INTRODUCTION
Haematopoietic stem cell transplantation (HSCT) provides an opportunity to treat malignant and non-malignant diseases in children, but these patients experience prolonged immunosuppression in the post-transplant period that gives rise to infectious complications [Reference Bjorklund1]. Infection due to varicella-zoster virus (VZV) is an important cause of morbidity after HSCT. VZV infection in children who undergo HSCT usually occurs within the first year post-transplantation [Reference Locksley2–Reference Atkinson4]. The majority of these infections have been demonstrated to be due to reactivation of pre-existing infection. Because of significant and prolonged immunosuppression, reconstitution of VZV immunity is delayed and these patients are at high risk of having severe VZV infection in the post-transplant period. The incidence of VZV infection in children following HSCT varies from 16% to 67% [Reference Locksley2, Reference Locksley3, Reference Locksley5–Reference Han11]. Antiviral agents have been reported to reduce the risk of reactivation and/or dissemination during the period of administration by 70% after transplantation [Reference Kim12]. However, after cessation of antiviral therapy, they may not completely prevent VZV infection. Five percent of patients still developed visceral dissemination while under prophylaxis.
In this study, we evaluated the incidence of VZV infection in children and adolescents who underwent HSCT at our centre. We also analysed the risk factors and immune reconstitution profile in a case-control study.
PATIENTS AND METHODS
We retrospectively analysed VZV infection following HSCT in children and adolescents aged <21 years at Hacettepe University, Department of Pediatrics, Division of Hematology and Bone Marrow Transplantation Unit. Charts of patients who received HSCT between January 2000 and December 2006 were reviewed. Of 125 patients whose charts were evaluated, 22 patients developed herpes zoster (HZ) and five patients developed chicken pox (CP) after transplantation. For every patient with VZV infection, preferably the two following HSCT cases were taken as the control group. Patients followed for at least 6 months after transplantation were enrolled and patients who died within 3 months after HSCT were excluded. Patients with severe combined immunodeficiency (SCID) were excluded due to regular intravenous immunoglobulin (IVIG) administration pre-transplant and/or lack of conditioning regimen and lack of varicella infection history. Therefore, a control group of 34 patients (1–2 controls per case depending on availability) was derived from the remaining population using the year of HSCT as the matching criterion. The characteristics of patients who developed HZ were compared with those who did not (control group).
Data were collected by chart review and consultation with the primary doctor, nurses in the Bone Marrow Transplantation Unit and/or parents. Diagnosis of CP was based on the characteristic cutaneous vesicular lesions and associated clinical symptoms. HZ was diagnosed by the presence of vesicular lesions in a dermatomal distribution. Visceral dissemination of either CP or HZ was defined as clinical evidence of internal organ involvement in the absence of other identified pathogens. Visceral dissemination was evaluated by chest radiographs and liver function tests. The characteristics of HZ and CP infections in those HSCT recipients were determined.
Pre-transplant serum samples of patients and donors that had been previously collected and stored at −80°C were analysed. Serological tests for VZV infection were performed with the enzyme-linked immunosorbent assay (ELISA) (Varicella IgG, IgM; Trinity Biotech, USA). Pre-transplant status of VZV infection in patients and donors was based on pre-transplant serology and/or history of VZV infection determined by physicians. The presence of either a positive infection history with VZV or a positive serology was considered as positive.
Immunological and haematological evaluations at 1, 3 and 6 months were reviewed. Data was not available for each patient at every time point. Lymphocyte populations were analysed by flow cytometry using a panel of monoclonal antibodies including CD3, CD4, CD8, CD3, HLADR, CD16/56, and CD19 levels (FACScan; Becton Dickinson, USA).
Transplantation procedures
Patients received conditioning regimens as outlined in Table 1. Briefly, in the HZ group, seven (32%) patients were conditioned using a myeloablative total body irradiation (TBI)-based regimen and 12 (55%) patients using a myeloablative non-TBI-based regimen; three (13%) patients received a reduced intensity conditioning regimen. The use of TBI is generally accepted for acute lymphoblastic leukaemia (ALL) whereas in our group besides four patients with ALL, one non-Hodgkin's lymphoma (NHL), one acute myeloid leukaemia (AML) and one patient with thalassemia major received TBI as conditioning. TBI-based conditioning regimen was given to the patient with thalassaemia major before her second transplantation after the first graft failure. None of the control group received a TBI-based regimen; 28 (82%) received a non-TBI-based regimen and six received a reduced intensity (18%) conditioning regimen. Graft vs. host disease (GVHD) prophylaxis following HSCT consisted of intravenous cyclosporin A (CsA), CsA plus short-course intravenous methotrexate (MTX) and CsA plus methylprednisolone. CsA was tapered after 2 months according to the risk of disease relapse. GVHD was treated with prednisone and CsA initially, but further immunosuppressive therapy was given as required. Post-transplant granulocyte colony-stimulating factor (G-CSF) support was not routinely given to the patients. Antiviral prophylaxis with acyclovir was given for 3 months at a dose of 30 mg/kg per day p.o., if the patient could not tolerate oral treatment they were given 500 mg/m2 per day i.v., and IVIG treatment was given for 6 months after transplantation.
Table 1. Characteristics of the patients with varicella zoster infections and the control group
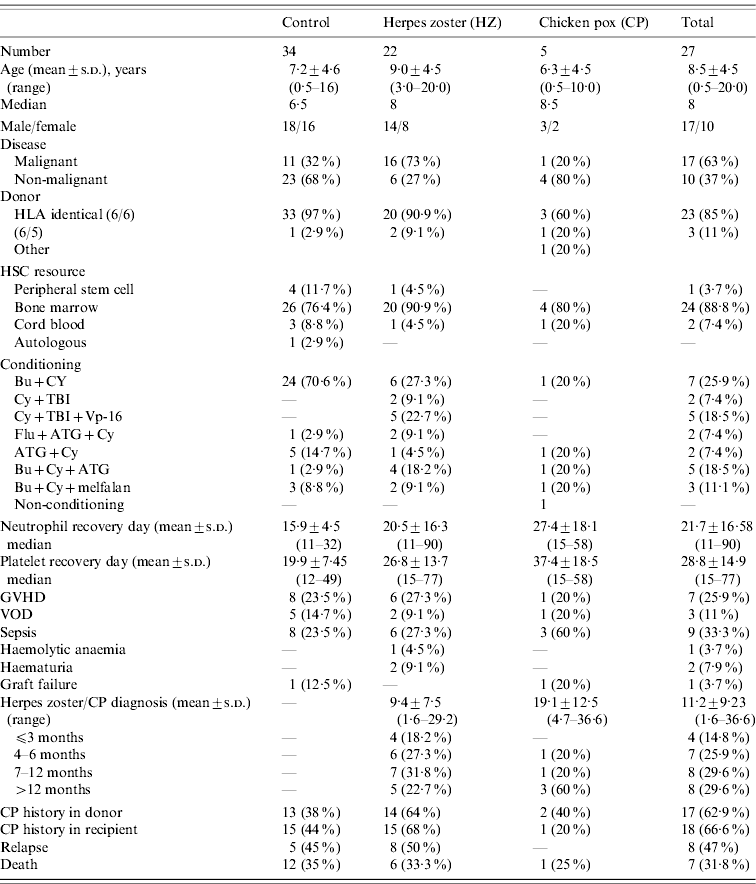
HLA, Human leukocyte antigen; HSC, human stem cell; GVHD, graft vs. host disease; VOD, veno-occlusive disease.
Statistical methods
Statistical Package for Social Sciences software version 11.0 (SPSS Inc., USA) was used for statistical analysis. The results are represented as mean±standard deviation and/or median (range) in both study and control groups. The differences between groups were analysed by Mann–Whitney U test and χ2 test or Fisher's exact test, where appropriate.
RESULTS
The cumulative incidence of VZV infection was 27/125 (20%; five CP, 22 HZ) during the follow-up period.
The mean time of onset after HSCT was 9·4±7·5 months (range 1·6–29·2) for HZ patients. The majority (17/22 for HZ) of cases occurred within the first year after HSCT and most cases (10/17) developed HZ in the early post-HSCT period within 6 months. Four patients were on acyclovir prophylaxis when HZ infection developed. Two patients were receiving methylprednisolone, nine patients IVIG treatment and 10 patients were on CsA treatment at the time of eruption. Two patients were not receiving any therapy.
A single dermatome was affected in all patients who developed HZ. The distribution of affected dermatomes was cervical region (n=9, 41%), sacral region (n=6, 27%), thoracic region (n=4, 18%), and lumbar region (n=3, 14%). Two patients developed post-herpetic neuralgia. In all cases, chest radiograph and liver function tests disclosed no evidence of disseminated zoster. No death occurred in relation to HZ infection.
Patient characteristics
HZ group
The ages of the patients in the HZ group (14 males, 8 females) ranged from 3 to 20 years (median 8 years). The majority of cases had a diagnosis of malignant disorder [four ALL, four AML, three chronic myeloid leukaemia (CML), four myelodysplastic syndrome (MDS), one NHL], while two had thalassaemia major, one adrenoleukodystrophy, one Fanconi's aplastic anaemia, and two idiopathic aplastic anaemia.
Control group
The ages in the control group (18 males, 16 females) ranged from 0·5 to 16 years (median 6·5 years). Underlying disorders included malignant disorders [n=11 (32%); three AML, one MDS, four CML, two neuroblastoma, one chronic myelomonocytic leukaemia (CMML)] and 23 patients had non-malignant disorders [seven thalassaemia major, three aplastic anaemia, four Fanconi's aplastic anaemia, two osteopetrosis, one congenital neutropenia, one α-mannosidosis, one hemophagocytic lymphohistiocytosis, one globoid cell leukodystrophy, two metachromatic leukodystrophy (MLD), one Griscelli syndrome].
CP group
CP was observed in five patients and none had a history of varicella infection. There were three boys and two girls in the CP group (median age 8·3 years, range 0·5–10). The underlying disease was malignant in one and non-malignant in four patients. The mean time of onset of CP infection after HSCT was 19·1±12·5 (range 4·7–36·6) months. Three of five children developed infection after the first year of HSCT. No patient relapsed after CP infection. There was one death directly attributable to CP pneumonia. Pre-transplant history and/or serology for VZV infection showed negativity in all patients with CP. Three of the donors in the CP group had no history of VZV infection whereas two donors had a positive history.
Donor characteristics
In the HZ group, of 22 HSCTs, 20 (91%) donor-recipient pairs were human leukocyte antigen (HLA) 6/6 identical and two were a one-antigen mismatch. On the other hand, in the control group, out of 34 transplants, 33 (97%) donor-recipient pairs were HLA identical, and one was a one-antigen mismatch. The most common stem cell source was bone marrow for the HZ patients and the control group.
Pre-transplant status of VZV infection in the HZ, control and donor groups
Pre-transplant history and/or serology for VZV infection showed positivity in 15 (68%) patients who developed HZ after HSCT. Initial serology was available in six (27%) of 22 patients in the HZ group and all seropositive patients' history were found to be positive. However, prior VZV infection status could not be determined in seven patients by history or serology. Fourteen (93%) of 15 donors of the patients with HZ had positive history and/or serology. There was no information about the remaining eight donors of patients with HZ.
Pre-transplant history and/or serology for VZV infection was positive in 15 (44%) patients in the control group and at the time of the study, none had experienced HZ infection. Initial serology was available in seven (21%) of 34 patients in the control group and 5/7 showed seropositivity with a positive history of previous VZV infection and two of the patients were seronegative with a negative history. Donor status of those 15 patients in the control group showed that 13 (86%) of 15 had a positive history and/or serology for VZV infection.
Comparison of clinical characteristics of HZ patients and the control group
Potential risk factors for the development of HZ infection in the study and control groups were compared (Table 1). Median ages of the patients at HSCT were not statistically different between groups (8 and 6·5 years, respectively; P=0·286). There were eight (36·4%) and 16 (47%) females, 14 (63·6%) and 18 (52·9%) males in the HZ and control groups, respectively. Presence of malignant disorders was significantly higher in HZ patients compared to the control group (73% vs. 32%, respectively; P=0·003).
Mean number of engraftment days were 20·5±16·4 and 15·9±4·5 for WBC and 26·8±13·7 and 19·9±7·5 for platelets in the HZ and control groups, respectively, and the difference was not statistically significant between the two groups. When the patients engrafted before 15 days were compared with those who engrafted later, there was no statistically significant difference between the HZ and control groups. No association was found between VZV infection and other clinical factors, including conditioning regimen and complications [GVHD, veno-occlusive disease (VOD), septicaemia, haemolytic anaemia, haematuria, graft failure] when both groups were compared. Six (27%) HZ patients and 12 (35%) patients in the control group died (P>0·05).
Comparison of some clinical and laboratory values in patients with a malignant vs. non-malignant condition
Of 61 patients, 28 received HSCT for a malignant disease and 17 (61%) of these 28 patients developed HZ (n=16) or CP (n=1), whereas only 10 (30%) of 33 patients with non-malignant diseases developed post-transplant VZV infection (P=0·022).
There were 16 and 11 patients with malignancy in the HZ and control groups, respectively. There were no statistically significant differences between patients with malignant disease with HZ and the control group according to clinical and laboratory values (age, gender, stem cell source, HLA compatibility, conditioning regimen, neutrophil engraftment days). However, platelet engraftment day was found to be statistically longer in the malignant group with HZ (P=0·02).
Relapse rate after HZ
There were 16 patients with malignancy in the HZ group of which eight experienced relapse, which occurred at a time interval of 7·85±6·15 (range 0·7–17·3) months following HZ. Relapse occurred in five (45%) of 11 patients with malignant disease in the control group. The time interval between HSCT to relapse was a median 14 and 16 months, respectively, in HZ patients and the control group (P>0·05). In the control and HZ groups 4/5 and 6/8 patients, respectively, died because of relapse.
Lymphocyte subsets
We investigated the association of lymphocyte recovery on the development of HZ and compared the absolute peripheral lymphocyte counts and specific lymphocyte subset analysis before and 1, 3 and 6 months after transplantation (Table 2). There were no statistically significant differences between groups in lymphocyte number or subsets at pre-transplant evaluation except for WBC counts. Initial WBC count was statistically lower in the HZ group (P<0·05). One month after HSCT, the mean percentage of CD4+ cells was statistically lower (P<0·01) and CD3+DR+ cells were higher (P<0·05) in the study group compared to the control group. Three and 6 months after HSCT, the mean percentage of CD8+ cells was found to be increased in the study group (P<0·05). CD4/CD8 ratios remained lower in the study group during the 6 months after HSCT and were significantly lower at the 6th month after HSCT compared to the control group.
Table 2. Comparison of immunological and hematological values between the study and the control group before HSCT and 1, 3 and 6 months after transplantation
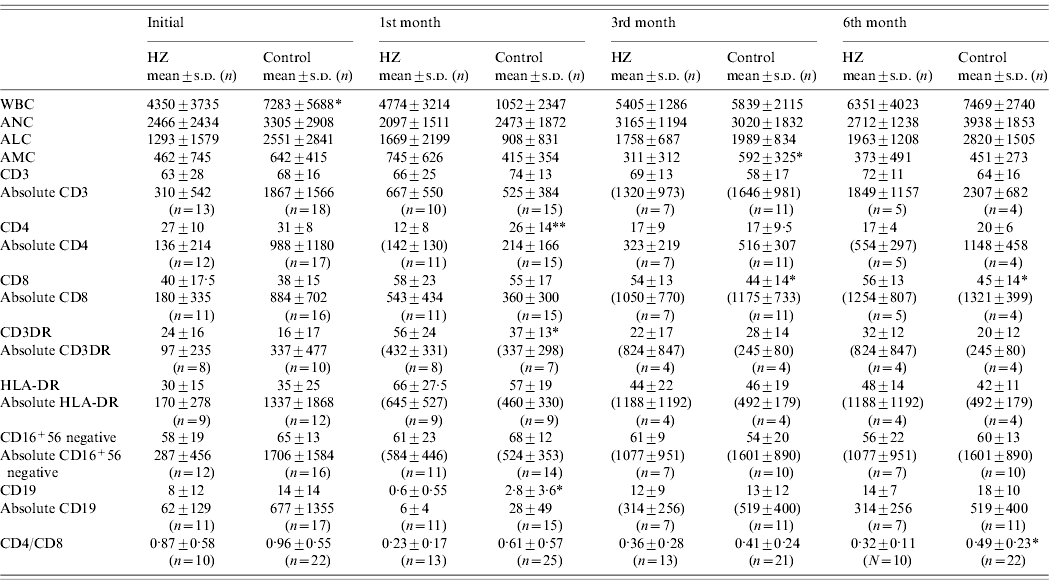
WBC, White blood cell count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count.
* P<0·05, ** P<0·01.
When the absolute numbers of peripheral lymphocyte subsets were compared, no statistically significant difference was noted between the study and control groups in CD3, CD4, CD8, CD3+DR+, CD16+56−, CD16+56·3 and CD19 subpopulations (Table 2). A comparison could not be made between patients with and without malignancy in the study and control groups due to the limited number of available patients in each group.
DISCUSSION
HZ is one of the common late infections in HSCT patients that occurs as a result of VZV reactivation in patients with a previous exposure to this virus. Typically, HZ occurs after engraftment, with the greatest risk between 2 and 10 months after HSCT [Reference Schuchter3, Reference Atkinson4, Reference Steer13]. A persistent susceptibility to reactivation of varicella, however, continues for up to 2 years but rarely thereafter (2–14%) [Reference Locksley2, Reference Tzeng10, Reference Han11, Reference Steer13]. In the present study, the cumulative incidence of HZ at 1 year was found to be 14% (17/125 patients) and at 3 years was 18% post-HSCT, which is consistent with the reported incidence in the literature, varying from 16% to 67% [Reference Locksley2, Reference Schuchter3, Reference Berman5–Reference Han11]. Without prolonged antiviral prophylaxis, the estimated median time of onset of VZV reactivation is 5–7 months. In previous studies, when prolonged antiviral suppression was not available, cutaneous and visceral dissemination rates of 23–36% and VZV-related mortality rates of 8–12% have been reported [Reference Locksley2, Reference Atkinson4]. Two randomized placebo-controlled studies indicated that 6 months of acyclovir prophylaxis after HSCT significantly reduced the incidence of varicella infection [Reference Ljungman14, Reference Selby15]. Recent studies have shown that acyclovir effectively and safely prevents VZV disease during the first year after HSCT; thus, a longer period of prophylaxis is recommended for patients under immunosuppressive treatment [Reference Boeckh16, Reference Erard17]. In the present study, patients with a malignant disease encountered HZ during the first 3 months of HSCT while on acyclovir prophylaxis. However, most cases of HZ were seen after cessation of acyclovir prophylaxis after a 3-month period. On the other hand, none of the patients developed dissemination despite of the cessation of antiviral prophylaxis long before the occurrence of HZ, and no deaths occurred related to HZ. In developing countries, CP infection is still endemic. In the present study, one patient with AML developed fatal CP infection while on acyclovir prophylaxis and on IVIG treatment, perhaps suggesting a role for prolonged antiviral prophylaxis particularly in malignant diseases.
Several retrospective studies have indicated some risk factors for development of varicella infection after HSCT. Older age at HSCT, underlying haematological malignancies, history of VZV seropositivity, allogeneic transplantation, conditioning regimen, acute or chronic GVHD, prolonged used of immunosuppressive therapy, and delayed immune reconstitution were some of the risk factors for VZV infection after HSCT [Reference Berman5–Reference Koc7, Reference Kawasaki, Takayama and Ohira9, Reference Kim12].
In the current study, the median age at HSCT in the HZ group was higher than in the control group, but the difference was not statistically significant (median age 8 vs. 6·5 years, respectively). Pre-transplant history and/or serology for VZV infection was positive in 68% (n=15) and 44% (n=15) of HZ patients and the control group, respectively. In 14 (93%) of 15 HZ donors and 13 (86%) of 15 control group donors, history and/or serology for VZV infection was positive (P>0·05), suggesting that donor status may not have an effect on development of HZ. However, initial serology was available for 27% and 21% of the HZ and control groups, respectively, and in our study we found that positive serology results correlated 100% with a positive history; nevertheless, the numbers tested serologically remain low and discrepancies between history and serology might be scarce in our study. It has previously been shown that negative histories of VZV infection should be seen as unreliable and found that positive history has a 100% positive predictive value [Reference Kanra18].
Previous studies have shown that prior conditioning with TBI was a significant risk factor for development of HZ [Reference Kawasaki, Takayama and Ohira9, Reference Han11]. None of the patients in the control group in our study had received TBI. Since the number of patients receiving TBI was low, it was not possible to draw a firm conclusion in this regard. Interestingly, it seems that three (50%) of seven patients who received a TBI-based conditioning regimen and one (6·7%) of 15 patients who did not contracted HZ within 3 months. VZV infection probably occurred earlier after the TBI-based conditioning regimen; however, this finding is not statistically significant (P=0·156). Moreover, no difference was noted between the study and the control groups regarding transplantation procedures, including stem cell source, HLA compatibility, engraftment days, or complications (GVHD, VOD, septicaemia, haemolytic anaemia, haematuria, graft failure) after HSCT.
We observed that patients with malignant disease who had underlying immunosuppression and chemotherapy prior to HSCT demonstrated a greater risk of HZ infection than those with non-malignant disease. Although some patients relapsed after HZ [8/16 malignant patients relapsed within an interval of 7·85±6·15 (range 0·7–17·3) months], a relation between HZ and relapse could not be determined due to the small number of patients in these groups with heterogeneous diagnoses.
Delayed lymphoid reconstitution may contribute to the development of HZ after HSCT. Studies on immune recovery after HSCT have been reported extensively in children and adults [Reference Kamani19–Reference Velardi23]. GVHD and immunosuppressive medications may affect the kinetics of immune reconstitution. Myeloablative regimens including busulfan and/or TBI have greater effect on decreasing lymphocyte count and result in delayed reconstitution of immune function [Reference Kamani19]. Kalwak et al. [Reference Kalwak24] showed that CD4+ T cell and B cell (CD19+) subsets recovered much faster at a younger age, and the CD4+/CD8+ ratio was also higher in those aged <5 years. These authors also demonstrated that the CD4+/CD8+ ratio remained inverted from day 30 until 2 years' post-HSCT. In another study, it was shown that CD8+ cells predominate in the early phase after HSCT [Reference Velardi23]. Slow recovery of CD4+ cells and inversion of the CD4+/CD8+ ratio have also been reported [Reference Foot21, Reference Velardi23]. Limited data on immune reconstitution are available in children with HZ in the literature [Reference Leung6, Reference Takaue25]. In our study, we observed a decreased CD4/CD8 ratio in HZ patients compared to the control group, which was statistically significant at 6 months (P<0·05). Lisse et al. [Reference Lisse26] reported significant changes in T lymphocyte subsets during the acute phase of CP infection, including suppression of CD4+ T cells and augmentation of CD8+ T cells in a healthy population. Additionally, in our study, this statistically significant difference between the study and control groups for the CD4+/CD8+ ratio may also have been due to viral infection, considering that 50% of the study group encountered HZ within 6 months of HSCT. As has been noted in previously published reports, the probability of VZV infection appears to be associated with a depressed CD4 count and low CD4/CD8 ratios, although the paucity of data in the present study is not helpful in either confirming or refuting these earlier reports.
In conclusion, in the present study, patients with malignant disorders tended to develop more VZV infections in the post-transplant period compared with those with non-malignant disease, and the CD4+/CD8+ ratio was diminished during the follow-up period after HSCT. The effect of donor status of VZV infection on post-transplant VZV infection of the patient may be determined in further prospective studies.
ACKNOWLEDGEMENTS
We thank the GlaxoSmithKline Company for their support in providing ELISA kits for analysing the VZV infection status of patients.
DECLARATION OF INTEREST
None.




