INTRODUCTION
Each year, many countries or regions experience a peak influenza virus activity. A large number of people suffer from the infection and many individuals are affected by small-scale outbreaks in schools. There are hundreds of such small-scale influenza outbreaks in mainland China every year, leading to a heavy disease burden which seriously impacts the operation of affected schools. Knowing the transmissibility of each outbreak in early stages has become a major concern for the public health policy-makers and primary healthcare providers. Thus, it is necessary to estimate the reproduction number R (a most commonly used indicator that quantifies the transmissibility of influenza) when the primary health departments receive an outbreak report. However, the R of small-scale outbreaks has not yet been well estimated in China.
R is defined as the average number of secondary infections that arise from a typical primary case [Reference Chen1, Reference Liu2]. From this definition, it is immediately clear that when R > 1, the disease is able to spread in the susceptible population. If R < 1, the infection will be cleared from the population. An individual-based model [Reference Longini3–Reference Yang5] or ordinary differential equation (ODE) model [Reference Liu2, Reference Chen and Liao6] has been commonly employed for the estimation of R. In these models, the natural history of influenza (the incubation period, the latent period, infectious, or recovered, etc.), the demographic characteristics of the affected population, the epidemic data, and the counter-measures for controlling the outbreak are always included, which press the model closer to the actual scenarios hiding below the outbreak. Unfortunately, because of the professional gap between public health and the mathematical model, it is difficult for the primary health department in China to estimate the R of an outbreak by using an individual-based model or an ODE model. It is imperative to confirm a practical and accurate transmissibility estimation method for the primary health department.
There are several simple approaches which could be used for the estimation of R from epidemiological data. These are defined as ‘susceptibles at endemic equilibrium’, ‘average age at infection’, ‘the final size equation’, ‘calculation from the intrinsic growth rate’ by Heffernan et al. [Reference Heffernan, Smith and Wahl7]. The epidemic model, normal distribution, delta distribution, and gamma distribution models by Wallinga et al. [Reference Wallinga and Lipsitch8], Heffernan et al. [Reference Heffernan, Smith and Wahl7] and Trichereau et al. [Reference Trichereau9] were also used. Heffernan's former three models are not easily used at the early stage of an outbreak because the parameters of these models are hard to obtain at that stage. The model ‘calculation from the intrinsic growth rate’ is the same as the epidemic model. Therefore, the epidemic model, along with the normal distribution, delta distribution, and gamma distribution models are the best choices for the model screening.
In this study, we collected all the small-scale outbreaks in Changsha from January 2005 to December 2013. The four simple and commonly used models [Reference Heffernan, Smith and Wahl7–Reference Trichereau9] were employed to calculate the R values of these outbreaks, and the results of each model were compared in order to commend an optimized model which could be used. We also collected the data of interventions employed in each outbreak and calculated the reproductive number with control measures (R con) of each outbreak to discover the effectiveness of the interventions.
METHODS
Data collection
In China, the criterion for an influenza outbreak has been defined as ⩾10 influenza-like illness (ILI) cases occurring in the same school, preschool, or other collective organization within 1 week, with laboratory-confirmed influenza viruses through virus isolation or real-time reverse transcriptase–polymerase chain reaction analysis. Our study subjects also included public health incidents that were defined as ⩾30 ILIs within 1 week. ILI refers to a fever (axillary temperature ⩾38 °C) accompanied by coughing or sore throat and a lack of a laboratory-confirmed diagnosis of the specific pathogen.
We built a dataset of seasonal influenza and influenza A(H1N1)pdm outbreaks by collecting information on all outbreaks reported from 1 January 2005 to 31 December 2013 in Changsha, China. Data included location type (e.g. primary school, middle school, high school, and prison), the school population, the date of the reported outbreak, the date of symptom onset of all cases, the subtype of influenza virus, and interventions including symptom surveillance, case isolation, symptomatic treatment of cases, antivirals for treatment or prophylaxis use, health education, environment disinfection, vaccination, social distance, and class, grade and school closure.
Symptom surveillance which focused on ILI cases was implemented every day from the reported outbreak date to the end of the outbreak. For case isolation, infected individuals were isolated in the hospital if they reported severe symptoms like pneumonia, or were isolated at home for mild cases until all the symptoms disappeared after 24 h or 48 h. For symptomatic treatment, cases were treated by medication (not antivirals) to relieve the symptoms in the hospital or at home.
Local CDC staff had overseen health education for the affected people who were taught to maintain personal hygiene and wear a gauze mask during the outbreak. Local public health providers also disinfected the potential environment or air contaminated by cases, and a chlorine-based disinfectant was employed to sterilize the fomites and a peracetic acid solution of 15% concentration was employed to disinfect the air through fumigation.
During the period of the class/grade/school closure, a teacher in charge of a class was required to monitor all the students in the class every day. At the same time, each student was asked to stay at home, take their own temperature and report their findings to their teacher by telephone.
Two medications (moroxydine and a traditional medicine called ‘Ban Lan Gen Chong Ji’) were used for prophylaxis. Moroxydine was prescribed at 0·1 g t.i.d. for 2 or 3 days for children aged <10 years and the dose was doubled for those aged >10 years. ‘Ban Lan Gen Chong Ji’, which is made from an herbaceous plant named Ban Lan Gen, was used at 10 g t.i.d. for 5 days.
In our study, 56 influenza outbreaks in school, prison and the community were collected (Table 1). To make the estimation of R more stable and reliable, the inclusion criterion was that the exponential growth phase of outbreak should be ⩾3 days. The exclusion criteria were as follows: (a) that the outbreaks had no illness onset date of each case, or only a part of the cases had an onset date, which may make the epidemic curve of the outbreak unavailable for the estimation of R; (b) that there was no influenza laboratory test results in the outbreak; (c) there was a combined infection of influenza subtypes/types in an outbreak; and (d) the virus was untyped. According to these criteria only the target datasets would be included in our study for estimating the R of each outbreak.
Table 1. General information of 56 influenza outbreaks in Changsha city, China, 2005–2013 (n = 56)
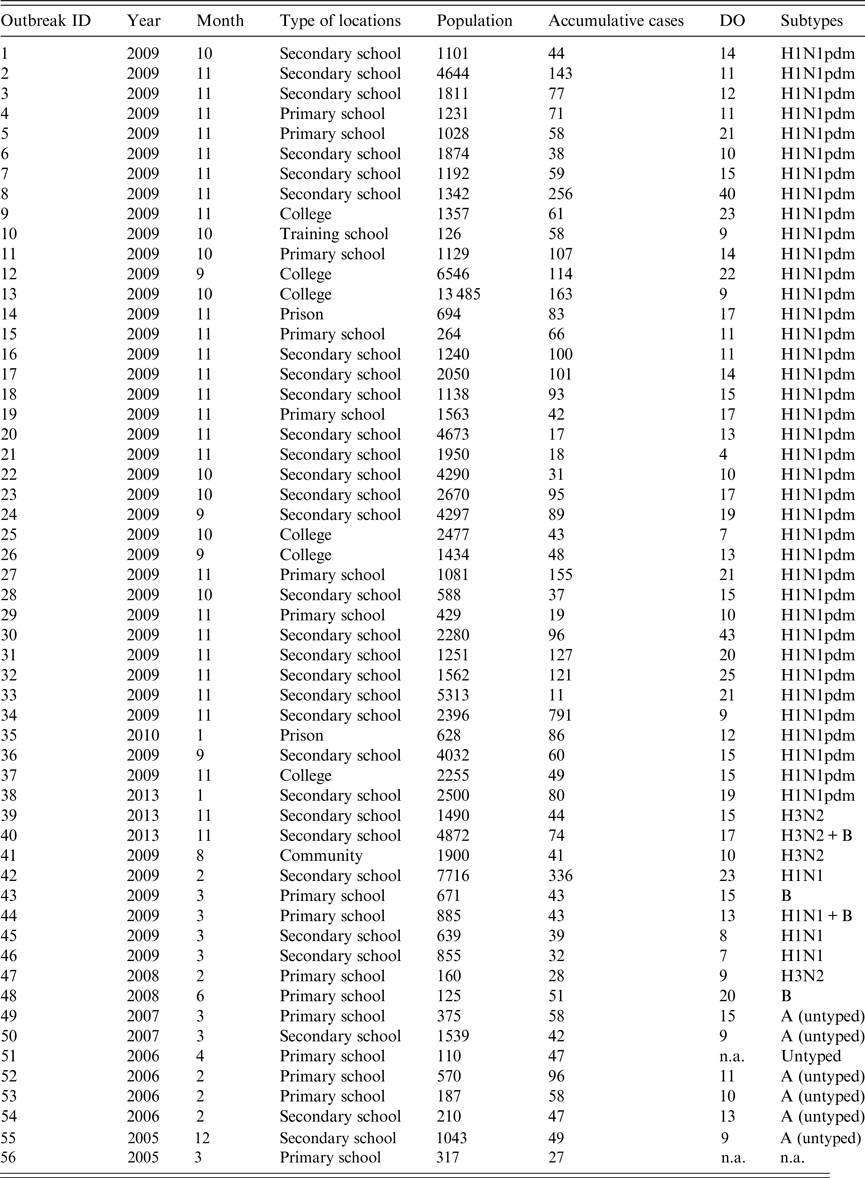
ID, Identification; DO, duration of outbreak; n.a., not available.
Estimation of reproduction number
To estimate the R of each outbreak, we employed the intrinsic growth rate method using the following four models [Reference Heffernan, Smith and Wahl7–Reference Trichereau9].
Epidemic model
In this model, the reproduction number, R, is expressed as follows:
In this equation, r and Tc refer to the epidemic growth rate and the duration of a generation interval.
Normal distribution
In this model, the generation intervals may approximate a normal distribution, making R as follows:
where Tc refers to the mean generation interval and σ refers to the standard deviation (s.d.).
Delta distribution
In this model, all secondary infections are exactly equal to the mean generation interval Tc. The model is expressed as follows:
Gamma distribution
For the gamma distribution Gamma (α, β), the relationship between r and R is expressed as follows:
During the exponential growth phase of an outbreak, the relationship between the growth rate r and daily incidence I(t) of symptom onset can be expressed as dI/dt = rI, where r is the exponential growth rate [Reference Heffernan, Smith and Wahl7, Reference McBryde10]. The exponential growth rate r was estimated from the daily epidemic curve of each outbreak we collected.
As Tc could not be estimated from our epidemic data, we used two different generation intervals derived from previous research [Reference McBryde10, Reference Hahné11]. The mean generation intervals and standard deviations were Tc = 2·7, σ = 1·1 [Reference Hahné11] and Tc = 2·9, σ = 1·4 [Reference McBryde10]. The two parameters α and β of a gamma distribution Gamma (α, β) were 4·2 and 0·68, respectively [Reference McBryde10]. The values of parameters (Tc = 2·9, σ = 1·4) and Gamma (4·2, 0·68) were calculated from the same data [Reference McBryde10].
Simulation methods
Berkeley Madonna v. 8.3.18 (University of California at Berkeley, USA) and Microsoft Office Excel 2003 (Microsoft, USA) software were employed for model simulation and figure development, respectively. The details of model-fitting methods run in Berkeley Madonna, such as the Runge–Kutta method of order 4 and root-mean-square deviation, were the same as the those in references [Reference Chen1, Reference Liu2, Reference Chen12].
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
After analysing 56 outbreaks, 32 outbreaks were enrolled for estimating R, because three outbreaks had no illness onset dates for each case and one had no laboratory test results. Moreover, the data of four outbreaks were not integrated, the combined infection of influenza subtypes/types were tested in two outbreaks, six outbreaks were untyped, and the exponential growth phase of nine outbreaks were <3 days. Of these 32 outbreaks, the growth rates of 15 were shown to have statistical significance by curve fitting (see Table 2). According to the generation interval used and its distribution, R estimations are shown in Table 3.
Table 2. The growth rate r of 32 influenza outbreaks in Changsha city, China, 2005–2013 (n = 32)
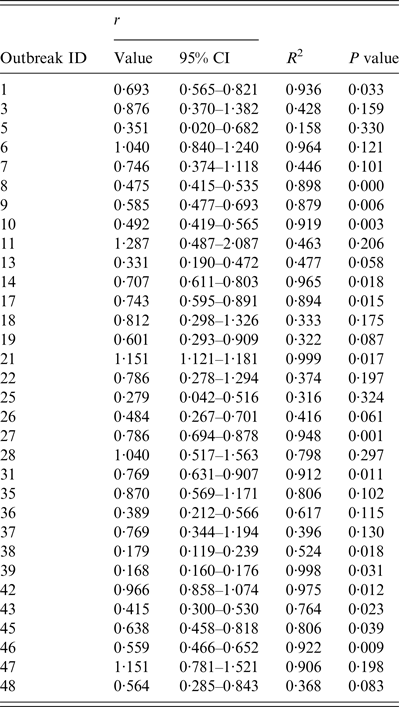
ID, Identification; r, epidemic growth rate; CI, confidence interval; R 2, coefficient of determination.
Table 3. The reproduction number R of 15 influenza outbreaks in Changsha city, China, 2005–2013 (n = 15)
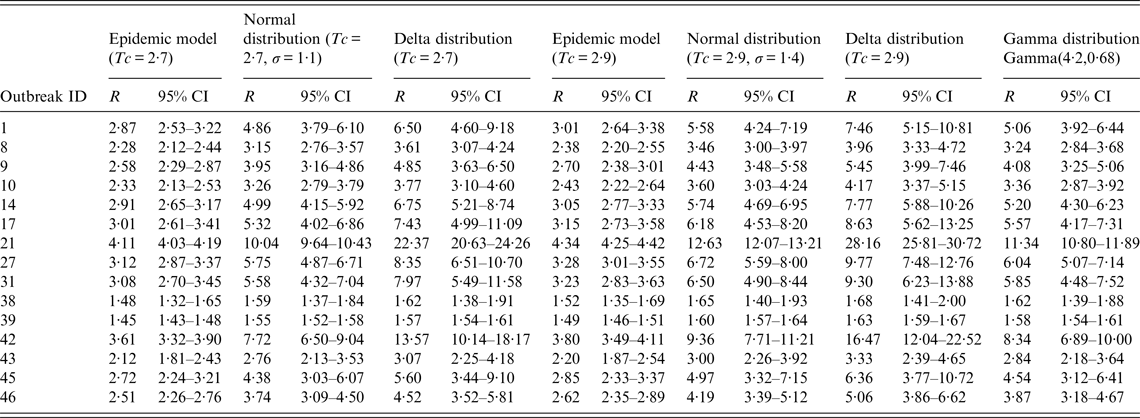
ID, Identification; Tc, mean generation interval; σ, standard deviation; R, reproduction number; CI, confidence interval.
Given that Tc = 2·7 and σ = 1·1, the mean R estimated by the epidemic model, normal distribution and delta distribution models were 2·68 (s.d. = 0·71), 4·58 (s.d. = 2·22) and 6·77 (s.d. = 5·28), respectively. Given that Tc = 2·9 and σ = 1·4, the mean R estimated by the three models were 2·80 (s.d. = 0·76), 5·31 (s.d. = 2·87) and 7·95 (s.d. = 6·73), respectively. The mean R estimated by gamma distribution was 4·84 (s.d. = 2·52) (see Table 3). The mean values of R estimated by normal distribution were higher than the those estimated by the epidemic model, even if Tc = 2·7 (t = −3·149, P = 0·006) or Tc = 2·9 (t = 3·265, P = 0·005). Similarly, the mean values of R estimated by delta distribution were higher than those estimated by the epidemic model, even if Tc = 2·7 (t = −2·974, P = 0·010) or Tc = 2·9 (t = −2·939, P = 0·011). However, there was no significance between the mean values of R estimated by delta distribution and those estimated by normal distribution, even if Tc = 2·7 (t = −1·483, P = 0·149) or Tc = 2·9 (t = −1·396, P = 0·174).
Because Tc = 2·9, σ = 1·4 and Gamma (4·2, 0·68) were from the same data, we only compared the results of the gamma distribution estimated with the results of the epidemic model, normal distribution and delta distribution models that had been tested under the same conditions. The mean R estimated by gamma distribution was higher than the mean R estimated by the epidemic model (t = −2·988, P = 0·008). However, the mean R estimated by gamma distribution was not significant compared to the mean R estimated by normal distribution (t = 0·478, P = 0·636) and delta distribution (t = 1·676, P = 0·105).
Of the 15 outbreaks, ten were H1N1pdm, one was H3N2, three were H1N1 and one was B subtype. There was no significance with the mean R of these subtypes based on the analysis of variance (ANOVA), even if R was estimated by the four models under different conditions (see Table 4).
Table 4. The results of ANOVA analysis in different subtypes of influenza virus (n = 15)

Tc, Mean generation interval; σ, standard deviation; R, reproduction number; CI, confidence interval; F, a statistic value in analysis of variance.
In the 15 outbreaks used for calculating R, we found that eight interventions, implemented individually or in combination, were employed after the reported outbreak date, except with social distance and antivirals like oseltamivir (see Table 5). These interventions included five non-pharmaceutical (symptom surveillance, case isolation, health education, environment disinfection and class/grade/school closure) and three pharmaceutical (symptomatic treatment, prophylaxis, vaccination) interventions. Symptom surveillance was implemented in 14 outbreaks. Case isolation, symptomatic treatment, health education and environment disinfection were employed in each outbreak. During four outbreaks (three H1N1pdm and one B subtype), class or grade closure was employed because cluster cases occurred in the class or grade, and school closure was employed in nine outbreaks (six H1N1pdm and three H1N1). Vaccination was employed in two outbreaks (one H1N1pdm and one H3N2). Prophylaxis was only employed in one H1N1 outbreak.
Table 5. The interventions in each outbreak and the reproduction number based on the epidemic model (n = 15)
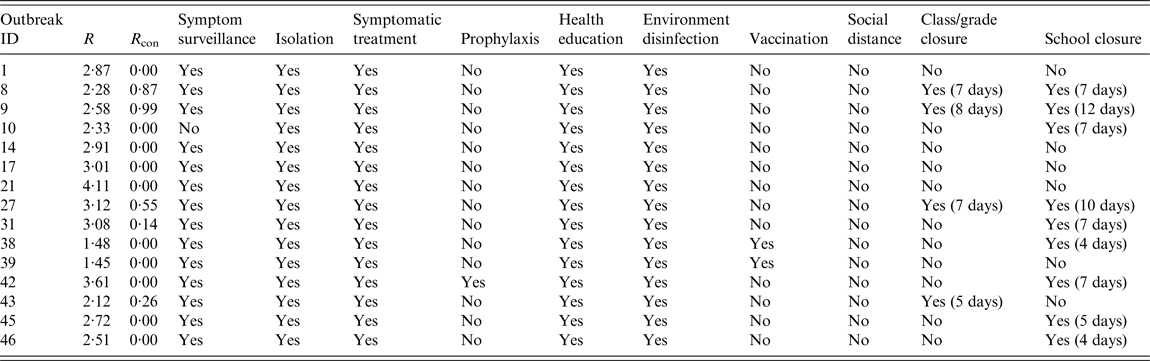
ID, Identification; R, reproduction number; R con, reproductive number with control measures.
According to whether the intervention was employed or not, we divided the 15 outbreaks into employed groups and non-employed groups. Then we compared the mean R of the two groups to infer if the intervention was employed based on a high R. There was no significance of class/grade closure (t = −0·492, P = 0·631), school closure (t = −0·286, P = 0·780) or prophylaxis (t = −1·405, P = 0·184). On the other hand, there was significance of vaccination (t = 3·479, P = 0·004), but before the countermeasure was chosen, the R of the employed group was lower than that of the non-employed group. These results reveal that the intervention was not employed based on whether the value of R was high or not.
The reproductive number with control measures (R con) of each outbreak was calculated by the epidemic model using the reported data which was collected after the reported date. We observed that R con was <1 after the interventions were implemented and most R con were 0 (see Table 5 and Fig. 1).

Fig. 1. Epidemic curves of 15 influenza outbreaks used for calculating R based on an epidemic model in Changsha city, China. ID, Identification; R, reproduction number; R con, reproductive number with control measures.
DISCUSSION
Our results reveal that the mean value of R in small-scale outbreaks was higher than in large-scale ones (outbreaks which occurred in a neighbourhood, city or province) in which R was <2·0 [Reference Liu2–Reference Yang5, Reference Trichereau9, Reference Germann13, Reference Tracht, Valle and Hyman14], whether or not Tc = 2·7 (t = 3·068, P = 0·007) or Tc = 2·9 (t = 3·494, P = 0·003), as calculated by the epidemic model. This implies that the attack rate and the peak incidence of a small-scale outbreak may be higher than those occurring in a city. The reasons for this difference are not clear, but may be caused by the high population density and contact frequency in a school or a prison compared to a neighbourhood. This may alert us to employ different countermeasures to control small-scale outbreaks rather than outbreaks occurring in a city at large.
Furthermore, the R values estimated by normal distribution, delta distribution, and gamma distribution models were higher than those calculated by the epidemic model. The former three models resulted in a higher s.d. and larger ranges of R, which might make the estimation more discrete and unstable. We conclude that the former three models seem to more easily overestimate the R of an influenza outbreak. And this overestimation may result in the control strategies being implemented excessively when the primary health department makes an emergency response policy. Thus, in the four models of the intrinsic growth rate method, the epidemic model might be the best one to recommend to estimate the transmissibility of influenza at the early stage of an outbreak.
We found that the commonly used interventions were symptom surveillance, case isolation, symptomatic treatment, health education and environment disinfection, followed by class/grade/school closure, vaccination and prophylaxis. They were not employed based on the transmissibility (the reproduction number R) of the outbreak. Although the transmissibility of most of the outbreaks decreased rapidly after the interventions, some of them were still close to 1, which would prolong an outbreak. Therefore, we recommend that local CDCs calculate the reproduction number of the outbreak by using the epidemic model and the data when they receive an outbreak report, thereby avoiding implementing countermeasures blindly.
It should be noted that this study has some limitations because of the lack of integrity of the data and the methodology of the model. Regarding the data we collected, epidemic curves of seven outbreaks were missing or not integrated, and there were 19 outbreaks showing no exponential growth in early stages, which meant that there was still more effort required from the primary health department to improve the quality of the data collection. If the data is collected more accurately through field investigation, the R estimation will be shown to be more precise. On the other hand, the epidemic model requires that the early stage of an outbreak must be ⩾3 days. The lower time span (especially <3 days) of the data may make the estimation unstable and unreliable. Finally, the epidemic model is only suitable to estimate the R of the outbreak in the early stage according to the principle of the model, which signified that we could not use the whole data if the outbreak was reported after the early stage. Therefore, after the exponential growth stage, particularly for the stage after the epidemic peak of the outbreak, other more complicated models should be utilized, such as an ordinary differential equation model or a stochastic individual-based model to conduct the transmissibility estimation.
In conclusion, we have shown the mean value of R of influenza outbreaks in Changsha, China, and our findings may show an appropriate example for the primary health department to assess the transmissibility of small-scale influenza outbreaks with a practical, yet accurate model at the early stage.
ACKNOWLEDGEMENTS
We thank Yelan Li and Zhi Xie from the Office for Disease Control and Emergency Response, Changsha Center for Disease Control and Prevention for helpful discussions. This work was supported by the National Natural Science Foundation of China (no. 81273192).
DECLARATION OF INTEREST
None.









