Sri Lanka has high endemic rates of leptospirosis infection, with the annual number of reported cases exceeding 4000 during 2007–2011 [Reference Agampodi1]. Leptospirosis cases in Sri Lanka are primarily due to occupational exposure affecting individuals involved in paddy farming activities [Reference Agampodi, Peacock and Thevanesam2]. Cases of urban leptospirosis are rare in Sri Lanka, with almost all leptospirosis contracted through agricultural activities in rural communities. This paper reports the first recorded recreational activity-related outbreak of leptospirosis in Sri Lanka.
On 7 September 2012, 20 office staff from a factory in Mihinthale, Sri Lanka participated in a 2-day recreational outing that involved white-water rafting and camping along the Kelani River in Kithulgala, located in Central Sri Lanka. This region of Sri Lanka is known to be a region with high endemic incidence of leptospirosis. Several of the office staff experienced fever about 2 weeks after the rafting trip and received treatment in hospital outpatient departments. One developed high fever (self-reported) 10 days after the event, lasting 7 days, with severe headache and myalgia lasting more than 10 days. On his third visit to the doctor, his blood was tested and found positive for Leptospira ELISA IgM. One month after the incident, on 6 October 2012, the Department of Community Medicine, Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka was invited to conduct an outbreak investigation.
We invited all the workers who participated in the white-water rafting trip to report on their experience. We used an interviewer-administered questionnaire for the initial interview with participants. Participants were also examined using a clinical data checklist. All the reported signs and symptoms in this paper are self-reported. All consenting participants provided 5 ml of venous blood for disease confirmation. The serum samples obtained were processed for transportation on the same day as the interview. Samples were stored at −4°C (24 h) until transportation to the Medical Research Institute, Colombo for confirmation. We performed the microscopic agglutination test (MAT) for leptospirosis using patoc-1 live antigen 1 week after obtaining the sample, the samples were stored at −20°C until required. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and serum creatinine tests were performed on patients who reported a history of fever from the fresh serum samples collected on the same day. The index case and a second patient who experienced clinical features suggestive of leptospirosis were not available for investigation at the time of the factory visit. These two individuals provided data through a telephone interview, but we were unable to obtain serum samples. However, both these patients had tested positive for Leptospira ELISA IgM in the private medical sector. A laboratory-confirmed case of leptospirosis in this study was defined as ‘Acute febrile illness, with headache, myalgia or prostration associated with any of the following: conjunctival suffusion/haemorrhage; meningeal irritation; anuria/oliguria/haematuria/proteinuria; haemorrhage – intestinal bleeding, lung bleeding or purpuric rash; cardiac arrhythmias/failure; plus a MAT titre ⩾1/400 or a positive Leptospira ELISA IgM’.
One female and 19 males participated in the white-water rafting and camping activity. We obtained data for 19 males. The female staff member was not involved in any outdoor recreational activity during the trip and refused to participate in the study. The participants age ranged from 24 to 47 years. All participants were involved in full-time office work at the factory and none had any outdoor activity exposure or exposure to surface water sources during the 4-week period prior to the investigation procedure (they were not involved in outdoor activities such as swimming, gardening, water sports, or agricultural work).
Of the 19 participants, six had moderate to severe febrile illness lasting 3–7 days which required medical care. Median incubation period of fever for six febrile patients was 10·5 days (range 6–22 days) after the recreational activity. All six fever patients had myalgia and headache, five had prostration, one had mild jaundice and four had conjunctival suffusion. None of them had oliguria, anuria, dyspnoea, or other features of end organ failures. A small increase in AST (range 49–56 units) and ALT (range 58–67 units) levels was observed in four febrile patients who were available at the time of investigation and serum creatinine was reported as normal (<1·2 mg/dl). None of the patients were hospitalized.
A total of four case-patients met the laboratory case confirmation criteria for leptospirosis: two with MAT titres ⩾1/400 and two with a positive IgM ELISA test result. While six persons reported a febrile illness, two of the six did not meet laboratory confirmation criteria. One had a MAT titre of 1/200 and one had a MAT that was non-reactive. An additional person did not have fever, but experienced mild headache, myalgia and anorexia, and was found to have a MAT titre of 1/400. Of the 17 participants who underwent serum screening for MAT, 15 had titres ⩾1/100; three with titres of 1/400, nine with titres of 1/200, and three with titres of 1/100 (Table 1). The two case-patients without MAT testing also had evidence of anti-leptospiral antibodies by IgM ELISA.
Table 1. Clinical description and laboratory findings for participants of a white-water rafting leptospirosis outbreak, Sri Lanka, 2012
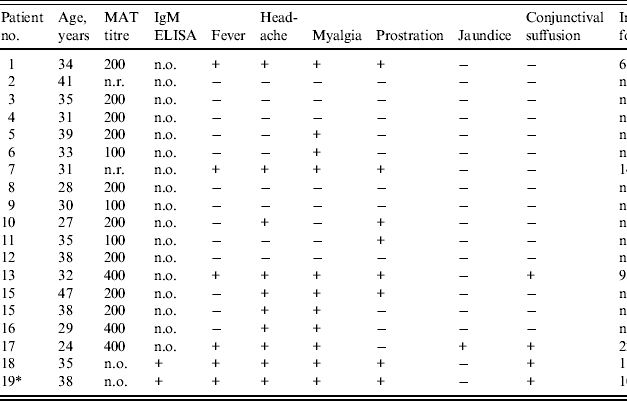
n.r., Not reactive; n.o., not obtained; n.a., not applicable.
* Index case.
Leptospirosis associated with white-water rafting is a well-known phenomenon in the global literature [3–Reference Stephan6]. White-water rafting on the Kelani River in Kithulgala, Sri Lanka has recently become a popular leisure activity among middle and upper class youth and white-collar workers. Although the rafting activities have been increasing in popularity for several years, this is the first report of leptospirosis associated with rafting in Sri Lanka. The delay in recognition of leptospirosis infection may be due to lack of reporting or under-diagnosis, due in part to the ambiguous presentation of mild leptospirosis infection. Less than 20% of patients with leptospirosis present with the classical textbook description of symptoms [Reference Agampodi7]. An interesting finding of this study was the presence of anti-leptospiral antibody in 17/19 (89·4%) of the participants. During the largest reported outbreak of leptospirosis in 2008, prevalence of anti-leptospiral antibody (titre >1:100) was only 30·1% in 167 probable cases of leptospirosis [Reference Agampodi8]. In the same year, another study performed in Sri Lanka assessed 889 febrile patients and found 27·1% with antibody titre suggestive of past infections [Reference Reller9]. The high MAT titres (1/400) observed in the present study are unlikely to be from past infection as the participants were white-collar workers who had no recent reported exposures to surface water sources.
One major limitation of this study is the use of a single genus-specific patoc strain as the sole antigen in the MAT. In Sri Lanka, this is the only MAT test antigen available. Even after having one of the largest global epidemics of leptospirosis in 2008 and a sustained outbreak of leptospirosis during 2008–2012 with more than 4000 cases each year, Sri Lanka is still without standard MAT diagnostic facilities for leptospirosis. Use of a genus-specific patoc strain could lead to non-specific results, which could be an alternative explanation for the high anti-leptospiral antibody seroprevalence detected in this study population.
This observation is a classical example of the changing epidemiology of diseases with the change in human behaviours. Raising awareness and implementing preventive strategies are necessary to minimize the health hazards of this type of water sport.
ACKNOWLEDGEMENTS
We acknowledge all third-year medical undergraduates who were involved in the data collection procedure as a part of their Community Medicine clerkship programme, and Jennifer Horton for her help in manuscript preparation.
DECLARATION OF INTEREST
None.



