on renal injury in the JCR:LA-cp model may be due to the improvement in hyperlipidaemia and insulinaemia previously reported from this study(Reference Lu, Borthwick and Hassanali12), as high circulating levels of lipids and insulin cause renal damage(Reference Abrass7, Reference Chen, Munter and Hamm8, Reference Wang, Jiang and Li10, Reference Schaffer11).
Dietary fish oil also could be nephroprotective via alterations in eicosanoid metabolism. Eicosanoids are biologically active molecules that play important and sometimes opposing roles in kidney function(Reference Hao and Breyer15, Reference Câmara, Martins and Landgraf27). PGD2, PGE2 and HETE are generally associated with pro-inflammatory effects, but PGE2 as well as PGI2 also are vasodilators and are important in maintaining normal renal plasma flow(Reference Kappor, Kojima and Yang51, Reference Mori, Saito and Sakamoto52). PGE2 is also involved in the regulation of Na re-absorption and renal cell over-proliferation and formation of fibrous tissue(Reference Remuzzi, Ruggeneti and Benigni14, Reference Hodeify and Kreydiyyeh53, Reference Kitahara, Eitner and Ostendorf54). Thromboxane A2 and 12-HETE are known to be vasoconstrictors that are involved in the pathogenesis of hypertension associated with long-term renal failure(Reference Darlametsos and Varnos30, Reference Larivière, Moreau and Rodrigue55), as well as being elevated in models of diabetic nephropathy(Reference Antonipillai, Nadler and Vu21–Reference Xu, Miao and Cui23).
The present findings in the JCR:LA-cp rat demonstrate that elevated renal HETE levels are associated with renal pathology in the MetS, supporting previous studies that indicated the importance of elevated HETE in other forms of renal injury(Reference Antonipillai, Nadler and Vu21–Reference Xu, Miao and Cui23). Several studies have shown that by blocking the levels of the 12/15 LOX enzyme using inhibitors or gene knockout strategies, glomerular volume, mesangial cell hypertrophy, extracellular matrix formation and proteinuria can be reduced in models of diabetic nephropathy(Reference Kim, Reddy and Lanting24–Reference Guo, Mia and Li26, Reference Dobrian, Lieb and Cole43). The similar effect of fish oil on HETE levels in the JCR:LA-cp rat indicates that these eicosanoids may also be key mediators of renal pathology associated with the MetS.
In contrast to the elevated HETE levels in MetS rat kidneys, renal prostanoid levels were not altered. This may have been due to the relatively early stage of renal injury present in this model, which would indicate that HETE alterations occur earlier in disease progression than perturbations in prostanoid levels. Nevertheless, dietary fish oil also reduced the endogenous levels and in vitro production of most prostanoids. The reduced levels of the 2-series prostanoids and HETE are consistent with previous reports that demonstrated that dietary n-3 PUFA can inhibit the production of eicosanoids derived from ARA. In these kidneys, the only eicosanoid products derived from n-3 PUFA detected were PGD3 and PGE3, demonstrating selectivity for the eicosanoids produced from EPA. Previous studies have shown that n-3 PUFA reduce the expression of COX messenger RNA and protein both in vivo and in vitro (Reference Mund, Pizato and Bonatto44–Reference Calviello, Di Nicuolo and Gragnoli46). n-3 PUFA accumulate in the plasma membrane and partially replace ARA as substrates for COX and LOX, resulting in reduced products from ARA and enhanced production from EPA(Reference Smith56, Reference Needleman, Wyche and Bronson57). Both protective and detrimental 2-series prostanoids were reduced in rats treated with fish oil, so their overall effect on the kidney is difficult to assess. However, the 3-series prostanoids tend to be much less bioactive(Reference Grimminger, Mayer and Kramer28–Reference Darlametsos and Varnos30), so the reduction of ARA-derived products in combination with the enhanced EPA-derived eicosanoids would probably have decreased the overall bioactivity of the eicosanoids present in rats treated with fish oil.
Caution regarding the use of high levels of fish oil for the treatment of kidney injury in the MetS is warranted, as the 10 % fish oil diet resulted in higher levels of tubular damage. Few studies have reported adverse effects of high-dose fish oil on the kidney, but the BHE/Cdb rat model of diabetic nephropathy also had worsened glomerular histopathology and shortened lifespan when given 9 % fish oil(Reference Berdanier, Johnson and Hartle58). The pcy mouse model of adolescent nephronophthisis also exhibits greater fibrosis and cyst growth when given high levels of dietary long-chain n-3 PUFA(Reference Sankaran, Lu and Bankovich-Calic59, Reference Aukema, Yamaguchi and Takahashi60). It is possible that whilst attenuation of HETE, which were elevated in the kidneys of MetS rats, may reduce renal damage, the reduction of protective 2-series prostanoids in the rats provided 10 % fish oil could have had detrimental effects on the development of renal pathology. It is not clear why the detrimental effect of 10 % fish oil is observed specifically with glomerulosclerosis and not with albuminuria, but may point to a greater sensitivity to lower prostanoid levels. Whilst treatment with drugs that inhibit prostanoid formation ameliorate kidney dysfunction in some models(Reference Norby, Wweidig and Ramwell61–Reference Arisz, Donker and Brentjens63), nephrotoxicity from prostanoid inhibitors has also been documented(Reference Hao and Breyer15, Reference Sankaran, Bankovich-Calic and Crow64). Hence, simultaneous analysis of both prostanoids and HETE is important in order to obtain a more comprehensive understanding of potential eicosanoid roles in renal injury.
Concluding remarks
The findings of the present study offer evidence of the importance of elevated HETE levels in renal injury in the MetS. Furthermore, supplementation of moderate levels of n-3 PUFA derived from fish oil in the JCR:LA-cp MetS rat reduces disease progression. We propose that long-term dietary intake of fish oil may improve glomerulosclerosis and kidney dysfunction associated with elevated renal HETE levels in the MetS.
Acknowledgements
The research was supported, in part, by a pilot grant from the Alberta Diabetes Institute (S. D. P.) and NSERC Discovery grants (S. D. P. and H. M. A.). S. D. P. is a HSFC New Investigator. The authors wish to declare no conflict of interest. We wish to thank Kristina MacNaughton and Sharon Sokolik for their excellent technical assistance throughout the present study. Thank you to Jennifer Wizbicki, Joy Gauthier and Tanja Winter for technical assistance with the eicosanoid analysis. S. D. P., J. L. and H. M. A. conceived and designed the experiments. J. L. performed the experiments. The data were analysed by J. L., S. D. P., H. M. A. and F. B. The manuscript was prepared by H. M. A., S. D. P. and F. B.