Ultra-processed food (UPF) is made by industrial processing or chemical synthesis, from processed substances extracted or refined from whole foods, and they are rich in additives used to imitate or enhance the sensory features of foods, such as colour stabilisers, flavour enhancers and non-sugar sweeteners(Reference Louzada, Baraldi and Steele1–Reference Monteiro, Cannon and Moubarac3). UPF is generally poor in micronutrient and fibres, but rich in fats, added sugar, salt and energy, and their packaging could be harmful to health(Reference Monteiro, Moubarac and Cannon2). The classification system NOVA (a name, not an acronym) is widely used at epidemiological level and rates foods according to the extent and purpose of processing into one of the following categories: (1) unprocessed or minimally processed foods; (2) processed culinary ingredients, processed foods and (3) UPF and drink products(Reference Monteiro, Cannon and Levy4). Excessive consumption of UPF has been associated with higher risk of metabolic conditions predisposing to increased health risk, such as obesity(Reference Rauber, Chang and Vamos5), hyperlipidaemia(Reference Rauber, Campagnolo and Hoffman6), hypertension(Reference Scaranni, Cardoso and Chor7), diabetes(Reference Levy, Rauber and Chang8) and metabolic syndrome(Reference Martínez Steele, Juul and Neri9).
Also, a numerous large prospective studies(Reference Kim, Hu and Rebholz10–Reference Bonaccio, Di Castelnuovo and Costanzo13) and meta-analyses(Reference Pagliai, Dinu and Madarena14,Reference Lane, Davis and Beattie15) provided evidence that a larger share of UPF in the diet leads to a rise in the risk of diet-related chronic disease.
The health impact of food processing has become a relevant and timely topic given the increasing volume of industrially processed food worldwide. Processed food constitutes a large part of the world’s food consumption and is remarkably high in non-Mediterranean countries, representing almost 60 % of total energies in the USA(Reference Baraldi, Martinez Steele and Canella16) and in the UK(Reference Rauber, da Costa Louzada and Steele17), 42 % in Australia(Reference Machado, Steele and Levy18) and 46 % in Canada(Reference Polsky, Moubarac and Garriguet19), with differences between adults and children/adolescents.
In a Mediterranean country such as Spain, the proportion of food that is ultra-processed is about 24 %(Reference Blanco-Rojo, Sandoval-Insausti and López-Garcia20), possibly because home cooking is part of a Mediterranean diet(Reference Blanco-Rojo, Sandoval-Insausti and López-Garcia20).
Despite the mounting epidemiological evidence at international level associating higher dietary share of UPF intake with adverse health outcomes, there is a lack of data in Italy regarding UPF consumption and also differences among socio-demographic strata, a major deficiency for developing effective public health policies.
Italy has been long characterised by a Mediterranean diet, the traditional diet of the olive tree-growing areas of the Mediterranean Sea that features whole or minimally processed foods and emphasises food preparation(Reference Poti, Braga and Qin21). The only available estimates for consumption of UPF in Italy derive from national household budget surveys collected in 1996 showing an average dietary share of UPF of 13·4 %(Reference Monteiro, Moubarac and Levy22), but individual-level consumption data are lacking. Quite recently, an analysis from the Moli-sani cohort estimated UPF consumption in the adult population of Molise, but data on children/adolescents were not available(Reference Bonaccio, Di Castelnuovo and Costanzo13).
To fill this knowledge gap, the aim of the present study was to describe the intake of UPF in Italian adults and children and to identify its main predictors. We thought that the traditionally high adherence of Italians to the Mediterranean diet might be associated to lower consumption of UPF, as compared with non-Mediterranean countries.
We took advantage of data collected by the Italian Nutrition & Health Survey (INHES study), a telephone-based survey conducted throughout Italy in 2010–2013 on 9139 participants aged 5–97 years.
Methods
Study population
The INHES cohort study is a 3-year telephone-based survey on nutrition and health specifically designed to collect information on dietary habits (quality, quantity and food patterns), food choice determinants and food health awareness of the Italian population according to geographical distribution, age, gender and socio-economic profile.
Between November 2010 and November 2013, 9319 men and women aged ≥5 years from all over Italy were enrolled. Details about this cohort have been previously described(Reference Pounis, Bonanni and Ruggiero23).
Briefly, 9106 subjects in the age range 35–79 years, recruited in the 2008–2012 wave of the Italian Cardiovascular Epidemiologic Observatory(Reference Giampaoli, Palmieri and Donfrancesco24) (participation rate 53 %, from 40 % to 85 % in the different regions), were invited to participate in the INHES survey. Once they accepted, participants were asked to invite one relative older than 79 or younger than 35 years to join the survey.
Finally, 5385 (59·1 %) from the original population and 3754 from their relatives were included in the survey for a total of 9139 participants.
The sampled subjects were distributed across four seasons (excluding Christmas, Easter and mid-August periods), and the survey calendar was organised to capture an adequate proportion of weekdays and weekend days at group level.
The recruitment was performed using computer-assisted telephone interviewing (CATI); data on diet and dietary-related behaviors, health status, common risk factors, anthropometry and health perception were collected.
For the purpose of the present study, we excluded subjects with missing values for BMI (0·2 %), smoking habits (0·4 %), socio-economic variables (occupation, education and marital status; 0·8 %) or reporting implausible energy intakes (<800 kcal/d in men and <500 kcal/d in women or >4000 kcal/d in men and >3500 kcal/d in women; 2·3 %). Finally, a total of 9078 subjects were included in the analyses.
Dietary assessment
Data on food intake were collected through a self-recorded diary, by using a computer-based 1-d 24-h dietary recall interview (24-HDR) software, and the Italian version of the European Food Propensity Questionnaire was also administered(Reference Leclercq, Arcella and Piccinelli25,Reference Illner, Harttig and Tognon26) .
For every eating occasion, subjects were asked to carefully record and recall: (a) time and place of consumption; (b) detailed description of foods (or beverages) and (c) quantity consumed and brand (for manufactured foods). Portion sizes were reported by subjects with the help of a picture booklet. Moreover, participants were asked if they were on a particular diet and if the consumption they had reported differed from their usual one.
Participants’ food consumption of single food items or recipes was ‘translated’ by the nutrition specialist during the interview into food items or recipes included in the food list of the data management system INRAN-DIARIO 3·1(Reference Leclercq, Arcella and Piccinelli25,Reference Sette, Le Donne and Piccinelli27) .
The final output database included information for the daily consumption of the 2000 single food items that were included in the software food list.
We used the NOVA classification(Reference Monteiro, Cannon and Levy4) to categorise each food item into one of the following categories according to the extent and purpose of food processing: (1) fresh or minimally processed foods (e.g. fruits and vegetables, meat and fish); (2) processed culinary ingredients (e.g. honey and butter); (3) processed foods with salt, sugar or oil (e.g. canned or bottled vegetables and legumes, canned fish) and (4) UPF containing predominantly industrial substances and little or no whole food (e.g. carbonated drinks and processed meat). For the purpose of this study, we used the latter NOVA group. We ultimately identified a total of twenty-five food groups that fell into the UPF category according to NOVA (see online Supplemental Table 1).
To calculate the proportion of energy from each group of the NOVA classification, we divided the energy content of each group by total energy intake. Quartiles of energy intake from UPF were also generated for analysis purposes.
Adherence to the Mediterranean diet in adults was evaluated by the Mediterranean Diet Score (MDS) as proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia28) and categorised into tertiles as good (MDS ≥ 5 points), average (4 points) and poor adherence (0–3 points). Adherence to the Mediterranean diet in children and adolescents was evaluated by the KIDMED index (Mediterranean Diet Quality Index) for children and teenagers(Reference Serra-Majem, Ribas and Ngo29), classified as follows: good (≥6 points, indicating an optimal adhesion to Mediterranean diet); average (4–5 points) and poor (≤3 points).
Meal patterns and eating behaviours
Meal patterns comprised both patterning of main meals (breakfast, lunch and dinner) and context of main meals, such as meals eaten out of the home, or time of consumption or eating meals in front of the television or when using PC. Information on daily amount of time spent in watching TV/using PC was also collected.
Socio-economic and psychosocial factors
Education was based on the highest qualification attained and was categorised as up to elementary school (corresponding to ≤ 5 years of study), lower secondary (>5 ≤ 8 years), upper secondary (>8 ≤ 13 years) and post-secondary (>13 years).
Present occupation was assembled into the following six groups: manual, non-manual, housewife, retired, student and unemployed. Marital status was defined as married/living in a couple, unmarried, separated/divorced and widowed.
Self-rated health was assessed through one-item question (‘in general, how would you rate your health status’), and responses were arranged along a four item Likert-type scale from ‘excellent’ to ‘poor’.
Information on psychosocial conditions during the previous 12 months were gathered by administering a standard set of questions(Reference Rosengren, Hawken and Ounpuu30).
Major adverse life events (yes/no) were assessed by asking participants whether in the past year they had experienced one or more of the following: (1) marital separation or divorce; (2) business failure; (3) major intra-family conflict; (4) death or major illness of a close family member; (5) loss of job or retirement, violence; (6) death of a spouse; (7) major personal injury or illness or (8) other major stress.
Psychological stress was assessed through two single-item questions relating to stress at work and home by asking participants how often in the past year they had felt stressed by indicating one of the following response options: (1) never; (2) sometimes; (3) most of the times; (4) often and (5) always.
Level of financial stress was defined as (1) little or none; (2) moderate or (3) high.
Perceived control on the job-related activities was assessed by asking participants to rate their level of autonomy in organising their own working days as (1) none; (2) little; (3) moderate; (4) good; (5) very good and (6) not currently working.
Finally, sleep quality was assessed through a single item question: ‘In general, how would you rate your sleep?’ with response options being: (a) restless and (b) restful.
Assessment of covariates
Urban or rural environments were defined on the basis of the urbanisation level as described by the European Institute of Statistics (EUROSTAT definition) and obtained by the tool ‘Atlante Statistico dei Comuni’ provided by the Italian National Institute of Statistics(31).
Subjects were classified as never (who has never smoked or who has smoked less than 100 cigarettes in the lifetime), current (smoking one or more cigarettes/d at the time of interview), former (who had quit smoking at the time of interview) or occasional smokers (smoking less than 1 cigarette/d at the time of interview).
History of CVD and cancer and previous diagnosis of diabetes, hypercholesterolaemia and hypertension was self-reported and categorised as no/yes.
In adults, BMI was calculated by using self-reported measurements of height and weight, calculated as kg/m2 and grouped into three categories as normal (≤25 kg/m2), overweight (>25 < 30 kg/m2) or obese (≥30 kg/m2). BMI in children/adolescents was categorised according to specific values for children considering sex and age(Reference Cole, Bellizzi and Flegal32). Sport activity was self-reported and used as a dichotomous variable (yes/no).
Statistical analysis
Main characteristics of the study population are presented as numbers and percentages for categorical values and means with sd for continuous variables (Table 1).
Table 1 Characteristics of participants from the INHES study cohort, Italy 2010–2013

Values are reported as number and percentages unless otherwise stated.
Beta-coefficients with 95 % confidence intervals (95 % CI) from multivariable-adjusted linear regression analysis were used to evaluate the association between socio-demographic characteristics, eating behaviours, psychosocial factors and dietary contribution of UPF (continuous-dependent variable).
Two models were ultimately fitted: Model 1 was adjusted for age, sex and energy intake, multivariable models 2 as in model 1 further adjusted for education, geographical area, place of residence, sport activity, occupation (adults), marital status (adults), smoking, BMI, CVD (adults), cancer (adults), hypertension (adults), diabetes (adults) and hyperlipidaemia (adults).
Missing data from categorical variables were assigned a missing indicator and were included in the models as dummy variables, similar to the way valid categories were represented.
Statistical hypotheses were tested using a two-tailed P < 0·05 level of significance.
Data analysis were generated using SAS/STAT software, version 9.4 (SAS Institute Inc.).
Results
A total of 9078 participants (53·3 % women/girls) were included in the present study with a mean age of 54·5 years (age range 5–97 years; sd ± 17·2 years).
Study participants reported an average percentage of energy intake from UPF of 17·8 % (95 % CI (17·5, 18·1); IQR: 8·3–25·0; Table 1) and a mean UPF intake of 154·8 g/d (95 % CI (151·9, 157·7); IQR: 40·0–221·6). More than three-quarters (82·2 %) of total energies derive from unprocessed, minimally or moderately processed foods (see online Supplemental Fig. 1).
Distribution of daily energy intake according to four categories of the NOVA classification across main socio-demographic indicators is reported in Supplemental Fig. 1.
Processed meat (32·5 %), bread substitutes (16·7 %) and sweet biscuits (15·2 %) were the top contributing foods to the total UPF consumed in our study sample (see online Supplemental Table 2).
Adult participants (20–97 years)
Among adults (n 8569), the average energy from UPF was of 17·3 % (95 % CI (17·1, 17·6); IQR: 8·0–24·4) for a total of 154·8 g/d of UPF eaten daily (95 % CI (147·5, 150·4); IQR: 40·0–211·2). Unprocessed or minimally processed foods provided nearly 40 % of total daily energies, processed foods an additional 24·4 % and processed culinary ingredients the remaining 19·6 % (Table 1).
The mean age of participants was 56·9 years (14·6), and they were prevalently women, residing in the Northern and Southern regions, with an average education and more frequently lived in pair and in urban areas (Table 1).
Processed meat (32·6 %), bread substitutes (17·2 %) and sweet biscuits (15·4 %) were the top contributing sources of total UPF eaten (Fig. 1).
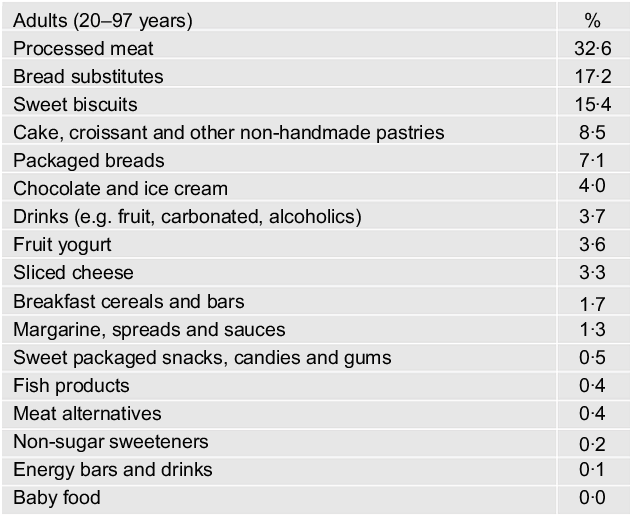
Fig. 1 Contributing food groups (%) to the total amount of ultraprocessed food consumed among adults (n 8569) from the INHES study cohort, Italy 2010–2013
Compared with the lowest (Q1), adult subjects in the highest quartile of UPF consumption (Q4) had higher intake of energy, sugar, protein, total fat, saturated fat, polyunsaturated fats, dietary cholesterol and Na, but lower intakes of total carbohydrate, fibre and monounsaturated fat (see online Supplemental Table 3).
Men (β = −1·28; 95 % CI (−1·89, −0·68); βs are dimensionless), older subjects (aged > 65 years v. 20–40 years; β = −3·10; 95 % CI (−4·40, −1·80)) and residents in Southern Italy (v. Northern Italy; β = −0·73; 95 % CI (−1·32, −0·14)) tended to consume less UPF as compared with their counterparts, while living in an urban environment was positively associated with UPF intake (β = 1·64; 95 % CI (0·87, 2·42); Table 2; Model 2).
Table 2 Demographic and socio-economic factors associated with ultra-processed food (UPF) intake in adults (20–97 years; n 8569) from the INHES study cohort, by means of adjusted regression coefficients (β) with 95 % confidence interval (95 % CI), Italy 2010–2013

* Multivariable-adjusted linear regression including age groups, sex and energy intake (continuous).
† Multivariable-adjusted linear regression including all variables in the Table.
‡ Missing data: hypertension (n 12), hyperlipidaemia (n 23), diabetes (n 21).
Housewives and retired people were less likely to consume UPF as compared with manual workers (β = −0·79; 95 % CI (−1·86, −0·29) and β = −1·87; 95 % CI (−2·83, −0·91), respectively), while unmarried, separated and widowed reported higher UPF intake as opposed to those living in pair; among lifestyles, practicing sport activities and being former smokers were associated with higher consumption of UPF (Table 2; Model 2).
Compared with poor adherence, good adherence to the Mediterranean diet was associated with a 4·86 % (95 % CI (4·20, 5·53)) lower energy intake from UPF (Fig. 2(a)).

Fig. 2 Contribution to total energy intake (%) of each food group according to its extent of processing (NOVA classification) by levels of adherence to the Mediterranean diet in (a) adults (20–97 years; n 8569) and (b) children/adolescents (5–19 years; n 509) from the INHES study cohort, Italy 2010–2013
Having breakfast and lunch out of home were associated with higher UPF intake, as compared with having these meals at home (β = 2·84; 95 % CI (1·46, 4·22); β = 3·14; 95 % CI (2·25, 4·04), respectively), and the same was true also for those individuals having meals while watching TV or PC; screen time (TV) was inversely associated with UPF intake, whereas snacking and frequent aperitifs were associated with more UPF eaten (Table 3).
Table 3 Association of meal patterns and eating behaviours with ultra-processed food intake in adults (20–97 years) and children/adolescents (5–19 years), by means of adjusted regression coefficients (β) with 95 % confidence interval (95 % CI), Italy 2010–2013

Numbers do not add up to 100 % due to missing values.
* For adults: multivariable-adjusted linear regression including age groups, sex, energy intake, geographical area, residence, educational level, occupation, marital status, smoking status, sport activity, CVD, cancer, hypertension, hypercholesterolaemia, diabetes and BMI.
† For children/adolescents: multivariable-adjusted linear regression including age groups, sex, energy intake, geographical area, residence, educational level, smoking status, sport activity and BMI.
Poor self-rated health status and reporting at least one adverse life event in the last year were associated with 5·32 % and 2·33 % higher contribution of UPF to total energy intake (95 % CI (2·66, 7·99) and 95 % CI (1·48, 3·18), respectively), as well as restless sleep (Table 4).
Table 4 Psychosocial factors associated with ultra-processed food (UPF) intake in adults (20–97 years; n 8569), by means of adjusted regression coefficients (β) with 95 % confidence interval (95 % CI), Italy 2010–2013

Numbers do not add up to 100 % due to missing values.
* Multivariable-adjusted linear regression including age groups, sex, energy intake, geographical area, residence, educational level, occupation, marital status, smoking status, sport activity, CVD, cancer, hypertension, hypercholesterolaemia, diabetes and BMI.
Finally, all types of stress showed inverse associations with the consumption of UPF, while job control positively correlated (Table 4).
Children/adolescents (5–19 years)
Among children/adolescents (5–19 years, n 509), the average energy from UPF was 25·9 % (95 % CI (24·8, 27·0); IQR: 17·0–34·1; Table 1), with a mean UPF intake of 277·6 g/d (95 % CI (259·5, 295·6); IQR: 125·1–381·0).
Processed meat (30·2 %), sweet biscuits (13·2 %), cakes and other non-handmade pastries (11·5 %) and drinks (9·3 %) were the foods mostly contributing to the total of UPF consumed (Fig. 3). Nutrient characteristics of young participants across quartiles of UPF differed for sugar, Na and energy intake that were higher in those consuming excessive UPF and for total carbohydrates and fibre that were lower (see online Supplemental Table 4).

Fig. 3 Contributing food groups (%) to the total amount of ultraprocessed food consumed among children/adolescents (n 509) from the INHES study cohort, Italy 2010–2013
Boys consumed two-point percent more UPF of the total energy compared with girls (β = 2·01; 95 % CI (0·20, 3·82)), while adolescents and former/occasional smokers tended to consume less UPF products, as compared with their counterparts. Higher educational level was associated with reduced energies from UPF in the diet (β = −2·57; 95 % CI (−4·74, −0·40)) (Table 5; Model 2).
Table 5 Demographic and socio-economic factors associated with ultra-processed food (UPF) intake among children/adolescents (5–19 years; n 509) from the INHES study cohort, by means of adjusted regression coefficients (β) with 95 % confidence interval (95 % CI), Italy 2010–2013

* Multivariable-adjusted linear regression including age groups, sex and energy intake (continuous).
† Multivariable-adjusted linear regression including all the variables in the Table.
Good adherence to the Mediterranean diet was associated with five-point percent less energy from UPF as compared with poor (β = −5·08; 95 % CI (−8·38, −1·77)) and nearly 6 % higher energy from unprocessed/minimally processed food (β = 5·98; 95 % CI (3·16, 8·80) for good v. poor adherence) (Fig. 2(b)).
Having breakfast regularly and out of home positively correlated with UPF intake, as well as regular afternoon snacking, while early lunch time was inversely related (Table 3).
Discussion
This study aimed to estimate the intake of UPF in a large cohort of 8569 adults and 509 children/adolescents residing in Italy and to investigate its major socio-demographic, psychosocial and behavioural correlates.
Average daily energies from UPF were 17·3 % and 25·9 % for adults and children/adolescents, respectively.
As expected, our UPF intake estimation in the adult sample was much lower than that reported in general populations from non-Mediterranean countries(Reference Baraldi, Martinez Steele and Canella16–Reference Polsky, Moubarac and Garriguet19), while being in line with data from another Mediterranean country such as Spain, where consumption of UPF was 24 % of the total energies eaten(Reference Blanco-Rojo, Sandoval-Insausti and López-Garcia20).
Consistently, the percentage of UPF consumed by children/adolescents aged 5–19 years in our cohort tended to diverge substantially from data collected in the UK where 65 % of energies eaten by primary and secondary school children were from UPF(Reference Martines, Machado and Neri33), and similar high dietary shares of UPF were documented in paediatric populations of the USA(Reference Neri, Martinez-Steele and Monteiro34) and Canada(Reference Moubarac, Batal and Louzada35).
Conversely, our estimations aligned with data from a Belgian cohort showing that the usual proportion of daily energy intake from UPF was 33·3 % for children and 29·2 % for adolescents(Reference Vandevijvere, De Ridder and Fiolet36) and with those provided within the SENDO project in Spain(Reference da Rocha, Rico-Campà and Romanos-Nanclares37).
UPF was mainly consumed by urban residents, young people and those practicing physical exercise, a finding aligned with others(Reference Rico-Campà, Martínez-González and Alvarez-Alvarez11,Reference Blanco-Rojo, Sandoval-Insausti and López-Garcia20) , possibly because active individuals tend to consume more frequently some highly processed foods, such as energy bars and drinks and health or slimming products.
As already documented in other populations(Reference Srour, Fezeu and Kesse-Guyot38), higher intake of UPF was associated with lower diet quality, being richer in Na, fat and poor in fibre, and inversely with the Mediterranean diet, both in adults and children/adolescents, as previously seen by others(Reference Rico-Campà, Martínez-González and Alvarez-Alvarez11,Reference da Rocha, Rico-Campà and Romanos-Nanclares37) . Since the traditional Mediterranean diet features unprocessed or minimally processed food and emphasises home cooking, its inverse association with UPF possibly accounts for the relatively lower energy from UPF in our participants as compared with much higher estimations reported in other countries, especially non-Mediterranean.
Consistently, Southern Italian regions were likely to consume less UPF, possibly because highly processed foods may have more difficulty in establishing in a dietary context characterized by a strong food heritage as reflected by the Mediterranean diet(Reference Ruggiero, Di Castelnuovo and Costanzo39).
In the last decade, several population studies reported the adverse effects of UPF on health; indeed, excessive consumption of industrially processed food was associated with elevated risk of diet-related chronic disease(Reference Martínez Steele, Juul and Neri9–Reference Fiolet, Srour and Sellem12,Reference Pagliai, Dinu and Madarena14,Reference Lane, Davis and Beattie15) and mortality(Reference Kim, Hu and Rebholz10,Reference Bonaccio, Di Castelnuovo and Costanzo13) , also through mechanisms that include altered inflammation(Reference da Silva, Felício and Caldas40).
Prospective studies following young children over time found that higher intake of UPF predicted higher total cholesterol, LDL cholesterol, tri-acyl glycerol and/or increased delta waist circumference(Reference Rauber, Campagnolo and Hoffman6,Reference Costa, Rauber and Leffa41,Reference Leffa, Hoffman and Rauber42) and supported the role of UPF in the obesity epidemic in Brazilian adolescents and adults(Reference Louzada, Baraldi and Steele1).
Among potential mechanisms linking UPF consumption to increased health risk, chemicals largely used in the packaging of these food products, such as bisphenols and phthalates, were found to promote inflammation and oxidative stress(Reference Ramadan, Cooper and Posnack43). In addition, food processing and particularly heat treatments produce neoformed contaminants (e.g. acrylamide) that are classified as genotoxic by the European Food Safety Agency(44).
More recently, a study on 139 adolescents in Iran showed a significant urinary biomarker of DNA oxidative damage associated with higher intake of UPF(Reference Edalati, Bagherzadeh and Asghari Jafarabadi45).
In the INHES cohort, both in adults and kids, processed meats were the top contributors to the dietary share of UPF, in accordance with estimations from the SUN cohort(Reference Rico-Campà, Martínez-González and Alvarez-Alvarez11) and the national Food Consumption Surveys in Belgium(Reference Vandevijvere, De Ridder and Fiolet36), differently from adults our children/adolescents tended to consume more sugar-sweetened beverages in line with others(Reference Vandevijvere, De Ridder and Fiolet36).
Among eating behaviours, regularly eating main meals out of home was associated with higher intake of UPF in adults, while for kids breakfast out of home was related to UPF intake suggesting that main foods included in the breakfast of Italian children are highly processed. Among adults, snacking likely led to consume more UPF, thus providing interesting suggestions on the type of foods preferably consumed by Italians between main meals.
Screen time while eating was directly associated with UPF intake in adults, a finding in accordance with prior evidence showing that TV watching possibly increases the amount eaten of high-density and palatable foods(Reference Blass, Anderson and Kirkorian46), while in children and adolescents such association was not observed, likely because this behaviour in our young sample is underrepresented.
An important aspect of our research is the observed association between several aspects of the psychosocial dimension and their relationship with consumption of UPF. We found that higher consumption of UPF is associated with poor self-rated health status, a finding in line with prior epidemiological studies suggesting a direct relation between UPF intake and risk of depression(Reference Pagliai, Dinu and Madarena14). We also found evidence of an association of adverse life events and UPF, in agreement with studies showing lower diet quality in stressed and neurotic individuals(Reference Schweren, Larsson and Vinke47). Finally, our findings revealed that participants reporting low sleep quality were more likely to consume UPF, in agreement with a recent study on 2499 Brazilian adolescents observing a direct relation between excessive UPF intake and poor sleep quality(Reference Sousa, Bragança and Oliveira48).
Strengths and limitations
This study has several strengths. First, results are from a large population sample of more than 9000 men and women recruited throughout Italy, with a complete assessment of diet, lifestyle and other covariates that minimise confounding. Also, our study shed light on correlates that, to date, had been less explored in other populations, such as meal patterns, eating behaviours and psychosocial factors.
Study limitations include the cross-sectional design and the telephone-based survey with potential interviewer bias and inability to use visual help. Also, the decline in the use of landlines may have resulted in an under-representation of respondents.
Furthermore, the study relies on self-reported health and dietary data that may lead to misreporting; however, data were collected by trained interviewers and each participant received by mail, beforehand, a short photograph atlas and guidance notes to estimate food portion sizes.
Confounding from unmeasured factors (e.g. additional psychological and socio-economic factors) cannot be fully ruled out. Also, dietary intakes were collected almost a decade ago, thus might not reflect the current UPF intake in the Italian population, although being the most updated data available so far, and in line with timeframes from the majority of studies in the field(Reference Baraldi, Martinez Steele and Canella16–Reference Machado, Steele and Levy18).
However, the analyses on correlates of UPF intake are independent of the time of data collection and actually provide useful information for public health policies aimed to minimize the share of highly processed food in the diet.
Finally, the NOVA classification we used is still debated, mainly because of its equivocal definition of UPF and also because it has been revised and refined over time(Reference Gibney49); however, its usefulness in nutrition research has been widely acknowledged(Reference Kelly and Jacoby50).
In any case, some caution is needed in generalising these findings to other populations.
Conclusions
Our results from a large cohort of adults and children/adolescents recruited in 2010–2013 throughout Italy showed that UPF constitutes less than 20 % of total energy intake among adults, while being approximately a quarter of the energies eaten by children/adolescents.
Such estimations are among the lowest recorded so far worldwide, but there is reason to believe that UPF intake is possibly on the rise as documented by worldwide trends(Reference Vandevijvere, De Ridder and Fiolet36,Reference Juul and Hemmingsson51) .
We did not observe substantial differences in UPF intake across socio-demographic strata, but rather identified some behavioural and psychosocial correlates that may promote increased UPF intake in the diet. In such, our findings provide relevant information to settle effective public health strategies at population levels by targeting groups at higher risk of unhealthy diets.
Finally, we observed that adherence to a Mediterranean diet, which features fresh or minimally processed foods and emphasises home cooking, possibly lowered the consumption of UPF in both adults and kids, thus potentially reducing the burden of major chronic diseases later in life.
Given the increasing dietary share of UPF worldwide and the growing epidemiological evidence on the adverse health effects of such foods, it is advisable to stress the importance of limiting UPF in dietary guidelines, as done in some(52,53) but not in the majority of countries.
Food policy initiatives to minimise consumption of UPF in the diet primarily include fiscal measures, which, however, should be accompanied by subsidies or incentives (such as VAT reduction for healthful foods) aimed at promoting purchase of healthier foods; indeed, diets rich in UPF are estimated to be cheaper than diets with a low inclusion of these products; thus, the economical affordability of minimally processed foods might be favourably improved(Reference Vandevijvere, Pedroni and De Ridder54).
Other actions would possibly include stricter regulations to reduce the advertisement and marketing of UPF, especially to children, front-of-package warning labels and targeted food policies to regulate access to and promotion of UPF in schools(Reference Popkin, Barquera and Corvalan55).
Acknowledgements
Acknowledgements: We thank the Research Group of the Cardiovascular Epidemiology Observatory/Health Examination Survey 2008 for making available the list of subjects recruited in their survey. Financial support: The enrolment phase of the INHES study was supported by a research grant from Barilla SpA. The present analyses were partially supported by the Italian Ministry of Economic Development (bando PON I&C 2014–2020; PLATONE Project – Platform for Integrated Health Life – n. F/080032/01-03/X35). E.R. is supported by Fondazione Umberto Veronesi that is gratefully acknowledged. These funders had no role in study design, collection, analysis and interpretation of data; in the writing of the report and in the decision to submit this article for publication. Conflict of interest: There are no conflicts of interest. Authorship: M.B., E.R. and S.E. designed the present research; S.C. and A.D.C. managed data collection; E.R. analysed the data; E.R., S.E. and M.B. wrote the paper; M.B.D., C.C., G.d.G. and L.I. were at the origin of the INHES study, discussed the data and critically reviewed the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committee of the Catholic University of Rome. Verbal informed consent was obtained from all subjects. Verbal consent was witnessed and formally recorded.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980021002767











