The contribution of a changing diet to increasing rates of chronic disease among Alaska Native people has long been a focus of research investigation( Reference Murphy, Schraer and Thiele 1 – Reference Johnson, Nobmann and Asay 3 ). We developed a particular interest in the role that changing diets of childbearing women may have on vitamin D deficiency and rickets in children after a recent study reported a high incidence of rickets in Alaska Native children relative to other populations( Reference Singleton, Lescher and Gessner 4 ), following a report of rickets diagnoses in Alaska in the 1990s( Reference Gessner, deSchweinitz and Petersen 5 ). Of the confirmed rickets diagnoses, 63 % were in children under 1 year of age( Reference Singleton, Lescher and Gessner 4 ), suggesting the importance of maternal vitamin D status. Previous dietary evaluations in Alaska Native people showed higher vitamin D intake in Alaska Native people eating a traditional subsistence diet( Reference Bersamin, Zidenberg-Cherr and Stern 2 , Reference Johnson, Nobmann and Asay 3 , Reference Luick, Bersamin and Stern 6 , Reference Fohner, Wang and Yracheta 7 ), mirroring trends found in the Canadian Arctic( Reference Kuhnlein, Receveur and Soueida 8 – Reference Sharma, Barr and Macdonald 10 ) and Greenland( Reference Andersen, Jakobsen and Laurberg 11 – Reference Rejnmark, Jorgensen and Pedersen 13 ).
A major survey of Alaska Native diets conducted in the late 1950s found that traditional foods contributed approximately 44 % of energy and 72 % of protein to the diet of adults in eleven communities spanning three regions of Alaska( Reference Heller and Scott 14 ). Currently Alaska Native diets include a much larger proportion of store-bought (hereafter, ‘market’) foods, averaging approximately 80 % of energy in the Yukon Kuskokwim (YK) Delta region( Reference Bersamin, Zidenberg-Cherr and Stern 2 , Reference Johnson, Nobmann and Asay 3 , Reference Bersamin, Luick and Ruppert 15 ). While the fact that diets have changed is evident, the time course of change from more traditional to more market-based diets has not been documented, as diet records spanning multiple decades and collected using consistent methods do not exist. Our group has recently validated chemical biomarkers of traditional and market food intake in a Yup’ik population in the YK Delta region of south-west Alaska, which allow retrospective dietary studies using banked specimens( Reference Nash, Bersamin and Kristal 16 – Reference O’Brien, Kristal and Nash 18 ). These biomarkers are based on naturally occurring differences in stable isotope ratios among foods( Reference Nash, Bersamin and Kristal 16 ) and can be measured in serum, plasma or red blood cells (RBC)( Reference O’Brien 19 ). The nitrogen isotope ratio (15N:14N, expressed relative to a standard as δ15N values) is high in fish and marine mammals( Reference Nash, Bersamin and Kristal 16 ), and provides a measure of traditional food intake because fish and marine mammals contribute over 75 % of total traditional food energy in that population( Reference Bersamin, Zidenberg-Cherr and Stern 2 ).
In the present study we tested the hypothesis that consumption of traditional marine foods by Alaska Native women of childbearing age has declined from the 1960s to the present, and that this decline is associated with reduced serum 25-hydroxycholecalciferol (25(OH)D3). We analysed serum samples collected during the 1960s, 1970s, 1980s and 1990s and stored in the Alaska Area Specimen Bank (AASB)( Reference Parkinson, Hennessy and Bulkow 20 ) for biomarkers of traditional marine diet (δ15N values) and 25(OH)D3. All samples were collected from women aged 20–29 years in the YK Delta region of south-western Alaska. To extend the record through to the present we used data from the same region that were collected by the University of Alaska Fairbanks (UAF) Center for Alaska Native Health Research (CANHR) in the 2000s and 2010s. We focused on the YK Delta region because it is well represented across all decades in the AASB and also has a modern data set for comparison. Our goal was to evaluate changes in traditional marine food intake over time, to understand potential contributors to the recent higher risk for rickets and vitamin D deficiency among YK Delta and other Alaska Native children.
Methods
Study population
Serum specimens from 1960–1999 were provided by the AASB, which is maintained at the Centers for Disease Control and Prevention’s Arctic Investigation Program and overseen by the AASB committee( Reference Parkinson, Hennessy and Bulkow 20 ). The present study used 100 serum samples collected from women of childbearing age in the YK Delta in the 1960s, 1970s, 1980s and 1990s, and stored at −20 to −30°C in the AASB. The samples were drawn originally for eight historical village-wide research studies on a variety of illnesses during the 1960s and 1970s, and for hepatitis B screening and follow-up studies (1980s, 1990s). Most of these were region-wide studies open to all village residents, making it unlikely that there was a bias towards or against a more traditional lifestyle. The samples were evenly divided with twenty-five from each of the decades, with similar numbers from coastal and upriver communities for each decade. These sample sizes were chosen to give >90 % power to detect inter-decade differences in serum δ15N values of 1·0 ‰, given an expected within-group sd of 1·1 ‰( Reference Nash, Bersamin and Kristal 16 ). All samples were from women aged 20–29 years at the time of blood draw, evenly split between 20–24 years of age and 25–29 years of age. All samples tested were drawn between September and November to control for seasonal variation. The selected specimens were randomly drawn from the pool of available serum specimens that met the above requirements. Serum samples were used for analysis of both the δ15N value and 25(OH)D3 concentration, as described below. The δ15N value has been validated as a measure of traditional marine food dietary intake in the YK Delta region in three ways: (i) by comparison with self-reported intake (Pearson’s r>0·5)( Reference Nash, Bersamin and Kristal 16 ); (ii) by comparison with RBC EPA, a marine-derived fatty acid that is a biomarker for EPA intake (Pearson’s r>0·8)( Reference O’Brien, Kristal and Jeannet 17 ); and (iii) by demonstrating that associations with disease risk mirror those found using RBC EPA( Reference O’Brien, Kristal and Nash 18 ).
Comparison measurements from 2000–2009 and 2010–present were provided by the CANHR, which has been conducting health research in the YK Delta region of Alaska since 2003( Reference Mohatt, Plaetke and Klejka 21 ). For dietary biomarker data, we used RBC δ15N values collected in the 2000s and 2010s. Where multiple blood samples had been collected from an individual woman, we used data from the most recent blood draw. Data were available for 219 Alaska Native women aged 20–29 years. From the 2000s, there were data from 110 women, fifty-nine in coastal communities and fifty-one in upriver communities. From the 2010s, there were data from 109 women, sixty-three in coastal communities and forty-six women in upriver communities. Measurements of serum 25(OH)D3 concentration were available for most of the samples from the 2010s (seventy-three women, thirty-five in coastal communities and thirty-eight in upriver communities), but were not available from 2000–2009. There were low numbers of CANHR samples drawn between September and November in coastal communities in the 2010s; therefore, CANHR data included all months of sample collection (primarily late spring and early winter) so that the distribution of community locations would be similar to the AASB samples. In upriver communities, the mean 25(OH)D3 concentration between September and November 2010 was 4·3 ng/ml higher than when all collection months were included.
The relationship between δ15N values measured in serum and RBC was tested using samples from eighteen CANHR participants for whom both serum and RBC δ15N values were available( Reference Nash, Kristal and Bersamin 22 ). For this sub-aim, participants were 56 % female and ranged in age from 14 to 56 years old.
Stable isotope measurements
Serum and RBC samples were prepared for analysis of nitrogen isotope ratios as described elsewhere( Reference Nash, Bersamin and Kristal 16 , Reference Nash, Kristal and Bersamin 22 ). Briefly, 1·9 μl of RBC and 3·5 μl of serum were pipetted into 3·5 mm×3·75 mm tin capsules (Costech) held in custom-machined aluminium ninety-six-well plates and autoclaved to remove blood-borne pathogen risk( Reference Wilkinson, Yai and O’Brien 23 ). Samples were dried at 60°C and tin capsules were crushed into balls for loading into an autosampler. Isotope ratios were determined at the UAF Alaska Stable Isotope Facility by continuous-flow isotope ratio MS, using a Costech ECS4010 Elemental Analyzer (Costech Analytical Technologies, Inc.) interfaced to a Thermo Delta V isotope ratio mass spectrometer via the Conflo IV interface (Thermo Fisher Scientific). Isotope ratios are conventionally expressed as delta values, which give the abundance of heavy isotope relative to an international standard, as follows: δ15N={[(15N:14N)sample–(15N:14N)standard]/[(15N:14N)standard]}×1000 ‰, in which the standard is atmospheric nitrogen (air, 15N:14N=0·0036765). Analytical precision was assessed as the sd of a laboratory working standard (peptone), which was run at the beginning and end of each batch of analyses and after every tenth sample. Precision was within 0·2 ‰. The isotope ratio 15N:14N is highly stable over time( Reference O’Brien 19 ) and thus can be measured in archived samples.
Vitamin D measurements
Quantification of 25(OH)D3 in human sera was achieved by protein precipitation, liquid–liquid extraction, derivatization and LC–MS/MS analysis, following a previously described general procedure( Reference Wang, Lin and Dickmann 24 ). Briefly, the calibration curve was generated by a series of dilutions of a standard mixture containing 100 ng 25(OH)D3/ml prepared in vitamin D-free human serum (Golden West Biologicals). After thawing, human sera (0·1 ml) or a standard curve set were accurately added into 2 ml capped PPE micro-centrifuge tubes and spiked with d 6-25(OH)D3 (5 ng) internal standard. Acetonitrile (0·2 ml, v/v 2:1) was added to precipitate proteins. After centrifugation (12 000 g , 5 min), the supernatant was transferred to capped glass tubes and concentrated under a nitrogen stream prior to extraction with ethyl acetate (3 ml). The organic layer was isolated after centrifugation and dried under a stream of nitrogen. Samples were then derivatized with 1 mg 4-phenyl-1,2,4-triazoline-3,5-dione/ml (100 µl) in acetonitrile for 1 h at room temperature, in the dark. The derivatized samples were transferred to a new glass tube and dried under a stream of nitrogen. The solid residue was reconstituted in 30 % v/v acetonitrile (60 µl) and transferred to 200 µl glass inserts for LC–MS/MS analysis. Ultra-high-performance LC was performed on a Hypersil Gold (2·1 mm×100 mm, 1·9 µm) column (Thermo Scientific) using methanol (B)/0·1 % v/v formic acid in water (A) as a mobile phase. The flow rate was 0·25 ml/min with a solvent gradient (v/v) as follows: 60 % solvent B holding for 1 min, then to 64 % B over 19 min, holding at 64 % B for 0·5 min, increasing to 90 % B in 3 min and then holding for another 1 min, decreasing back to 60 % B until 25 min, followed by 3 min for equilibrium back to initial conditions. MS/MS analysis was carried out using positive mode electrospray ionization on an Agilent 6410 QQQ equipped with a UPLC1290 infinity system (Agilent Technologies). Multiple Reaction Monitoring channels of m/z 558→298 and 564→298 were set to detect 25(OH)D3 and d 6-25(OH)D3, respectively. This assay was validated in-house using the National Institute of Standards and Technology (NIST) standard reference material for vitamin D metabolites (SRM972), which contains four low-to-high levels of 25(OH)D3 in frozen human serum. All the measured 25(OH)D3 concentrations at each of these four pre-specified levels were within 15 % of the CV, as compared with the reference concentrations provided with the NIST standard. In addition, inter-day assay variability was assessed by daily analysis of two quality control samples that had been prepared in replicate prior to the start of the study and stored at −80°C. All measured concentrations of the quality control samples differed by <10 % from the expected nominal (15 and 25 ng/ml) concentrations.
The present study involved the analysis of serum samples that had been archived for up to 55 years. Other investigators have demonstrated the stability of 25(OH)D3 in frozen serum/plasma samples kept for extended periods of time( Reference Lissner, Mason and Posen 25 – Reference Agborsangaya, Toriola and Grankvist 27 ).
Statistical analyses
Statistical analyses were performed using the statistical software packages JMP version 11 and Stata version 10. We tested the association between δ15N values in RBC and serum using linear regression, and used the results to convert δ15N values of CANHR RBC samples to equivalent values for serum. Those values were used for comparison with AASB sample data.
We tested whether δ15N values and 25(OH)D3 concentrations changed between the decades using ANOVA, with independent variables=decade, coastal (yes/no) and age class (20–24 years/25–29 years), and all second-order interactions. Non-significant interaction terms were removed from the final models. Pairwise differences among decades were tested using Tukey’s honest significant differences at P<0·05. Normality of residuals was tested using the Shapiro–Wilks test, and serum δ15N values and vitamin D were log-transformed for parametric analyses to improve normality. The effect of location (coastal/inland) stratified by decade was tested with Student’s t test.
The association between serum vitamin D and δ15N values was tested using Spearman rank correlation. We report the number/percentage of participants with serum 25(OH)D3 concentrations of <12 ng/ml and 12–20 ng/ml, as these are highlighted by the Institute of Medicine as meaningful thresholds for vitamin D status in their 2011 report( 28 , Reference Rosen, Abrams and Aloia 29 ).
Results
Serum δ15N values were strongly and linearly related to RBC δ15N values (R 2=0·9, P<0·0001), with an average offset of +1·5 ‰ (Fig. 1). A correction of +1·5 ‰ was applied to the RBC δ15N values from the CANHR study to convert them to comparable serum δ15N values, for comparison with the AASB serum samples.

Fig. 1 Linear relationship between red-blood-cell (RBC) δ15N values and serum δ15N values (R 2=0·90), from eighteen participants in the Center for Alaska Native Health Research study (ten females, eight males, aged 14–56 years)
Intake of traditional marine foods, as measured by serum δ15N values, decreased significantly each decade from the 1960s through the 1990s, then remained constant from the 1990s through the present (F 5,306=77·4, P<0·0001; Table 1, Fig. 2(a)). Women aged 25–29 years had slightly higher δ15N values than women aged 20–24 years (F 1,306=5·5, P=0·02). Women from coastal communities had higher δ15N values than women from inland communities (F 1,306=35·4, P<0·0001) and there was a significant interaction between community location and decade (F 5,306=5·3, P=0·0001; Fig. 2(a)). When δ15N values were analysed by decade, the effect of community location was greatest in the 1960s (t=−5·7, P<0·0001), but also significant in the 2000s (t=−2·8, P=0·006) and the 2010s (t=−2·2, P=0·03; Fig. 2(a)). There was no significant interaction between community location and age class or between decade and age class.

Fig. 2 Differences in (a) mean serum δ15N values and (b) mean serum 25-hydroxycholecalciferol (25(OH)D3) concentrations, with their standard deviations represented by vertical bars, by decade and community location (![]() , upriver;
, upriver; ![]() , coastal), in 20–29-year-old women from the Yukon Kuskokwim Delta region of south-west Alaska. Samples from 1960–1999 derive from the Alaska Area Specimen Bank(
Reference Parkinson, Hennessy and Bulkow
20
), samples from 2000–present derive from the Center for Alaska Native Health Research Study(
Reference Mohatt, Plaetke and Klejka
21
). In (b), ——— indicates serum 25(OH)D3 concentration of 20 ng/ml and - - - - - indicates serum 25(OH)D3 concentration of 12 ng/ml (meaningful thresholds for vitamin D status according to the Institute of Medicine(
28
,
Reference Rosen, Abrams and Aloia
29
))
, coastal), in 20–29-year-old women from the Yukon Kuskokwim Delta region of south-west Alaska. Samples from 1960–1999 derive from the Alaska Area Specimen Bank(
Reference Parkinson, Hennessy and Bulkow
20
), samples from 2000–present derive from the Center for Alaska Native Health Research Study(
Reference Mohatt, Plaetke and Klejka
21
). In (b), ——— indicates serum 25(OH)D3 concentration of 20 ng/ml and - - - - - indicates serum 25(OH)D3 concentration of 12 ng/ml (meaningful thresholds for vitamin D status according to the Institute of Medicine(
28
,
Reference Rosen, Abrams and Aloia
29
))
Table 1 Serum δ15N values and 25-hydroxycholecalciferol (25(OH)D3) concentrations, by decade, in 20–29-year-old women from the Yukon Kuskokwim Delta region of south-west Alaska

AASB, Alaska Area Specimen Bank( Reference Parkinson, Hennessy and Bulkow 20 ); CANHR, Center for Alaska Native Health Research Study( Reference Mohatt, Plaetke and Klejka 21 ); min., minimum; max., maximum; GMC, geometric mean concentration.
a,b,c,dMean values with unlike superscript letters were statistically different in post hoc comparisons (Tukey’s honest significant differences; P<0·05).
* δ15N values were log-transformed for statistical analysis; however, geometric means differed from means by <0·2 ‰, thus here only means and sd are presented.
Serum 25(OH)D3 concentrations also decreased from the 1960s to the present (F 4,162=26·1, P<0·0001). Serum 25(OH)D3 concentrations in the 1980s, 1990s and 2010s were lower than in the 1960s and 1970s, but concentrations in the 1980s, 1990s and 2010s did not differ significantly from each other (Table 1, Fig. 2(b)). Serum 25(OH)D3 concentrations did not differ by age (F 1,162=0·6, P=0·42) or by community location (F 1,162=0·003, P=0·96); however, there was a significant interaction between community location and decade (F 4,162=2·8, P=0·029; Table 1, Fig. 2(b)). When analysed by decade, the effect of community location was significant only in the 2010s and, unlike the δ15N value, 25(OH)D3 concentration was higher in inland communities (t=3·43, P<0·001; Fig. 2(b)). In the 1960s and 1970s, none of the women had vitamin D concentrations ≤20 ng/ml, whereas in the 1980s, 1990s and 2010s, 24 %, 28 % and 27 % of the women had vitamin D concentrations ≤20 ng/ml, and 2 %, 0 % and 4 % had vitamin D concentrations <12 ng/ml, respectively (Table 1, Fig. 2(b)).
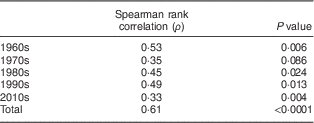
Serum 25(OH)D3 concentrations were significantly associated with serum δ15N values, both in the complete data set (Spearman ρ=0·61, P<0·0001; Fig. 3) and by decade (Table 2, Fig. 3).

Fig. 3 Association between serum 25-hydroxycholecalciferol (25(OH)D3) concentrations and serum δ15N values, by decade (![]() , 1960s;
, 1960s; ![]() , 1970s;
, 1970s; ![]() , 1980s;
, 1980s; ![]() , 1990s;
, 1990s; ![]() , 2010s), in 20–29-year-old-women from the Yukon Kuskokwim Delta region of south-west Alaska. Samples from 1960–1999 derive from the Alaska Area Specimen Bank(
Reference Parkinson, Hennessy and Bulkow
20
), samples from 2000–present derive from the Center for Alaska Native Health Research Study(
Reference Mohatt, Plaetke and Klejka
21
)
, 2010s), in 20–29-year-old-women from the Yukon Kuskokwim Delta region of south-west Alaska. Samples from 1960–1999 derive from the Alaska Area Specimen Bank(
Reference Parkinson, Hennessy and Bulkow
20
), samples from 2000–present derive from the Center for Alaska Native Health Research Study(
Reference Mohatt, Plaetke and Klejka
21
)
Table 2 Spearman rank correlation of serum δ 15N values and 25-hydroxycholecalciferol concentrations, by decade, in 20–29-year-old women from the Yukon Kuskokwim Delta region of south-west Alaska
Discussion
Traditional marine food intake, as assessed by the serum δ15N value, declined linearly from the 1960s through the 1990s in young (20–29-year-old) Alaska Native women and then remained constant from the 1990s through the present. Vitamin D levels, measured as serum 25(OH)D3 concentrations, also declined significantly between the 1960s–1970s and the 1980s–2010s, and were associated with assessed intake of traditional marine foods both within and across decades. These data demonstrate a nutrition transition from traditional foods to market foods in Alaska Native people. Other studies have inferred this transition( Reference Murphy, Schraer and Thiele 1 – Reference Johnson, Nobmann and Asay 3 , Reference Nobmann, Byers and Lanier 30 ); however, our approach of measuring a dietary biomarker and vitamin D in archived historical specimens provides a quantitative temporal record, covering a period of nearly 60 years. These declines in traditional food intake and vitamin D have significant implications for public health, as none of the women studied had vitamin D concentrations ≤20 ng/ml in the 1960s and 1970s, whereas 24–28 % did in the 1980s, 1990s and 2010s. By some criteria, these concentrations are considered vitamin D insufficient( 28 ), whereas by others they are considered vitamin D deficient( Reference Holick, Binkley and Bischoff-Ferrari 31 ); either way, they demonstrate a decrease in vitamin D status of these women over time.
It is likely that the sharp decline in traditional marine food intake by women of childbearing age from the 1960s to the 1990s in the YK Delta is one of the main factors causing significant declines in vitamin D concentrations in this sample of women, similar to recent findings from Greenland( Reference Nielsen, Jorgensen and Friis 12 ). This dietary transition may also have been a factor contributing to increasing reports of rickets in Alaska Native children starting in the 1990s( Reference Gessner, deSchweinitz and Petersen 5 ). Many traditional subsistence foods provide an excellent and economical source of vitamin D( Reference Bersamin, Zidenberg-Cherr and Stern 2 , Reference Holick 32 ), whereas market foods rich in vitamin D are often expensive, unavailable or not preferred. The importance of traditional dietary intake to vitamin D status of Alaska Native people and people of the Canadian Arctic and Greenland has been well documented in recent years( Reference Bersamin, Zidenberg-Cherr and Stern 2 , Reference Johnson, Nobmann and Asay 3 , Reference Luick, Bersamin and Stern 6 , Reference Sharma, Barr and Macdonald 10 , Reference El Hayek, Egeland and Weiler 33 – Reference Kolahdooz, Barr and Roache 35 ). Two recent studies highlight this point for the contemporary Yup’ik population of the YK Delta: one study found that 90 % of self-reported dietary vitamin D intake was from fish( Reference Luick, Bersamin and Stern 6 ) and another found that traditional dietary intake (measured as the RBC δ15N value) explained 21 % of inter-individual differences in serum 25(OH)D3 concentration( Reference Fohner, Wang and Yracheta 7 ). At the latitude of Alaska, pre-cholecalciferol production in the skin essentially ceases from October through March( Reference Webb, Kline and Holick 36 – Reference Chaplin and Jablonski 38 ), making Alaskans and other high-latitude populations particularly dependent on dietary sources of vitamin D( Reference Sharma, Barr and Macdonald 10 , Reference Andersen, Jakobsen and Laurberg 11 ).
Our group recently described high rates of rickets incidence and hospitalization in Alaska Native children relative to the general US rate( Reference Singleton, Lescher and Gessner 4 ). Rickets diagnoses dated from the 1990s( Reference Singleton, Lescher and Gessner 4 , Reference Gessner, deSchweinitz and Petersen 5 ), when traditional food intake by childbearing aged women in the present study had reached a constant, low value. Given the evidence of low traditional food consumption in childbearing-aged Alaska Native women, vitamin D supplementation may be important in women and infants to prevent infantile rickets. Consistent with this, the American Academy of Pediatrics recommends that regardless of sunlight and food intake, all breast-fed infants/children and those receiving <1 litre of infant formula daily receive vitamin D supplementation of 10 µg/d (400 IU/d)( Reference Wagner and Greer 39 ). Although 10 µg/d d is adequate to prevent rickets in most infants, those born to severely vitamin D-deficient women may develop congenital rickets( Reference Paterson and Ayoub 40 ); therefore, a traditional marine diet and vitamin D supplementation to prevent deficiency may be particularly important for pregnant Alaska Native women.
The present study is distinctive for its approach of using archived specimens to create a continuous, 60-year historical record of traditional dietary intake and vitamin D status in Alaska Native people, which was made possible by the unique tribal–federal partnership of the AASB( Reference Parkinson, Hennessy and Bulkow 20 ). While the fact that Alaska Native people are undergoing a nutrition transition is widely inferred( Reference Johnson, Nobmann and Asay 3 , Reference Bersamin, Luick and Ruppert 15 , Reference Bersamin, Luick and King 41 ), direct evidence of this transition has been lacking, and the timing and trajectory of such a transition in different regions of Alaska have not been documented given the lack of historical dietary records. A number of studies have documented dietary differences between youth and elders( Reference Murphy, Schraer and Thiele 1 , Reference Bersamin, Zidenberg-Cherr and Stern 2 , Reference Bersamin, Luick and Ruppert 15 , Reference Nash, Bersamin and Kristal 16 ); however, whether those differences persist throughout the lifespan or whether youth adopt the diet of their elders as they age is not known. We note that biomarker measurements of traditional dietary intake by women aged 20–29 years in the 1960s and 1970s from the current study are similar to those of modern elders in the YK region( Reference Fohner, Wang and Yracheta 7 , Reference Nash, Bersamin and Kristal 16 ). It would be interesting to extend the record to the 1950s and prior, given reports of major dietary change prior to the early 1960s( Reference Murphy, Schraer and Thiele 1 ); however, these dates are not represented in the AASB( Reference Parkinson, Hennessy and Bulkow 20 ).
The study has some limitations. The randomly selected AASB samples may not be representative of the population of women 20–29 years of age in the YK Delta during those decades; however, because most were collected during region-wide studies open to all village residents, we believe it is unlikely that there was a sampling bias towards or against women who followed a more traditional lifestyle. Furthermore, because the design takes advantage of sampling from the same population during several successive decades, there is the possibility that bias to the reported serum 25(OH)D3 concentration could be introduced by sample desiccation, as discussed previously( Reference Longnecker, Zhou and Klebanoff 42 ). The effect would likely have been greatest with the longest storage times and thus may contribute to higher measured 25(OH)D3 concentrations in the oldest samples. We attempted to control for this potential bias by measuring and normalizing the 25(OH)D3 concentration data for serum Na concentration. Both aberrantly high and low Na concentrations were measured using a standardized automated clinical laboratory protocol, as reported by others( Reference Longnecker, Zhou and Klebanoff 42 ). However, some of the oldest serum samples had been stored in glass tubes for a significant period of time and leaching of Na from the glass into the serum could also have contributed to aberrantly high Na concentrations. Furthermore, there was particulate (removed by centrifugation) in some of the serum samples after thawing, which could trap Na and contribute to aberrantly low Na values, as reported previously( Reference Longnecker, Zhou and Klebanoff 42 ). Without full confidence in all Na measurements, we elected to not correct the 25(OH)D3 data set. The δ15N value is not affected by desiccation, as it measures 15N:14N, and the fact that it was correlated with serum 25OHD3 across all decades suggests that any sample desiccation that did occur was random. Of note in this regard, the same positive and significant correlation between δ15N and serum 25(OH)D3 was seen in a large contemporary study conducted in the same population, where precautions were taken to avoid desiccation( Reference Fohner, Wang and Yracheta 7 ).
In summary, we found a linear decline in traditional marine food intake from the 1960s through the 1990s in young (20–29-year-old) Alaska Native women in the YK Delta region of south-west Alaska, concomitant with a drop in serum vitamin D levels. Prior to this decrease, none of these 20–29-year-old women were vitamin D deficient; however, after the decrease (1990s and 2010s) 28 % were vitamin D deficient. Traditional food intake remained low and constant from the 1990s through the present, a period which has also been marked by the appearance of rickets in Alaska Native children( Reference Singleton, Lescher and Gessner 4 ). Studies to evaluate traditional dietary practices and optimal vitamin D supplementation during pregnancy to prevent early childhood rickets are needed.
Acknowledgements
Acknowledgements: The authors thank Jynene Black for laboratory support; Karen Rudolph and Carolynn DeByle, Arctic Investigations Program, for AASB specimen processing; and Andrea Bersamin for helpful input. Financial support: This work was supported by the National Institutes of Health (K.E.T. and Z.W., grant numbers U01 GM092676 and R01 GM063666; B.B.B., S.E.H. and D.M.O., grant number P30 GM103325); and the UAF Office of Undergraduate Research and Scholarly Activity (D.M.O. and B.C., UAF Summer Undergraduate Research Award). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Conflict of interest: None. Authorship: D.M.O., K.E.T., L.R.B. and R.S. designed the study; D.M.O., K.E.T., Z.W. and B.C. conducted the laboratory analyses; D.M.O. and L.R.B. conducted the statistical analyses; D.M.O., R.S. and K.E.T. wrote the first draft of the manuscript; all authors contributed to the final version of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Alaska Area Institutional Review Board, the UAF Institutional Review Board, the Centers for Disease Control and Prevention, and the Yukon Kuskokwim Health Corporation Board of Directors.








