Clostridioides (formerly Clostridium) difficile is one of the most frequent causes of hospital-acquired infections in both adult and pediatric patients.Reference Langley, LeBlanc, Hanakowski and Goloubeva1, Reference Magill, Edwards and Bamberg2 The incidence and healthcare burden of C. difficile infection (CDI) in the hospitalized pediatric population has increased in the past 20 years,Reference Nylund, Goudie, Garza, Fairbrother and Cohen3–Reference Zilberberg, Tillotson and McDonald6 mostly attributed to the emergence of the new, hypervirulent strain B1/NAP1/027.Reference Toltzis, Kim, Dul, Zoltanski, Smathers and Zaoutis7 Although most children recover without long-term sequelae, CDI in hospitalized children is associated with increased mortality, length of stay, and hospital cost,Reference Sammons, Localio, Xiao, Coffin and Zaoutis5 and it is an independent predictor of subsequent colectomy and discharge to short- or long-term care facility.Reference Gupta, Pardi, Baddour and Khanna8
In adult patients, CDI is associated with discrete risk factors including advanced age, antibiotic exposure, prolonged hospitalization, proton-pump inhibitor use, immunocompromised state, and other medical comorbidities.Reference McDonald, Gerding and Johnson9 In comparison, risk factors for CDI in children are less well-defined. Notably, antibiotic and proton pump inhibitor (PPI) use have only been variably associated with CDI risk in children.Reference Sandora, Fung and Flaherty10–Reference Turco, Martinelli and Miele12 Additionally, current understanding of pediatric CDI is further complicated by the fact that up to 70% of infants <1 month and up to 2 years of age are colonized with C. difficile but do not develop clinical illness until 12–24 months of age.Reference McFarland, Brandmarker and Guandalini13 Therefore, closer examination of currently available evidence is needed to better understand the significance and implications of potential risk factors for pediatric CDI. The aim of this meta-analysis and systematic review was to evaluate the association of previously identified risk factors with CDI in hospitalized children.
Methods
All procedures used in this study were consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Reference Moher, Liberati, Tetzlaff and Altman14
Data sources and searches
Two investigators (S.A. and Z.H.) systematically searched the literature independently using the following predetermined inclusion criteria: (1) observational studies (including case control and cohort) evaluating risk factors for primary CDI, (2) pediatric patients (≤19 years), (3) study population includes >10 pediatric patients, (4) in-patients admitted to a hospital, and (5) studies evaluated >1 CDI risk factor. Studies were excluded if (1) they exclusively studied CDI in children <2 years, in which the role of C. difficile was unclear and testing was not routinely recommended,Reference McDonald, Gerding and Johnson9 and (2) if they exclusively studied CDI patients in an outpatient setting. The following databases were searched from January 1975 to August 2017: MEDLINE (PubMed), EMBASE, Web of Science, and Scopus. The following search terms were used: C. diff infection, Clostridium difficile infection, CDI, Clostridium difficile associated infection, CDAD, pediatric, paediatric, children, infants, adolescents, risk, risk factors, predictor, and marker. We also conducted a modified search from September 2016 to August 2017 including Clostridioides difficile in our search criteria, but we did not find any additional eligible studies (data not shown). The electronic PubMed search strategy is available in the supplemental appendix online.
Study selection and data extraction
A list of retrieved articles that met the inclusion criteria was reviewed by 2 investigators independently (S.A. and Z.H.). These investigators also independently extracted data from the full text of the included studies. The data collected included study design, study population, patient demographics, clinical characteristics, and identified risk factors for CDI. Any disagreement was resolved in consensus with a third investigator (A.D.). Authors were contacted if relevant information was not available for a particular study. The Cohen’s interrater κ statistics for inclusion agreement and data extraction were 0.85 and 0.90 respectively, which indicated excellent interrater agreement.
Quality assessment
The quality of the observational studies (including cohort and case-control) was assessed independently by 2 investigators (S.A. and Z.H.) using the Newcastle-Ottawa Scale (NOS).Reference Wells, Shea and O’Connell15 Studies with NOS scores >7 were considered high-quality studies, and those with NOS scores of 5–7 were considered moderate-quality studies. Any disagreements or discrepancies were resolved by consensus with a third investigator (A.D.). The Cohen’s interrater κ statistic for study quality assessment was 0.90, which indicated excellent interrater agreement.
Data synthesis and analysis
Due to the diversity of risk factors evaluated in studies of C. difficile, we decided a priori that all risk factors reported in ≥3 studies were eligible for inclusion in the meta-analysis.
For all studies, when possible, we extracted the adjusted odds ratios (ORs) and relative risks. When adjusted data were not available, crude odds ratios and relative risks with their 95% confidence intervals (CIs) were calculated from the number of events.
We decided a priori that adjusted data would be used for all meta-analyses with ≥3 studies. When adjusted data were not available for ≥3 studies, we combined the adjusted and unadjusted data. DerSimonian and Laird random-effects models were used for all meta-analyses.Reference DerSimonian and Laird16 The meta-analysis was performed using the inverse variance method to obtain pooled ORs and 95% confidence intervals (CIs). We assumed similarity between the OR and other relative measures, such as relative risk or rate ratios, because of low disease frequency and prevalence of CDI in this population. We evaluated statistical heterogeneity using the Cochran χ2 (Cochran Q) and the I2 statistic. We defined significant heterogeneity as a χ2 <0.10 or an I 2 statistic >66%.Reference Deeks, Higgins, Altman, Higgins and Green17 Moderate heterogeneity was defined as an I2 statistic between 33% and 66%. Low heterogeneity was defined as an I2 statistic <33%.
Assessment of publication bias
To check for publication bias, we generated funnel plots and used Egger’s regression asymmetry test. Where asymmetry was detected, we assessed the potential impact of the publication bias using the Duval and Tweedie nonparametric “trim-and-fill” method.Reference Duval and Tweedie18
We used Review Manager software (RevMan, version 5.3 for Windows, Oxford, UK; The Cochrane Collaboration, 2014) for our statistical analyses.
Results
Study characteristics
The preliminary literature search identified 2,032 publications (Fig. 1). After removing duplicates and screening titles for potentially relevant articles, 127 studies were considered relevant. On further screening of the abstracts of these potentially relevant studies, 56 were selected for full text review. Finally, a total of 14 articles met the full inclusion criteria and were included in the systematic review. The reasons for excluding the remaining 42 articles are listed in Figure 1.
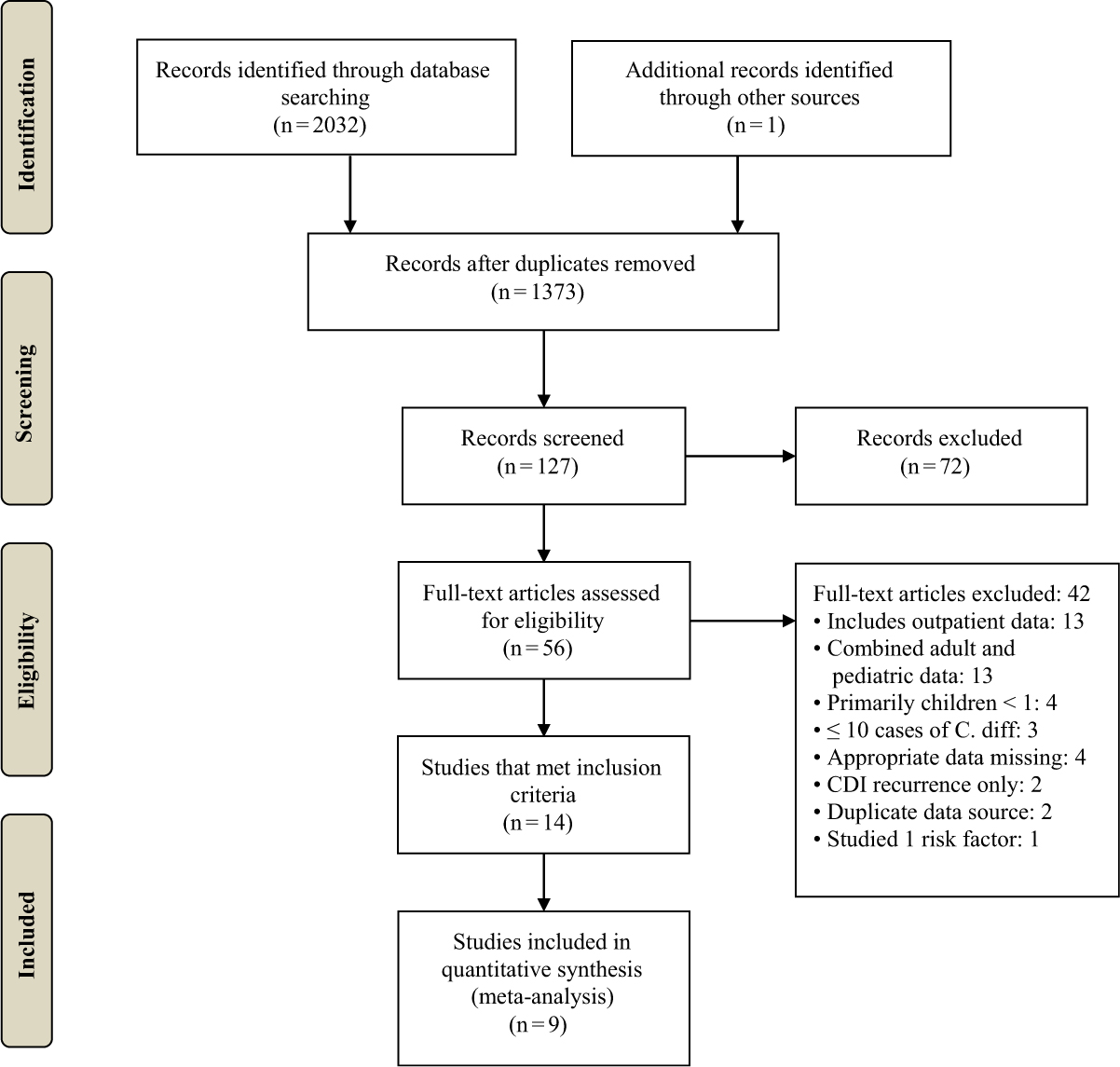
Fig. 1. Flow chart for study inclusion in the systematic review and meta-analysis.
The main characteristics of the included studies are summarized in Supplemental Table 2. The final study population consisted of 10,531,669 children, of which 22,320 patients developed CDI. In analyzing the 14 included studies, 7 were retrospective cohort studies and 6 were retrospective case-control studies, while the remaining study was a prospective cohort study. Six of the studies were conducted in the United States, 2 were conducted in Italy, with a single study was conducted in each of the following countries: Canada, China, Croatia, Japan, Spain, and Turkey. The testing methodology for CDI consisted of a C. difficile toxin assay for 10 studies. Clostridium difficile culture and/or toxin assay for 2 studies and the use of the International Classification of Diseases Ninth Edition (ICD-9) code 008.45 or other billing codes were used in the remaining 3 studies.
Quality assessment
Using the NOS scale, all included studies were identified as moderate or high in quality (Supplemental Table 1 online). Most included studies clearly identified the study population and defined the outcome and outcome assessment. Most studies identified important confounders that were used for adjustment of the association exposures and risk of CDI. We found considerable variation in the selection of available confounding variables for adjustment. A few confounding variables may not have been fully identified and recorded. The most common confounders adjusted were age, gender, and antibiotic exposure. Information on the dose and duration of antibiotic therapy prior to the diagnosis of CDI was limited. Various methods were used to identify antibiotic use, including review of patient medical records, patient prescription records, and ICD-9 codes.
Meta-analyses of risk factors for CDI
Exposure to antibiotics
A total of 7 studies reported data on prior antibiotic exposure (5 unadjusted and 2 adjusted studies). Because <3 studies provided adjusted data, we combined studies reporting the adjusted and unadjusted data. Meta-analysis of the 7 studies demonstrated a significantly increased risk of CDI with prior exposure to any antibiotic class (OR, 2.14; 95% CI, 1.31–3.52; P = .003) (Fig. 2). There was moderate heterogeneity among these studies (I2 = 57%). We also performed subgroup analysis for the adjusted and unadjusted studies. The meta-analysis of the unadjusted studies showed a significantly increased risk of CDI with prior exposure to any antibiotic class (OR, 2.34; 95% CI, 1.27–4.31; P = .006). There was significant heterogeneity among these studies (I2 = 68%). The meta-analysis of the adjusted studies also showed an increased risk of CDI with prior exposure to antibiotics, but the results were not statistically significant (OR, 1.49; 95% CI, 0.66–3.34; P = .34). There was low heterogeneity between the 2 adjusted studies (I2 = 1%). Risk factors for CDI with individual antibiotic subclasses were not reported by >2 studies and were therefore not included in the meta-analysis.
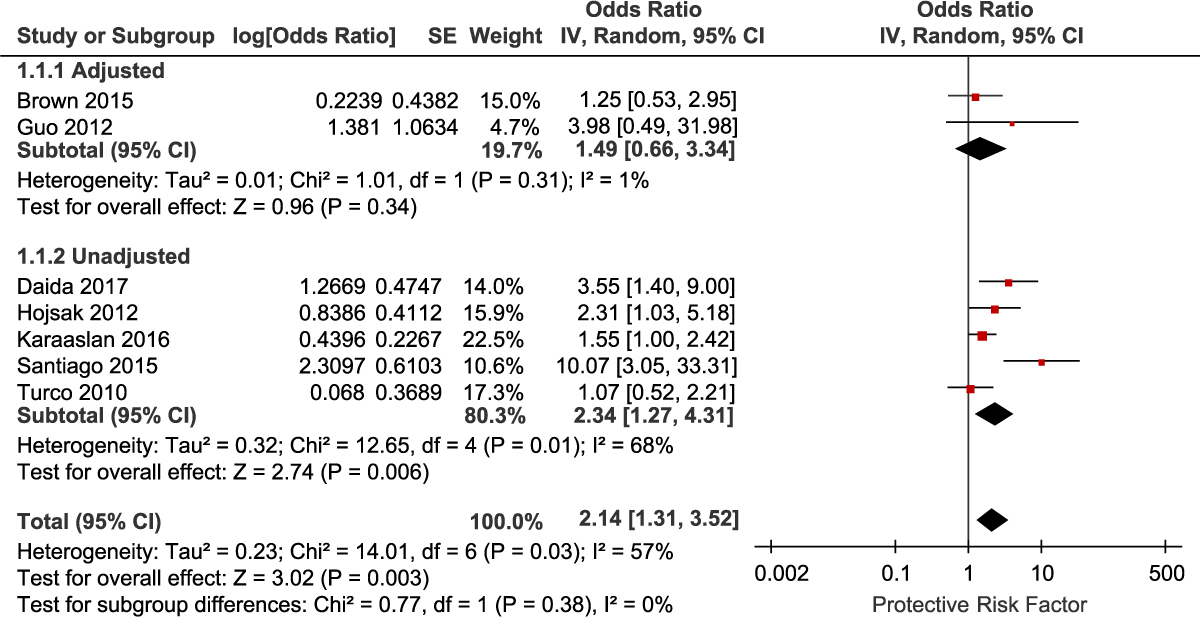
Fig. 2. Forest plot of the association between antibiotic use and CDI. Vertical line corresponds to no difference point between the 2 groups. Squares correspond to risk ratios. Horizontal lines represent the 95% confidence intervals. The diamond indicates the pooled relative risk ratios. Note. df, degrees of freedom; M-H, Mantel-Haenszel.
Gastric acid suppression
We identified 4 studies that reported adjusted data on PPI use as a risk factor for CDI. Meta-analysis of the 4 studies showed increased risk of CDI with PPI use (OR, 1.33; 95% CI, 1.07–1.64; P = .01) (Fig. 3). There was moderate heterogeneity among these studies (I2 = 36%). Adjusted data for the risk of CDI with H2 receptor antagonist (H2RA) use was obtained from 3 studies. Meta-analysis of the 3 studies examining H2RA use also showed increased risk of CDI associated with H2RA use, but the result was not statistically significant (OR, 1.36; 95% CI, 0.31–5.98; P = .68) (Fig. 4). There was significant heterogeneity among these studies (I2 = 68%).
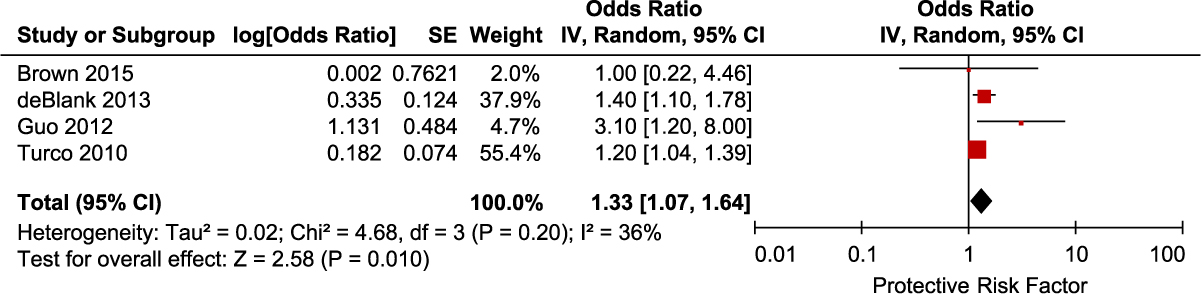
Fig. 3. Forest plot of the association between PPI use and CDI. Vertical line corresponds to no difference point between the 2 groups. Squares correspond to risk ratios. Horizontal lines represent the 95% confidence intervals. The diamond indicates the pooled relative risk ratios. Note. df, degrees of freedom; M-H, Mantel-Haenszel.
Gender
We identified 4 studies that reported adjusted data on gender as a risk factor for CDI. Meta-analysis of the 4 adjusted studies did not show a significantly increased risk of CDI associated with female gender (OR, 0.87; 95% CI, 0.74–1.03; P = .10) (Fig. 5). There was significant heterogeneity among these studies (I2 = 76%).

Fig. 4. Forest plot of the association between H2RA use and CDI. Vertical line corresponds to no difference point between the 2 groups. Squares correspond to risk ratios. Horizontal lines represent the 95% confidence intervals. The diamond indicates the pooled relative risk ratios. Note. df, degrees of freedom; M-H, Mantel-Haenszel.
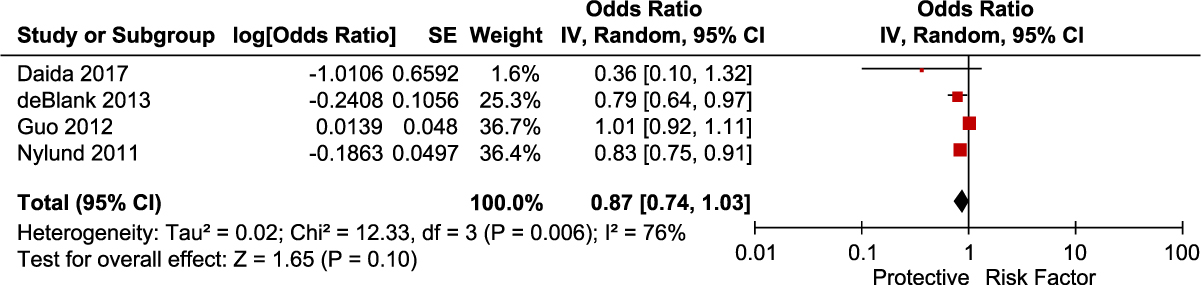
Fig. 5. Forest plot of the association between gender and CDI. Vertical line corresponds to no difference point between the 2 groups. Squares correspond to risk ratios. Horizontal lines represent the 95% confidence intervals. The diamond indicates the pooled relative risk ratios. Note. df, degrees of freedom; M-H, Mantel-Haenszel.
Other risk factors
Several additional risk factors associated with pediatric CDI were not included in the meta-analysis because they were reported in <3 studies. These risk factors and their corresponding estimated effect sizes have been listed in Supplemental Table 2 (online). Notably, underlying comorbidities that have been previously reported such as inflammatory bowel disease (IBD), solid organ transplant, and malignanciesReference Sandora, Fung and Flaherty10, Reference Kim, Smathers, Prasad, Leckerman, Coffin and Zaoutis19,Reference Pant, Anderson and Deshpande20 were also reported by multiple studies included in this review (Supplemental Table 2 online).
Publication bias
We did not assess publication bias because there were <10 included studies (for each risk factor that was meta-analyzed). A minimum number of 10 studies is suggested when assessing publication bias using a funnel plot or other more advanced regression-based methods. However, we constructed a funnel plot for 2 of the variables, antibiotic exposure and PPI use, because these risk factors had a large number of patients included from 7 and 4 studies, respectively (Supplemental Fig. 1 online).
Discussion
In this meta-analysis of 14 studies, prior antibiotic exposure and PPI use were significantly associated with increased risk of developing CDI. Children with prior antibiotic exposure have approximately twice the risk of developing CDI compared to patients without a recent history of antibiotic exposure. However, the association was not statistically significant after pooling studies providing adjusted data.
Antibiotic exposure was a significant risk factor in the pediatric inpatient population in our meta-analysis. This finding is consistent with results from the adult population, where antibiotic exposure has been observed to be the most important modifiable risk factor for the development of CDI.Reference McDonald, Gerding and Johnson9 These findings are consistent with the observation that usage of antibiotics can eliminate the natural gut microbiota and establish a favorable environment for C. difficile.Reference Dethlefsen, Huse, Sogin and Relman21 Multiple classes of antibiotics have been independently associated with CDI in the adult population.Reference Slimings and Riley22 In hospitalized pediatric patients, several antibiotic classes were independently associated with CDI. Specifically, carbapenems were identified as a significant risk factor by 2 studies,Reference Adams, Eberly, Rajnik and Nylund23, Reference Hojsak, Ferenc and Bojanić24 while aminoglycosides and cephalosporins were identified by only 1 study.Reference Hojsak, Ferenc and Bojanić24 Individual studies included in this systematic review demonstrated significant risks with use of carbapenems, aminoglycosides, and thrd- or fourth-generation cephalosporins (Supplemental Table 2 online). However, none of the individual antibiotic classes were evaluated by at least 3 different studies and were therefore deemed ineligible for the meta-analysis. Although certain antibiotic classes may in fact be independently associated with increased risk for CDI, more studies performed in the pediatric population are needed to further evaluate these associations. Specifically, design of future studies should clarify on the duration of antibiotic or gastric acid suppression treatment and identify specific antibiotic classes used.
Although our findings are consistent with current acceptance of antibiotic exposure as a risk factor for pediatric CDI, the significance of our results should be considered with caution. Due to the limited availability of studies on the risk factors of CDI in pediatric inpatients, our meta-analysis of antibiotic exposure included <10 studies for antibiotic exposure. Additionally, our analysis of antibiotic exposure is subject to confounding due to the inclusion of unadjusted studies because few studies provided adjusted data. Furthermore, the 2 studies that provided adjusted data for antibiotic exposure did not demonstrate a significant association. The loss of significance may be attributed to adjustments for age, sex, chemotherapy, and use of PPIs, H2Ras, and steroids.
Previous investigations of gastric acid suppression as a risk factor for pediatric CDI have been conflicting. Biologically, there is strong plausibility for gastric acid suppression as a risk factor for CDI because the loss of acidity may disrupt the normal gastrointestinal microbial diversityReference Seto, Jeraldo, Orenstein, Chia and DiBaise25 and prolong the survival of spores,Reference Jump, Pultz and Donskey26 both of which may predispose the host to susceptibility for C. difficile. PPI use was significantly associated with development of CDI in the pediatric inpatient population in this meta-analysis, although the effect size was small. Most recently, Oshima et alReference Oshima, Wu, Li, Fukui, Watari and Miwa27 reported a 3-fold increase in risk of CDI with PPI use in their meta-analysis of pediatric patients. The discrepancies between our results may be explained by differences in our study methodologies. Specifically, Oshima et al included studies that examined community acquired CDI in the outpatient setting and exclusively used raw data from chosen studies that were not adjusted for potential confounding factors. In contrast, we avoided unadjusted data in our analysis of PPI use, and we used only data that had been adjusted by multivariable logistic regression in the original studies. In particular, we obtained unpublished multivariate data for the association of PPI use with CDI from the authors of Brown et alReference Brown, Knoderer, Nichols and Crumby28 to include in this meta-analysis, whereas Oshima et al used unadjusted data from the same study. Nonetheless, the 2 meta-analyses are consistent in demonstrating some degree of association between PPI use and CDI in the pediatric population.
For many of the risk factors assessed by the included studies, we were unable to pool the data due to either an insufficient number of studies or variability in reporting. For example, the presence of IBD as an underlying comorbidity was reported to be a statistically significant risk factor, but was reported by only 2 studies.Reference Nylund, Goudie, Garza, Fairbrother and Cohen3, Reference Pascarella, Martinelli, Miele, Del Pezzo, Roscetto and Staiano29 The association between IBD and CDI might be mediated by the increased use of immunosuppressive agents, antibiotics, and healthcare services, as well as the disruption of the gut mucosal barrier and flora that underlie the pathophysiology of IBD.Reference Sinh, Barrett and Yun30 Similarly, solid organ transplantation was also reported to be an independent risk factor by 2 separate studies, potentially due to the chronic immunosuppressive therapy in these patients.Reference Nylund, Goudie, Garza, Fairbrother and Cohen3, Reference Pant, Deshpande and Desai31 Malignancies in general as well as specific subtypes of tumors were reported to be independent risk factors in several studies. Again, the association may be mediated by the immunosuppressive and antimicrobial effects of chemotherapy, which was reported to be a statistically significant risk factor itself in 1 study.Reference de Blank, Zaoutis, Fisher, Troxel, Kim and Aplenc32 In general, additional studies examining these risk factors in the hospitalized pediatric population are needed to validate these findings.
Our meta-analysis has several limitations. First, relatively few studies were identified for our meta-analysis, and they were exclusively observational. To address this limitation, we searched 4 separate databases and included >2,000 articles in our original search. Unfortunately, studies of risk factors for CDI in the pediatric population are limited in nature, and they often include community acquired-CDI in combination to hospital acquired-CDI. Due to differences in the acquisition of these 2 conditions, we did not feel that using studies of exclusively outpatient CDI infections would be representative of the risk factors for hospitalized patients. As a result, the number of eligible studies was limited in our analysis. Second, contemporary practice guidelines recommend the use of nucleic acid amplification test over the use of other modalities in the diagnosis of CDI.Reference McDonald, Gerding and Johnson9 Due to the paucity of new studies of CDI in pediatric patients, however, most of the studies included in this meta-analysis are older and utilized previously accepted methods such as toxin EIA (Table 1). Third, we evaluated antibiotic exposure as a composite variable without clarifying the specific antibiotics used and duration of treatment because few studies had reported this information. In reality, select antibiotics may have greater effects on CDI compared to others.Reference Adams, Eberly, Rajnik and Nylund23 Fourth, although our meta-analysis incorporated studies representing >10 million patients overall, several studies with large sample size, particularly that of Nylund et alReference Nylund, Goudie, Garza, Fairbrother and Cohen3 did not provide data on important risk factors such as antibiotic exposure and PPI use. A final limitation is the utilization of unadjusted studies that are especially prone to bias and confounding by additional variables. We performed subgroup analyses with only adjusted studies where possible, but we felt the limited number of studies justified the use of unadjusted studies for a preliminary examination of these risk factors. Additional adjusted studies of risk factors for pediatric inpatient CDI would provide the data for a more robust meta-analysis of these risk factors in the future.
Table 1. Characteristics of Included Studies

Note. N/A, not available; RCC, retrospective case-control; RC, retrospective cohort; NCC, nested case-control; EIA, enzyme immunoassay; DRG, Diagnosis-Related Group; ICD-9, International Classification of Disease, 9th edition; PCR, polymerase chain reaction; CTA, cytotoxicity assay; LAMP, loop-mediated isothermal amplification; qPCR, quantitative polymerase chain reaction.
a Presence of billing codes for Toxin A/B EIA and/or PCR, as well as metronidazole (PO or IV) or vancomycin (PO) within the period of 1 day before or 2 days after diagnostic test.
In conclusion, we found that antibiotic exposure and PPI use may be risk factors for CDI infection in hospitalized pediatric patients. Clinicians should continue to utilize antibiotics judiciously in hospitalized patients to minimize the risk for CDI, and similar considerations may be beneficial prior to administration of PPIs. Higher-quality adjusted studies of risk factors in the pediatric population with better defined study parameters and definitions for risk factors are needed to validate these results and to further explore other potential risk factors, including the risk associated with specific antibiotic classes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.23.
Author ORCIDs
Scott Anjewierden, 0000-0001-5542-1599; Chaitanya Pant, 0000-0003-4599-7644; Abhishek Deshpande, 0000-0001-5522-2995
Acknowledgments
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Abhishek Deshpande has received research support from 3M, Clorox, and STERIS unrelated to this study. All other authors report no conflicts of interest relevant to this article to disclose.








