Introduction
The Udzungwa Forest Partridge Xenoperdix udzungwensis was described in 1994 after months of fieldwork in 1991–1992, including two of the authors (LD and EM). It was assessed as ‘Vulnerable’ on the IUCN Red List upon its discovery (McGowan Reference McGowan, del Hoyo, Elliot and Sargatal1994), uplisted to ‘Endangered’ by Stattersfield et al. (Reference Stattersfield, Crosby, Long and Wege1998), changed back to ‘Vulnerable’ in 2000, justified by a small population, but inferred to be stable and within well-protected areas (BirdLife International 2000, Fuller et al. Reference Fuller, Carroll and McGowan2000) and uplisted again to ‘Endangered’ in 2004. Several of the later assessments included a tiny new population discovered in the Rubeho Mountains still within the Eastern Arc and approximately 150 km north of the Udzungwas (Fjeldså and Kiure Reference Fjeldså and Kiure2003). This population was subsequently elevated to a separate species (Bowie and Fjeldså Reference Bowie and Fjeldså2005), the Rubeho Forest Partridge Xenoperdix obscuratus; we concur and regard this forest partridge population as representing a separate species.
The rate of species extinction at the global level is hundreds or perhaps thousands of times higher than the average rate over the past 10 million years, and it is accelerating (Barnosky et al. Reference Barnosky, Matzke, Tomiya, Wogan, Swartz, Quental, Marshall, McGuire, Lindsey, Maguire, Mersey and Ferrer2011, Pimm et al. Reference Pimm, Jenkins and Abell2014, Ceballos et al. Reference Ceballos, Ehrlich, Barnosky, Garcia, Pringler and Palmer2015). The main causes are habitat loss and exploitation, but climate change, invasive alien species, and pollution are also important drivers of species extinction, often in combination (IPBES 2019). Many species may be “living dead” (Tilman et al. Reference Tilman, May, Lehman and Nowak1994, Kuussaari et al. Reference Kuussaari, Bommarco, Heikkinen, Helm, Krauss, Lindborg, Ockinger, Partel, Pino, Roda, Stefanescu, Teder, Zobel and Steffan-Dewenter2009, Halley et al. Reference Halley, Monokrousos, Mazaris, Newmark and Vokou2016), and hundreds of thousands of terrestrial plants and animals may be in an extinction debt (Hoskins et al. Reference Hoskins, Harwood, Ware, Williams, Perry, Ota, Croft, Yeates, Walter, Golebiewski, Purvis and Ferrier2019). There is a call to increase our knowledge at the species level to help understand and reverse this situation (e.g. Kuussaari et al. Reference Kuussaari, Bommarco, Heikkinen, Helm, Krauss, Lindborg, Ockinger, Partel, Pino, Roda, Stefanescu, Teder, Zobel and Steffan-Dewenter2009, Hylander and Ehrlén Reference Hylander and Ehrlén2013).
Here we investigate the case of the partridge in the Udzungwa Mountains in south-central Tanzania. The species was discovered in two separate evergreen montane forests, Ndundulu-Luhombero and Nyumbanitu, and occurred between 1,350 and 1,900 m (Figure 1). It was placed in its own genus with no close relatives in Africa (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994, Crowe et al. Reference Crowe, Bloomer, Randi, Lucchesi, Kimball and Groth2004). This study presents the first population survey based on playback and a first attempt to evaluate its main habitat requirements in a systematic way.
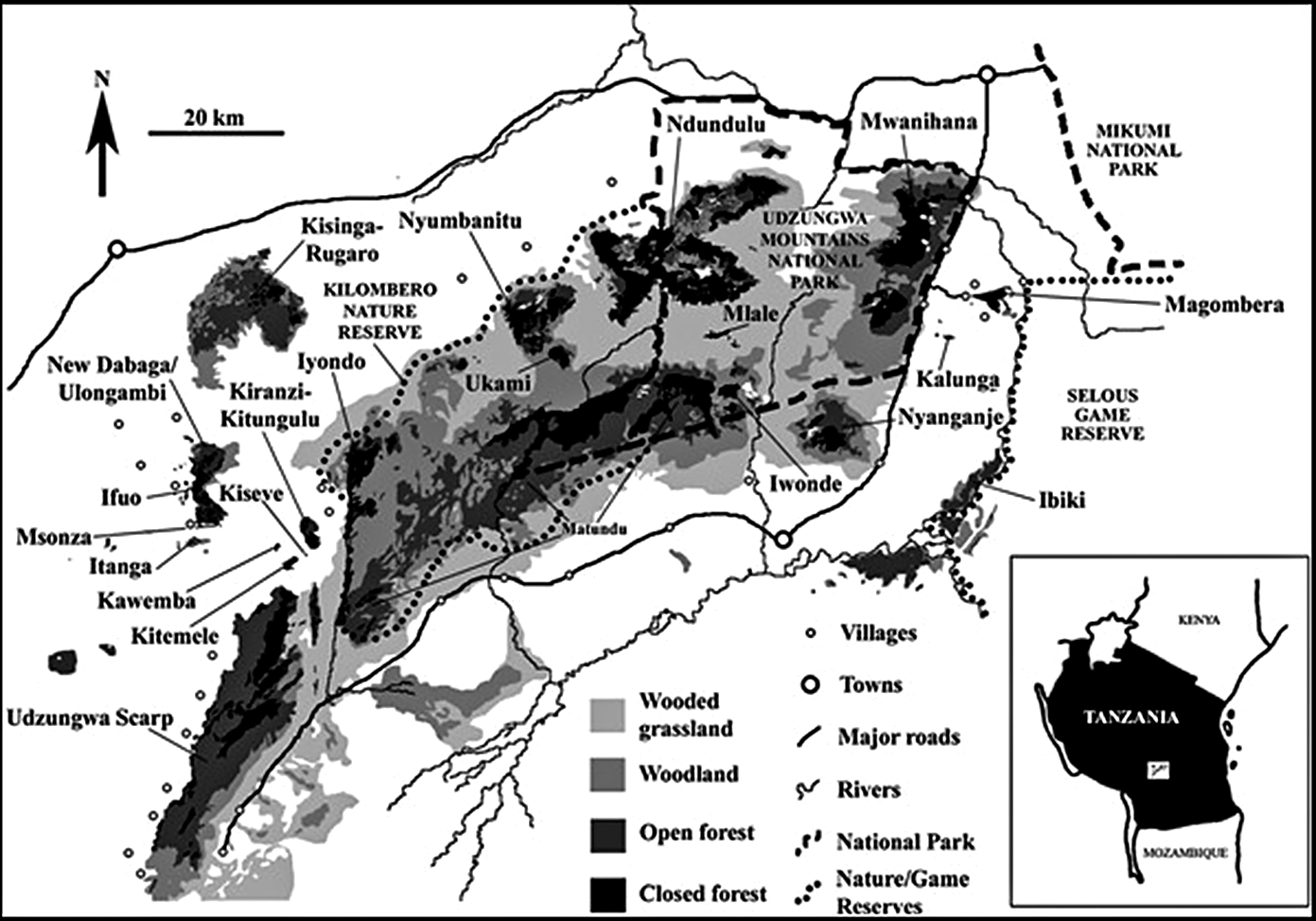
Figure 1. Forests in the Udzungwa Mountains based on Landsat imagery (from Marshall et al. Reference Marshall, Jørgensbye, Rovero, Platts, White and Lovett2009). Areas of unclassified habitat are mostly agriculture and bushland.
Methods
Field sites
Ndundulu-Luhombero forest: Data collection was undertaken from 9 November to 1 December 2016 from three campsites. This forest extends from the mountains of the Ndundulus in the west beyond Mt. Luhombero in the east, the highest peak in the Udzungwas at 2,576 m (see Figure 1). The evergreen forest covers an estimated area of 171 km2 between 1,350 and 2,400 m (own calculations using 2D World Map) of which 152 km² are within the known altitudinal range of the partridge. Our area calculations are based on a flat projection and close to the 161 km² flat projection (Marshall et al. Reference Marshall, Jørgensbye, Rovero, Platts, White and Lovett2009, Jensen et al. Reference Jensen, Dinesen, Hansen, Moyer and Mulungu2020) based on satellite imagery and verifying canopy cover for about 50% of the forest area from ground survey and aerial overflights. The true extent of the forest area will be larger due to the undulating mountain terrain including forest-covered mountain ridges and steep slopes. The eastern part of the forest is approximately 100 km² and situated within the Udzungwa Mountains National Park. The park comprises 1,990 km² of the Udzungwas (Marshall et al. Reference Marshall, Aloyce, Mariki, Jones, Burgess, Kilahama, Sawe, Nashanda, Massao, Rovero and Watkin2007; Figure 1).
The western forest edge is situated approximately 8 km from the village of Udekwa (centre) and approximately 11 km from the village of Ikula in the north-west. These two villages are the closest human population centres to this forest, but scattered farms are located closer to the forest edge. The Tanzanian National Park Authority (TANAPA) has a ranger post in the grassland between Udekwa and the forest edge about 11 km before the park boundary.
The forest varies in structure and tree species composition over short distances depending on soil and orientation (Frontier-Tanzania Reference Tanzania, Doody, Howell and Fanning2001a). Disturbance from African buffalo Syncerus caffer and elephant Loxodonta africana now occurs in the eastern part of the forest near or within the Udzungwa Mountains National Park (formerly they were much more widespread; Frontier-Tanzania Reference Tanzania, Doody, Howell and Fanning2001b, authors’ pers obs.).
The Nyumbanitu forest: Data collection was conducted from 25 November to 3 December 2018 from two campsites. The area of closed canopy montane forest is estimated to be 28 km² between 1,350 and 2,350 m and (Marshall et al. Reference Marshall, Jørgensbye, Rovero, Platts, White and Lovett2009, Jensen et al. Reference Jensen, Dinesen, Hansen, Moyer and Mulungu2020; see below). The entire forest is included in the Kilombero Nature Reserve and outside the National Park (Figure 1). The forest edge is approximately 5 km from Udekwa (centre) to the north and the larger village of Ifuwa approximately 12 km to the west of the forest (Fig. 1).
The two surveyed forests are separated by approximately 5 km of grassland uninhabited by people and consist of montane evergreen forests above 1,350 m intermixed with drier deciduous forest and areas of mountain bamboo Sinarundinaria alpina especially on local peaks or ridges and at higher elevations. Distinct tree communities included Hagenia spp. and Tecomaria nyassae dominant at higher altitudes and Neoboutonia, Aphloia, Afrocarpus, and Cassipourea at mid-altitude and with Cola and Craterispermum at several altitudes (Frontier-Tanzania Reference Tanzania, Doody, Howell and Fanning2001a). The evergreen forests cover steep mountain terrain and comprise tall trees up to 40–50 m but with thick forest and shrub in many places and develop into shrubby and tall grass vegetation towards the edges surrounded by large fire-maintained grassland. Hunting is strictly prohibited in both forests.
Although the long-term presence of edaphic montane grasslands is documented in the Eastern Arc (Finch and Marchant Reference Finch and Marchant2010), large areas of secondary grassland in the Udzungwas separate the forest tracts and are a result of relatively recent clearing and burning of the forest by humans for agriculture (Newmark Reference Newmark1998, authors’ pers. obs.). Although such activities have taken place since the arrival of Bantu agriculturalists in the area several thousand years ago, most of the forest loss and isolation of forests patches is expected to have occurred, although not continuously, within the last 200 years (Kjekshus Reference Kjekshus1977, Schmidt Reference Schmidt, Hamilton and Bensted-Smith1989, Newmark Reference Newmark1998).
Mean annual rainfall in the Udzungwas along the south-eastern scarp is c.2,000 mm, decreasing to 900 mm on the western plateau (Ehardt et al. Reference Ehardt, Jones and Butinski2005). At Mwanihana in the east where there is a continuous elevational range of moist forest from 450 to 1,760 m mean annual rainfall at the foot of the escarpment has been measured at 1,747 mm (at 366 m altitude with 14 years of records) (Lovett Reference Lovett1996). Rainfall on the escarpment slopes is higher, probably 2,000–2,500 mm per year. Rainfall and local climate vary between years, as does the dry season, often with periods of drought. The wet season starts in November, lasting to May, with on average greater than 100 mm of rain each month (Lovett Reference Lovett1996). Temperatures drop rapidly with altitude in forested areas with a lapse rate of about 0.6˚C per 100 m (Pocs Reference Pocs1976).
Data collection
To document the presence of forest partridges, we used point counts (Sutherland Reference Sutherland1998, Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000) and playback of the advertisement call at the observation point. During previous testing of the method by FPJ and EM, the partridge was found to react strongly to playback. Therefore, playback was regarded as the only feasible way to collect standardized and adequate data because the species is very elusive and difficult to observe (Allen et al. Reference Allen, Finkbeiner and Johnson2004, Fuller et al. Reference Fuller, Akite, Amuno, Fuller, Ofwono, Proaktor and Ssemmanda2012). The period between November and December was chosen for the fieldwork because it is the onset of the breeding cycle (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994), and the partridges segregate into pairs. Only males are expected to defend territory and reply with their advertisement call to playback (McGowan Reference McGowan, del Hoyo, Elliot and Sargatal1994).
During testing of the playback method, we found that calling forest partridges could be heard to a distance of c.100 m. To avoid double recording, we therefore spaced the observation points at least 200 m apart, measured in the field. To record the partridge at each point, we played the advertisement call for two minutes, followed by two minutes of silence repeated by playback for 30 seconds followed by another 30 seconds of silence. Only birds recorded from the point within these five-minute intervals were used in our data analysis.
Three of the authors (LD, FPJ and EM) conducted the fieldwork with a local guide. We moved as a team because it is difficult to navigate in the dense forest. We undertook point transects using routes in different directions from camp to cover as large a forest area as possible. Preliminary routes of transects were drawn on a map before we began the count. However, in some cases we deviated from the transects due to topographical barriers or dense forests. The location of the point counts is shown on the map (Figure 2). Data collection started in the morning and usually stopped at noon, but in some instances, we continued into the early afternoon to conduct as many counts and cover as large an area as possible. When males were responding to the playback, we estimated the distance to the bird when first heard. We feel rather confident that our estimations were within ±10 m accuracy because we cross-checked our independent estimates between three observers, and moreover, estimates were based on the extensive experience from conducting an earlier playback survey in a comparable census of the endemic Junin Rail in a marsh in the high Andes (Dinesen et al. Reference Dinesen, Chamorro, Fjeldså and Aucca2017). We covered the species’ known altitudinal span from the forest edge at c.1,350 m up to 1,950 m but also included a number of survey points up to 2,060 m.

Figure 2. Distribution of point records of the Udzungwa Forest Partridge in the surveyed part of the Ndundulu-Luhombero and Nyumbanitu forests in 2016 and 2018 with no records = small black dot; one responding individual (n = 36, white circle with small black dot); and two responding individuals (n = 13, circle with large black dot). The survey points provide an overview of the routes undertaken. The counts in the Ndundulus were primarily conducted using existing animal trails, which eased access. In the Nyumbanitus few trails existed due to the lack of large mammals. Thus, access was more difficult and cutting a path was necessary in some cases.
At each point, we noted the date and time and the exact position and altitude (using GPS Garmin etrex 20). The following data were estimated to characterize the habitat: 1) maximum canopy height (i.e. tallest trees in metres), 2) percentage forest canopy cover, including the cover of lower trees and bushes (understorey), 3) average slope in degrees, 4) the portion of the ground covered by a) dead forest leaves (leaf litter), b) grass, but noting bamboo vegetation separately, c) sedges Cyperus spp. as well as d) ginger family: Afromomum vegetation. Since these data were recorded at the survey point, they did not necessarily correspond to the location of the responding partridge. Nevertheless, we find that this information most likely represents an indication of the bird’s habitat.
Signs of illegal hunting inside the forests, such as snares, were noted, including their position, and further information on hunting was obtained from our local guides. Moreover, the indications of the presence of elephants and buffalos were recorded as an indicator of general hunting activity. Habitat change around the campsites used during the first field visits in the 1990s (LD and EM) and today were noted as well.
Data analysis and the extension of suitable habitat
We estimated the population size by simply calculating the density (males/km2) in the area we covered (number of survey points x the area of a survey point). We then multiplied this density with the suitable forest area within the altitudinal range where we recorded the partridge. Finally, we multiplied the number by two, assuming that the number of females is at least the same as males (see Results). The collected data did not meet the underlying assumptions of a distance sampling analysis, because only a few individuals were recorded close to the point (Figure 3). We do not have specific data on the sex ratio of the species. We also calculated the forest extension (area in km2 2D) and the distance from each survey point to the nearest village using Arc GIS software and World Map.

Figure 3. Distribution of the Udzungwa Forest Partridge in the Ndundulu-Luhombero forest. White circles with small or large black dots represent positive records. Black line outlines the forest part in northwestern Ndundulu where the partridge may be absent. Approximately 71 km2 of the Ndundulu Luhombero (171 km2) evergreen forest is situated west of (outside) the National Park.
To identify the habitat parameters determining the presence/absence of Xenoperdix we fitted logistic models with all combinations of explanatory variables. These variables were altitude, leaf litter, sedge cover, grass cover, shrub cover, Afromomum vegetation cover, canopy cover, canopy height, and distance to a village. The leaf litter and grass cover variables were zero-inflated. Therefore, we converted the variables to binary character states (i.e. presence/absence of leaf litter and grass cover). To minimize issues with overfitting, a model could maximum contain five explanatory variables (one for every 10th Xenoperdix record). We then used Akaike Information Criterion with correction for small sample sizes (AICC) to select the best fitting models. Following Burnham and Anderson (Reference Burnham and Anderson2002), models for which ΔAICC was ≤2.0 compared to the lowest AICC were considered equally fit – hereafter called minimum adequate models (MAMs). We found no single best combination of explanatory variables as our analyses identified 13 MAMs. Therefore, we averaged the estimates from all model combinations weighted by Akaike weights (wi). We additionally present the summed Akaike weights for all models containing a focal predictor variable (Σwi; Burnham and Anderson Reference Burnham and Anderson2002). This metric ranges from 0 to 1 and is called the variables’ relative importance. All model selection and averaging were conducted using the ‘MuMIn’ package in R (Barton Reference Barton2015).
Finally, we compared the habitat composition of Ndundulu-Luhombero forest with Nyumbanitu. For this purpose, we merged the data from the two sites and used principal component analysis (PCA) on all explanatory variables. The first two principal components jointly explained 40.1% of the variation in the data. The low percentage means that we can only use the data as a crude assessment of the habitat differences. As the Nyumbanitu forest is closer to the village than Ndundulu-Luhombero, we conducted a second PCA after removing the ‘distance to village’ variable. The first two principal components were plotted using the R package ‘ggbiplot’ (Vu Reference Vu2011).
Results
Survey results
In the Luhombero-Ndundulu forest the Udzungwa Forest Partridge was recorded at 49 (29%) out of 168 points. A total of 62 responding males were recorded; only two birds were actually seen. One male responded at 36 survey points and two at 13 survey points (Figure 2).
We had records between 1,360 m and 2,060 m (Table 1), which is an expansion of its known upper altitudinal range from 1,900 m (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994, Fuller et al. Reference Fuller, Carroll and McGowan2000). Moreover, there were records of the partridge from all 100 m altitudinal bands between 1,300 and 2,100 m, although fewer between 1,500 and 1,700 m (Table 1) for which we have no immediate explanation. Partridges were recorded from most parts of the surveyed forest (Figure 2) at varying densities except from the north-western part of Ndundulus closest to the village of Udekwa (Figure 3; see discussion).
Table 1. The number of playback points at different altitudes, forest partridges recorded, and percentage of points with positive feedback in Ndundulu-Luhombero and Nyumbanitu forests.

We found the partridge to occur at an average density of 12.45 males per km2 (SE ±1.63) in the surveyed parts of the Ndundulu-Luhombero forest. Average densities ranged from 10.19 males per km2 (±SE 3.99) within the National Park to 14.57 males per km2 (SE ±2.81) in the northern parts of the forest (Figure S1 in the online supplementary material). These estimates were not affected by reduced bird activity during the afternoon. After excluding survey points initiated after 15h00, the average density changed to 12.61 males per km2² (SE ±1.64; Figure S2, Appendix S1). The species has also previously been recorded in the north-eastern parts of the forest (WWF 2011) and on the southern slope of the Luhombero peak inside the National Park (Fuller et al. Reference Fuller, Carroll and McGowan2000, Butynski and Ehardt Reference Butynski and Ehardt2003), suggesting the species does occur throughout the Ndundulu-Luhombero forest.
Using a detailed GIS calculation of the extension of the forest within its altitudinal range, we estimate the male population to number between 840 and 1,930 individuals in 136 km2 of the Ndundulu-Luhombero forest. This estimate is based on the range of male densities outlined in Figures S1, S2, Appendix S1 (i.e. 6.20-17.66 males per km2). There are no studies of the sex ratio of the Udzungwa forest partridge. However, the plumages of the sexes are very similar, suggesting a 1:1 ratio (McGowan Reference McGowan, del Hoyo, Elliot and Sargatal1994). Thus, by assuming equal numbers of males and females, the total population estimate becomes 1,680-3,860 individuals. Within the last two decades, the partridge has been documented throughout this forest, which is generally undisturbed by human activity. However, an area of 16 km2 in the north-western corner of the Ndundulu-Luhombero forest where no partridges were recorded was deducted from the estimated total forest area of 152 km2. The partridge generally occurs in flocks of up to seven birds outside the breeding season – presumably family groups - and segregate in pairs from November to breed (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994). Hence, the population at the time of the survey is assumed to comprise birds one year old or older and regarded as mature individuals.
Because so few partridges were recorded closer than 20 m from the survey point, some birds were probably disturbed by the recording team (Figure 4). However, they are expected to remain inside the survey area and to be recorded, but further away from the point. On the contrary, a potential bias also results from birds that are attracted by the playback and move towards the recording team before calling back (see Fuller et al. Reference Fuller, Akite, Amuno, Fuller, Ofwono, Proaktor and Ssemmanda2012). The lower numbers we recorded in the 80–100 m zone (Figure 4) could indicate that we were subject to this bias. This bias is limited by estimating the distance when a bird was first heard. The majority of the males responded soon after the initiation of playback. On two occasions, birds responded in the last half-minute of the five minutes count after a second playback session. By using a radius of 100 m in calculating density, we provide for a more conservative estimate (compared to using 80 m, within which the majority of the males actually were recorded).

Figure 4. Distances from the survey point to Xenoperdix within the Ndundulu-Luhombero (n = 60; 2 bird records missing distance).
In general, perdicine birds are known to be more vocal in the morning and afternoon (see McGowan Reference McGowan, del Hoyo, Elliot and Sargatal1994). In the Ndundulu-Luhombero forest, where we recorded the partridge, 92% of the counts were undertaken before 13h00 and 80% before 12h00. The latest responding partridge on a day was at 14h16 hours and the latest count 15h35. Thus, we generally aimed to undertake most point counts before noon. However, some transect counts were continued into the afternoon to cover a larger area and to collect more data. In the Nyumbanitus with no partridges recorded, we spent more time in the afternoon to search for the partridge. Here 58% of the point counts were undertaken before 12h00 and 71% before 13h00 and the latest count here was conducted at 17h40. We believe that the far majority of birds have responded to playback regardless of the time but the continuation of counts late in the day may provide an underestimate of the true number.
No partridges were recorded in Nyumbanitu, although we spent two weeks of fieldwork here and undertook 98 playback sessions (Figure 2). However, we did not manage to cover the full range of this forest; hence a site selection bias cannot be excluded (Fournier et al. Reference Fournier, White and Heard2019). It was, however, a surprise that we did not encounter any partridges. Our point transects covered the altitudinal range between 1,400 and 1,900 m (Table 1) in areas where LD and EM recorded the species in the 1990s and which included pairs with chicks. Subsequent visits by EM in 2005 and 2012 revealed that the partridge was present but in very low numbers i.e. only single records at 1,500 m and 1,800 m. Our results suggest the species is absent currently and may have been extirpated within the last few years primarily due to hunting (see Discussion).
Habitat parameters and exploitation
Most survey points were within the evergreen forest, with only a few at forest edges and none in the grassland where the species has never been recorded. Our results show that the occurrence of the partridge correlated most with leaf litter from the deciduous forest trees and the presence of 0.5–1 m tall sedges on the forest floor. However, the model selection procedure identified 13 “best fitting” model, which indicate overall poor model fit (Table 2, Table S1). This result implies that the partridge may have few preferences for specific microhabitats within the Ndundulu-Luhombero forest and that probably the entire evergreen forest area is environmentally suitable. It is a surprise that numbers are correlated to a more open canopy, which is assumed to be a methodological artifact (Table 2; see Discussion). Neither understorey woody shrubs, terrain slope, or village distance were significant for the partridge’s presence in the Ndundulu-Luhombero forest.
Table 2. Model selection and averaging logistic models results predicting the presence/absence of Xenoperdix within the Ndundulu-Luhombero forest. Σwi: summed Akaike weights for all models containing the explanatory variable, also called the variables’ relative importance. Averaged: the standardized coefficients averaged across all models containing the explanatory variable, weighted by Akaike weights (wi). Minimum adequate model (MAM): the standardized coefficients of variables present in the model with the lowest AICc. NMAM: number of minimum adequate models. See methods for details regarding the explanatory variables

The average cover of leaf litter at the survey points where the partridge was recorded was 92 % (60–100%; n = 48), and the grass covered 2.3%; n = 49. The average estimated coverage of sedges on the forest floor providing cover for the birds was 49% in the records with the presence of the partridge (0–100%, n = 48). Our transect counts in the Ndundulu-Luhombero forest were undertaken with an average minimum canopy cover of 65 % (30–100%; n = 48) and with a middle storey layer contributing further to provide for a forest floor with dead leaves and no grass or herbs.
Principal component plots comparing the habitat composition in Ndundulu-Luhombero forest with the Nyumbanitu forest revealed little difference in partridge habitat (Figure 5). This result corresponds to our visual impression of the two forests with regard to what we considered optimal partridge habitat in terms of e.g. levels of foliage and sedges on the forest floor.

Figure 5. Principal component plots comparing the habitat compositions in Ndundulu-Luhombero forest and Nyumbanitu. Panel A comprises all habitat variables plus ‘distance to village’, whereas B has ‘distance to village’ removed. Arrows represent the strength and direction of relationships between each variable and the two first principal component axes.
Visual observation of the forest structure by LD and EM at two campsites c.500 m from the forest edge at the beginning of the 1990s compared to 2016 and 2018 revealed a change in forest structure. At one campsite in the Nyumbanitus the closed canopy forest was converted to bamboo with a uniform layer of bamboo leaves on the ground, and at the other site, evergreen forest had turned into a thick shrub with only scattered tall trees. It is believed that it is caused by fires in combination with a forest edge effect causing a retreat of forest habitat. According to our findings of the habitat requirement of the partridge in the current study, these habitat types at the forest edges are thus becoming less favourable. In the 1990s, we had recorded chicks or juveniles at both of these sites. Table 3 compares the frequency of partridge recordings with the distance to the nearest village. It shows that we did not record partridges closer than 12 km to a population centre while the recordings were relatively consistent at around 40% further away (12–19 km).
Table 3. Distance from Udekwa village center to survey point in the two forest fragments of Ndundulu-Luhombero forests and with positive records (48) and numbers (n = 61) of the Udzungwa Forest Partridge (one record excluded). Note that there is no forest closer than 7 km to the village.

In the Ndundulu-Luhombero forest, we saw few indications of hunting with only a single snare which was probably targeted at ground-dwelling birds or small mammals. The grassland that surrounds the forest at 1,800 m was recently burned locally most probably due to illegal hunting. This prevents forest regeneration and may also have a negative effect on the quality of the forest edge habitat. However, we have no data to support this. In the Nyumbanitus there were many indications of hunting activities in the form of snares targeting small and medium-sized birds and mammals. In the 1990s we recorded both elephant and buffalo in this forest but during the present survey we did not observe signs of any of these large mammals. Hunting may be seasonally concentrated e.g. outside the wet season, and our guides reported that intensive hunting took place in Nyumbanitu by teams with shotguns and dogs.
Discussion
Conservation status
Extensive surveys in other Udzungwa montane forest over the last four decades has failed to record the forest partridge from other forest tracts than Ndundulu-Luhombero and Nyumbanitu (Jensen and Brøgger-Jensen Reference Jensen and Brøgger-Jensen1992, Fjeldså Reference Fjeldså1999, Butynski and Ehardt Reference Butynski and Ehardt2003, Dinesen et al. Reference Dinesen, Lehmberg, Rahner and Fjeldså2001, Jensen et al. Reference Jensen, Dinesen, Hansen, Moyer and Mulungu2020, Hansen pers. comm.). This study suggests that the partridge is now confined to only one forest (Ndundulu-Luhombero) and presumably extirpated in Nyumbanitu within the last few years.
In the 1990s, the population was roughly estimated at 3,700 birds including a Nyumbanitu population of c.450 individuals (Dinesen et al. Reference Dinesen, Lehmberg, Rahner and Fjeldså2001). However, these assessments were based on visual observations of birds at random trips in the forests and not systematic playback, as in this study, which is much more effective in detecting the partridge. Today, we estimate the population to c. 2,800 birds (1,680–3,860) within now less than 150 km2 of montane forest although this figure is not directly comparable with the previous estimate as mentioned due to the different methods. Our findings suggest that the species range is significantly reduced since its discovery in the early 1990s. Although it was never found to be common in Nyumbanitu, the loss of this population as well as in the north-western part of the Ndundulus suggests that also the total population is now smaller.
The eastern half of the partridge’s distribution in Ndundulu-Luhombero is inside Udzungwa Mountains National Park. The other half of this forest, situated closer to populated areas, is outside, but included in Kilombero Nature Reserve. However, it is still guarded by staff from Tanzanian National Parks (TANAPA) because they have a ranger post situated in this area. The lack of records of partridges from the montane forest closest to a village (8–12 km away) may suggest that human activities in the forest involving hunting could have an impact on the remaining population. Thus, we did not record forest partridges in the north-western part of Ndundulu-Luhombero comprising 16 km2 of closed evergreen closest to the villages where comprehensive logging activities was observed 22 years earlier (Figure3; Dinesen and Lehmberg Reference Dinesen and Lehmberg1996), and which is outside the patrol area of TANAPA rangers.
Our findings suggest that the partridge should be maintained as ‘Endangered’ (IUCN 2017, BirdLife International 2019). Unfortunately, the species is moving towards the category of ‘Critically Endangered’, i.e., facing an extremely high risk of extinction (IUCN 2017). The remaining population will be vulnerable to stochastic events such as humans, epidemics, or possibly access to new predators following increased human activity levels or poaching for larger game and where the partridge is snared on a random basis. The breeding ecology of the partridge is poorly known, and its nest has never been found. Flock size in what is expected to be family groups range between one and eight birds with an average of 3.4 birds in the study in 1991-92 (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994). Most groups are composed of four (comprising two adults and two chicks) but several comprising three, five or six birds were also recorded. Hence, the Udzungwa Forest Partridge may have a low reproductive potential, like several other tropical forest species and much lower than many other Galliformes (McGowan Reference McGowan, del Hoyo, Elliot and Sargatal1994) and thus a single tiny population is more vulnerable to stochastic events.
Causes of change
The bird’s plumage makes it well camouflaged when foraging on the forest floor and previous surveys have found the partridge to feed on invertebrates and seeds among leaf litter (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994). This study documents the importance of a closed or semi-closed evergreen forest canopy providing a significant leaf litter with forest seeds and invertebrates. Moreover, good coverage of sedges is believed to provide cover against predators. It is not known at present which tree species provide seeds for the partridge and to which degree such trees may depend on the species as a seed disperser. The correlation in this study of partridge habitat with an open canopy does not reflect its dependence on foliage on the floor and our general impression. It may be an artifact that is blurred by a “canopy cover” provided by lower forest strata, which may have a similar effect, and for which data was not collected.
The forest habitat of the Nyumbanitus, where we did not record the partridge, still seems suited for the species (Figure 5). Because the individual forest fragments in the Udzungwas today are small and rather isolated, combined with easier access to them we believe that a crucial factor in the partridge decline is illegal hunting in the Nyumbanitus (Ehardt et al. Reference Ehardt, Jones and Butinski2005, Nielsen Reference Nielsen2011), in particular the threat posed by snares, perhaps in combination with hunting with dogs. In contrast to the Luhombero-Ndundulu forest, the Nyumbanitu forest is not guarded by staff from TANAPA. The clear indications of a large hunting pressure in Nyumbanitu as opposed to the situation in the Luhombero-Ndundulu forest, make us believe that hunting is probably a key driver of the decline and its presumable extirpation in this forest.
Our results show that about one-third of the partridge’s present distribution is outside the protection of the National Park. The designation of the park in 1992 included the eastern half of Luhombero-Ndundulu forest (see also Rodgers and Homewood Reference Rodgers and Homewood1982). Since the park boundary was established, the forest partridge and other endemic and globally threatened species have been described adjacent to but outside the park, including the highland mangabey Lophocebus kipunji (Jones et al. Reference Jones, Ehardt, Butynski, Davenport, Mpunga, Machaga and De Luca2005), the giant rufous elephant shrew Rhynchocyon udzungwensis (Rovero et al. Reference Rovero, Rathbun, Perkin, Jones, Ribble, Leonard, Mwakisoma and Doggart2008) as well as a large number of globally threatened bird species (Dinesen et al. Reference Dinesen, Lehmberg, Rahner and Fjeldså2001, Jensen et al. Reference Jensen, Dinesen, Hansen, Moyer and Mulungu2020). High numbers of endemic invertebrates must be expected as well (e.g. see Scharff Reference Scharff, Lovett and Wasser1993). The Udzungwas is a central part of a global biodiversity hotspot comprising one of the highest concentrations of endemic and threatened species on earth (Myers et al. Reference Myers, Mittermeier, da Fonseca and Kent2000).
The threat now posed by hunting in the Udzungwas is linked to fragmentation and the small size of the remaining forest fragments as well as the capacity of the authorities responsible for their conservation. The remaining population in the Luhombero-Ndundulu forest, where we found the partridge to be present and locally not uncommon, is either National Park or at least guarded by TANAPA and is the largest remaining forest fragment in the Udzungwas. Jones et al. (Reference Jones, Hawes, Norton and Hawkins2019) found that snaring activity and distance to ranger posts were significant predictors of encounter rates of mammals in the Udzungwas and a significant progression in abundance (and species richness) among mammals going from low to high conservation status i.e. from Forest Reserves to Nature Reserves to areas with National Park status.
Securing the Udzungwa Forest Partridge for the future?
We consider the Udzungwa forest partridge to be a flagship species (sensu Caro et al. Reference Caro, Doherty and O’Doherty1999, Smith and Sutton Reference Smith and Sutton2008) with a potential to raise funds and awareness for the conservation of Udzungwa forest habitats crucial to biodiversity (see e.g. Myers et al. Reference Myers, Mittermeier, da Fonseca and Kent2000). The partridge is illustrated on the cover of Important Bird Areas in Tanzania (Baker and Baker Reference Baker and Baker2002) and in tourist brochures (TANAPA 2020). However, this study shows that the species distribution is in serious decline, and there is a serious risk that it may go towards extinction without notice. An extinction may occur with a substantial delay following e.g. habitat loss or degradation (Newmark et al. Reference Newmark, Jenkins, Pimm, McNeally and Halley2017). Accumulating evidence suggests that such extinction debts pose a significant but often unrecognized challenge for biodiversity across the board of taxa and ecosystems (Tilman et al. Reference Tilman, May, Lehman and Nowak1994, Hylander and Ehrlén Reference Hylander and Ehrlén2013, Halley et al. Reference Halley, Monokrousos, Mazaris, Newmark and Vokou2016, Newmark et al. Reference Newmark, Jenkins, Pimm, McNeally and Halley2017).
Small remnants of evergreen montane forest on steep slopes away from the larger Udzungwa forests indicate that the entire mountain range historically has been forest covered (Newmark Reference Newmark1998). Bantu agriculturalists probably settled in the Udzungwa mountains several thousand years ago (Schmidt Reference Schmidt, Hamilton and Bensted-Smith1989). The large areas of secondary grassland separating Udzungwa forests patches today are most likely a result of forest clearing and burning (Dinesen et al. Reference Dinesen, Lehmberg, Rahner and Fjeldså2001) and the evergreen forest is thought to have experienced continuous degradation and fragmentation especially within the last few centuries (Newmark Reference Newmark1998, Schmidt Reference Schmidt, Hamilton and Bensted-Smith1989). Recent human population growth and immigration have meant that villages around the Udzungwas have grown considerably and new ones established as recently as in the late 19th century such as e.g. the village of Udekwa (Dinesen and Lehmberg Reference Dinesen and Lehmberg1996) next to the core partridge areas.
Thus it is most likely that the Udzungwa Forest Partridge had a wider distribution just a few hundred years ago. Today it is surprisingly absent from other Udzungwa forests (Jensen et al. Reference Jensen, Dinesen, Hansen, Moyer and Mulungu2020) with apparently suited habitat and now also in the Nyumbanitus where it was discovered 30 years ago (Dinesen et al. Reference Dinesen, Lehmberg, Svendsen, Hansen and Fjeldså1994).
Tilman et al. (Reference Tilman, May, Lehman and Nowak1994) postulated that the effect of habitat destruction may be the selective extinction of habitat specialists such as the Udzungwa Forest Partridge. The Udzungwas offers conditions for high persistence of habitat specialists due to old and eco-climatically stable mountains formed 25–100 million years ago (Hamilton et al. Reference Hamilton, Ruffo, Mwasha, Mmari, Lovett, Hamilton and Bensted-Smith1989, Griffiths, Reference Griffiths, Lovett and Wasser1993, Lovett Reference Lovett, Wasser and Lovett1993, Ehardt et al. Reference Ehardt, Jones and Butinski2005), which are a good competitor in a stable environment and may be efficient user of resources and controllers of ecosystem functions e.g. seed dispersal. However, they remain vulnerable to rapid changes.
The time it takes for an extinction debt to be paid off provides an opportunity to reverse declines (Kuussaari et al. Reference Kuussaari, Bommarco, Heikkinen, Helm, Krauss, Lindborg, Ockinger, Partel, Pino, Roda, Stefanescu, Teder, Zobel and Steffan-Dewenter2009, Newmark et al. Reference Newmark, Jenkins, Pimm, McNeally and Halley2017). An effective stop to hunting is crucial, as well as allowing natural forest regeneration and expansion of evergreen forest in the largely uninhabited areas of formerly fire-maintained grassland. The latter may take time because the partridge is dependent on a dark and humid evergreen forest environment providing leaf litter with feeding opportunities on the forest floor. Creating the conditions for increasing the partridge habitat and thereby its population including opportunities for birds to spread to new forest habitat seem to us essential for its long-term survival.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0959270921000319.
Acknowledgements
Bjørn Hermansen assisted with the preparation of maps and area calculations. The Bøje Benzon Foundation supported part of the fieldwork of LD and FPJ. JS was supported by research grant no. 25925 from VILLUM FONDEN. Søren Wium-Andersen, Louis A. Hansen, Neil Burgess and Jon Fjeldså provided comments on earlier drafts. Finally, we thank two anonymous referees for their valid comments.










