Acute malnutrition represents a major cause of childhood morbidity and mortality worldwide. The number of children under the age of 5 years with severe acute malnutrition (SAM) at any time is currently estimated from prevalence data to be nearly 19 million, with the burden or number of incident cases occurring each year presumably higher( Reference Black, Victora and Walker 1 ). SAM contributes to over a million child deaths annually, as children with SAM are estimated to have an approximately ninefold increased risk of death compared with well-nourished children( Reference Olofin, McDonald and Ezzati 2 , 3 ).
Traditionally, treatment for SAM was conducted exclusively in in-patient settings, an approach that was both costly and limited access to, and impact of, such programmes. In 2007, a new model for the community-based management of acute malnutrition (CMAM) was endorsed by the WHO, UNICEF, World Food Programme and the UN System Standing Committee on Nutrition, in which children with uncomplicated cases of SAM and appetite could be treated on an out-patient basis with the provision of ready-to-use therapeutic foods and weekly or biweekly follow-up( 3 ). Increasing evidence and operational experience have demonstrated that community-based treatment of uncomplicated SAM is effective( Reference Ciliberto, Sandige and Ndekha 4 – Reference Manary, Ndkeha and Ashorn 7 ) and cost-effective( Reference Bachmann 8 , Reference Wilford, Golden and Walker 9 ).
Current guidelines recommend admission to therapeutic feeding if a child aged 6–59 months meets any one of the following three criteria: (i) weight-for-height Z-score (WHZ) of <−3 using the 2006 WHO Growth Standards; (ii) mid-upper arm circumference (MUAC) <115 mm; or (iii) presence of bilateral oedema( 10 ). Following increasing experience with CMAM and a focus on simplifying and scaling up therapeutic feeding, the possibility of using MUAC as the sole anthropometric criterion for admission to therapeutic feeding has been raised. Arguments in support of a broader use of MUAC include the simplicity of measurement, potential for improved coverage based on ease of implementation and low cost. In addition, MUAC has been found to be more sensitive at high specificity levels than WHZ in predicting mortality among children( Reference Berkley, Mwangi and Griffiths 11 – Reference Briend, Maire and Fontaine 13 ). Transition to a single MUAC criterion for admission to therapeutic feeding, however, is complicated by the fact that MUAC and WHZ are known to select different children at risk for acute malnutrition( Reference Ali, Zachariah and Shams 14 ), and operational experience from programmes using MUAC as a sole admission criterion remains limited.
The objective of the present study was to better understand the operational implications of using MUAC as a single admission criterion for therapeutic feeding. Using routine programme data from a Médecins Sans Frontières (MSF) therapeutic feeding programme (TFP) in Aweil, Northern Bahr El Ghazal, South Sudan, we compared population characteristics and treatment outcomes, including mortality, of children who would have been admitted for therapeutic feeding using MUAC<115 mm alone v. children admitted for therapeutic feeding under the current WHZ<−3 and/or MUAC<115 mm criteria but who would have been excluded from therapeutic feeding if MUAC<115 mm alone was used as a single anthropometric admission criterion.
Methods
Programme description and study population
South Sudan has one of the highest rates of malnutrition globally; the rate of acute malnutrition is approximately twice that seen in other countries in sub-Saharan Africa( Reference Harvey and Rogers-Witte 15 ). In August 2008, MSF opened a TFP in Aweil, Northern Bahr El Ghazal, a region characterized by historically high rates of SAM. The programme primarily served a Nilotic population (75 % Dinka and 25 % Luo ethnic groups), who are typically tall and slim. In such populations there has been discussion surrounding the suitability of WHZ to diagnose and monitor treatment response among children with SAM( Reference Ruff and Walker 16 , Reference Myatt, Duffield and Seal 17 ).
Children aged 6–59 months were screened for malnutrition using weight, height (or length for children <87 cm), MUAC (for children with height >65 cm) and the presence of bilateral oedema; measurements were taken in accordance with WHO standard techniques( 18 ). Those children meeting at least one of two anthropometric criteria – WHZ <−3 or MUAC <115 mm – and presenting with appetite and no medical complications were eligible for outpatient treatment, where home rations of ready-to-use therapeutic food (Plumpy’nut®, approximately 837 kJ/kg body weight (BW) per d (200 kcal/kg BW per d)) were provided and weekly follow-up was conducted with physical examination, anthropometric assessment and distribution of the next therapeutic ration until discharge. Children were referred to in-patient treatment for deterioration of clinical status, including poor appetite, increasing or new oedema, or weight loss or lack of weight gain for three consecutive visits. Children were discharged as ‘recovered’ if they achieved WHZ≥−2 and MUAC>115 mm at two consecutive visits, were free of oedema for >7 d, and were clinically well for at least two consecutive follow-up visits.
The study population for the present analysis included all children aged 6–59 months admitted to out-patient care in the MSF TFP in Aweil, South Sudan with WHZ<−3 or MUAC<115 mm from January 2010 to December 2010. Children with oedema were excluded from the analysis as the assessment of weight in the presence of oedema is not informative.
Statistical analysis
We performed a retrospective analysis of routine programme data of children aged 6–59 months admitted to the MSF outpatient TFP in Aweil, South Sudan in 2010. To describe the potential implications of using MUAC as the single anthropometric admission criterion in this setting, we compared patient characteristics and treatment outcomes between two groups in the 2010 programme population: (i) children admitted to the programme with MUAC<115 mm, regardless of WHZ (referred to as the ‘MUAC+’ group), to represent those children who would have been included for treatment if using MUAC as the single anthropometric admission criterion; v. (ii) children admitted with WHZ<−3 and MUAC≥115 mm (referred to as the ‘MUAC–/WHZ+’ group), to represent children potentially excluded from treatment when using MUAC as the single anthropometric admission criterion.
Treatment outcomes were defined for all children as follows.
-
1. Death: death from any cause during follow-up.
-
2. Default: children who failed to appear for two or more consecutive follow-up visits.
-
3. Transfer: children for whom out-patient management was deemed to be insufficient, including children with chronic illness, deterioration in clinical status, increasing or new oedema, or weight loss or failure to gain weight for at least three consecutive visits.
-
4. Recovered: children with WHZ≥−2 and MUAC >115mm at two consecutive visits and who were free of oedema for >7 d.
To compare baseline characteristics between the groups, the χ 2 test was used to compare proportions, including age and sex, and a t test was used to compare continuous variables, including nutritional status at admission. Binomial regression, adjusting for age and sex, was used to compare the risk of each treatment outcome between groups. For children classified as ‘recovered’, length of stay, change in MUAC and change in WHZ were calculated and compared between groups using linear regression, adjusting for age and sex. The level of significance was set at P≤0·05. All data were analysed using R software version 2·9·2( 19 ). The data analyses were exempted from review by the MSF Ethical Review Board given the use of de-identified, routine programme data.
Results
Characteristics of the study population
A total of 2601 records were available for children admitted for out-patient treatment of uncomplicated SAM in 2010. For the present analysis, 396 (15·2 %) records were excluded: 107 (4·1 %) for failing for meet stated programme admission criteria, sixty-five (2·5 %) for oedema on admission and 224 (8·6 %) for missing data on age, sex or anthropometry. Of the 2205 remaining eligible records, median age was 14 months (interquartile range=10–24 months) and 53 % were male. In the overall cohort, mean MUAC at admission was 117·5 (sd 9·1) mm and mean WHZ was −3·9 (sd 0·7; Table 1). Of the 2205 children included for analysis, 698 were admitted with both MUAC<115 mm and WHZ≤−3 (31·6 %; MUAC+/WHZ+), twenty-one were admitted based on MUAC alone (1·0 %; MUAC+/WHZ−) and 1486 were admitted based on WHZ alone (67·4 %; MUAC−/WHZ+).
Table 1 Patient characteristics by admission criteria, children aged 6–59 months, Aweil, South Sudan, January–December 2010

MUAC, mid-upper arm circumference; WHZ, weight-for-height Z-score.
MUAC+: includes children admitted based on single MUAC<115 mm criterion, regardless of WHZ.
MUAC–/WHZ+: includes children currently admitted but excluded from treatment using a single MUAC<115 mm admission criterion (WHZ<−3 and MUAC≥115 mm).
* Proportions compared using χ 2 test and continuous measures compared using t test.
To better understand the programmatic implications for admission based on MUAC<115 mm alone, we described and compared treatment outcomes of two groups: MUAC+ (n 719) v. MUAC−/WHZ+ (n 1486). Children admitted using MUAC as a single anthropometric admission criterion (MUAC+) were more likely to be female (55 % v. 43 %; P<0·0001) and younger (59 % v. 45 % less than 12 months of age) than children who would not have been admitted with the single MUAC<115 mm criterion (MUAC−/WHZ+). Children in the MUAC+ group had significantly lower MUAC (107·3 mm v. 122·5 mm; P<0·0001) and WHZ (−4·3 v. −3·8; P<0·0001) on admission, compared with children in the MUAC−/WHZ+ group.
Treatment outcomes
Children in the MUAC+ group were less likely to recover (54 % v. 69 %; P<0·0001) and experienced a higher risk of death (4 % v. 1 %; P<0·0001) and transfer to in-patient care (12 % v. 7 %; P<0·0001) compared with the MUAC−/WHZ+ group. In both groups, the risk of default was higher than the SPHERE standard of <15 %( Reference Goossens, Bekele and Yun 20 ), with the risk being significantly higher among the MUAC+ group compared with the MUAC−/WHZ+ group (30 % v. 23 %; P<0·0001; Table 2). Among recovered children, treatment response was more favourable among the MUAC+ group than the MUAC−/WHZ+ group: the MUAC+ group had a greater mean weight gain (6·01 g/kg BW per d v. 5·12 g/kg BW per d; P<0·0001) and greater mean MUAC gain (0·45 mm/d v. 0·25 mm/d; P<0·0001). Mean length of treatment was approximately 51 (sd 29·01) d overall and did not differ significantly between groups.
Table 2 Treatment outcomes by admission criteria, children aged 6–59 months, Aweil, South Sudan, January–December 2010
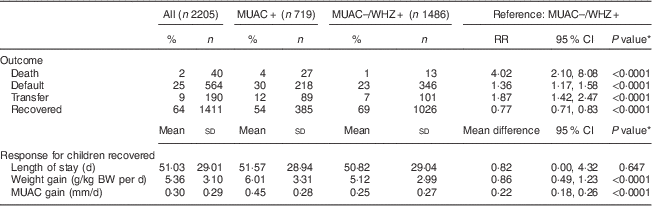
MUAC, mid-upper arm circumference; WHZ, weight-for-height Z-score; RR, relative risk; BW, body weight.
MUAC+: includes children admitted based on single MUAC<115 mm criterion, regardless of WHZ.
MUAC–/WHZ+: includes children currently admitted but excluded from treatment using a single MUAC<115 mm admission criterion (WHZ<−3 and MUAC≥115 mm).
* Proportions compared using binomial regression and continuous measures using linear regression; all models are adjusted for age and sex.
A total of forty deaths (2 %) were recorded in the study population, of which twenty-seven (67·5 %) were seen in the MUAC+ group and thirteen (32·5 %) in the MUAC−/WHZ+ group (Fig. 1(a)). Adjusting for age and sex, the risk of death was over four times higher among children in the MUAC+ group compared with the MUAC−/WHZ+ group (risk ratio=4·02; 95 % CI 2·10, 8·08). Figure 1(b) indicates the distribution of both MUAC and WHZ on admission for recorded deaths, showing that MUAC<115 mm as the single anthropometric admission criterion would have missed 33 % of deaths in the cohort. MUAC<125 mm as the single anthropometric admission criterion would have captured all but two deaths in the cohort and all deaths would have been captured using MUAC<130 mm.

Fig. 1 (a) Death as outcome by anthropometric criteria on admission and (b) distribution of deaths in the study population by MUAC and WHZ upon admission, children aged 6–59 months, Aweil, South Sudan, January–December 2010 (MUAC, mid-upper arm circumference; WHZ, weight-for-height Z-score)
Discussion
We analysed data from an MSF TFP to describe patient characteristics and treatment outcomes of children who would have been admitted for out-patient treatment using MUAC<115 mm as a single admission criterion v. those who would have been excluded using MUAC<115 mm as a single admission criterion. We found that children who would have been admitted with a single MUAC<115 mm criterion were more likely to be female and of younger age. These children were more severely malnourished, as indicated by lower MUAC and lower WHZ on admission. They were also at greater risk of default and death, but responded well to treatment in terms of weight and MUAC gain.
The MSF TFP in Aweil, South Sudan was opened in response to the high risk of SAM and other complicating factors in this setting. Some experience suggests, however, that WHZ may not be well suited for the diagnosis and follow-up of children with tall, slim body shapes, characteristic of some Nilotic populations. MSF programme experience in Aweil noted some difficulty of children admitted using WHZ criterion to meet discharge criteria for nutritional recovery, which generated interest in the possibility to adapt programme admission criteria to a single MUAC-based measure.
More broadly, MUAC has been proposed as a single admission criterion for several reasons, including its simplicity of use and correlation with increased risk of death. It is recognized that MUAC is more easily implemented than WHZ in field-based settings and facilitates community-based screening. Myatt et al. reviewed various indicators for case detection in the context of SAM( Reference Myatt, Khara and Collins 21 ). The authors scored various indicators on the basis of a set of properties (including simplicity, acceptability, cost, objectivity, independence of age, precision/reliability, accuracy, sensitivity, specificity and predictive value) and concluded MUAC<110 mm or the presence of bipedal oedema was the most appropriate screening criterion.
Despite support for broader use of MUAC as a single admission criterion, programmatic experience with MUAC-based admissions is limited. Few reports have been published to share experience from programmes admitting children with SAM to therapeutic feeding using MUAC only( Reference Goossens, Bekele and Yun 20 , Reference Defourny, Minetti and Harczi 22 , Reference Nielsen, Valentiner-Branth and Martins 23 ). In these studies, treatment outcomes, including mortality, were similar to those recorded in our study. A study of the effect of a supplemental food programme in Guinea-Bissau found a mortality of 1 %( Reference Nielsen, Valentiner-Branth and Martins 23 ). Defourny et al. evaluated the use of MUAC<110 mm as an admission criterion for a TFP in Niger( Reference Defourny, Minetti and Harczi 22 ). The programme reported risks of death, default and non-response as 1·8 %, 4·7 % and 1·1 %, respectively. Average weight gain was 5·1 g/kg BW per d and average length of treatment was 44·4 d, similar to outcomes seen in our programme (weight gain of 5·12 g/kg BW per d and median length of treatment of 50 d).
The decision regarding the use of WHZ v. MUAC for admission to therapeutic feeding is complicated by the fact that each measure may select different children for treatment. Depending on the setting and geographic location, 40–90 % of children are not classified as severely malnourished based on both criteria( Reference Berkley, Mwangi and Griffiths 11 , Reference Myatt, Duffield and Seal 17 , Reference Fernandez, Delchevalerie and Van Herp 24 – Reference Roberfroid, Hammami and Lachat 26 ). Existing literature suggests that MUAC-based programmes tend to identify significantly more girls and younger children than those identified by WHZ( Reference Berkley, Mwangi and Griffiths 11 ). Receiver-operating characteristic curves have also demonstrated that MUAC may identify children at a higher risk of mortality( 18 ). Our data confirm these findings.
We found that a MUAC cut-off of <115 mm would have excluded 33 % of the children who died in the programme, whereas a WHZ cut-off of <−3 identified almost all (98 %) children who died. This finding is in contrast with community studies in which low WHZ is less predictive of mortality than low MUAC( Reference Briend, Maire and Fontaine 13 , Reference Pelletier 27 ). The greater sensitivity of WHZ<−3 to identify nearly all deaths observed in the present study population may be specific to the study context, which as a programmatic cohort represents a sub-sample of the general community that presented for and received treatment, and is drawn from a Nilotic population whose tall and slim body shape is associated with low WHZ. Nearly all children included in the present analysis were identified by low WHZ (only 1 % of children were admitted with WHZ≥−3) and as a result, WHZ may be expected to more completely capture deaths here. As suggested by a previous analysis also carried out in a selected sample of treated children that aimed to compare the accuracy of WHZ and MUAC to predict death in a large nutritional programme in Niger( Reference Lapidus, Luquero and Gaboulaud 28 ), predicting death among children admitted to treatment is less useful than identifying which children in the community would benefit most (e.g. experience the greatest reduction in the risk of mortality) from treatment. Such comparison of mortality according to anthropometric criterion (e.g. WHZ v. MUAC<115 mm v. MUAC<125 mm) to determine which has superior accuracy and efficiency to identify children at risk of death in the absence of treatment would, however, be unethical. The present analysis should be viewed as a step to help to address this question in South Sudan, but cannot be used to draw firm conclusions.
While MUAC<115 mm did not identify the majority of deaths in this setting, our results highlight how an upward adjustment of the MUAC admission threshold to MUAC<125 mm could be applied to identify nearly all deaths (e.g. 95 %) in this setting. Any such upward adjustment would yield a more sensitive, but less specific, criterion for admission and consequently have important programmatic implications, including increased workload and costs. MSF experience in Burkina Faso in 2007–2009, where children were admitted to TFP with MUAC ≤118 mm or oedema, suggests children with MUAC of 116–118 mm benefited from treatment, as evidenced by rates of weight gain similar to those typically seen in TFP( Reference Goossens, Bekele and Yun 20 ). The authors conclude that the despite the ‘programmatic price’ of increased caseloads, a more sensitive MUAC cut-off may still be appropriate. The extent to which an upward adjustment in the MUAC admission threshold to MUAC<125 mm would increase programme size and costs cannot be derived from the present data and would depend on the patient population (including age) and other operational factors, and therefore would require evaluation across various contexts. Additional experience with the use of MUAC for admission, monitoring and discharge is also needed. To date, few studies present information of children with SAM who were identified, monitored and discharged by weight and MUAC( Reference Berkley, Mwangi and Griffiths 11 , Reference Binns, Dale and Banda 29 , Reference Dale, Myatt and Prudhon 30 ).
Several limitations of the present analysis warrant mention. We note that the study population represents a selected sample of the general population (i.e. children admitted to the MSF TFP). Therefore, the results are not representative of the population at large and do not allow a clear conclusion about the relevance of using WHZ in addition to MUAC to select those children who have the highest risk of death in the community. Additional limitations of the analysis include the retrospective data collection, a risk of programme default exceeding the SPHERE standard of 15 % and missing data. Each of these limitations may impact the internal and external validity of our results. However, the analysis uniquely presents data from a large programmatic cohort from a context with a high burden of SAM to provide further insight into the key implications of using MUAC as a sole anthropomorphic admission criterion in terms of treatment response, recovery, transfer and death.
Conclusion
In the present retrospective study we found that children who would have been admitted to an out-patient TFP using a single MUAC<115 mm criterion were more likely to be more severely malnourished, female and of younger age than children who would have been excluded if a single MUAC<115 mm criterion were adopted. Children who would have been admitted using a single MUAC<115 mm criterion had higher mortality, but responded well to treatment, as demonstrated by greater weight and MUAC gain. In this setting, we have shown that MUAC<115 mm as a single admission criterion for community-based treatment programmes for SAM would have failed to include one-third of the children who died in the programme. Our experience in the field supports continued efforts to better understand the implementation and implications of MUAC-based programming across a variety of programmatic settings, and the present analysis suggests that admission criteria will need to be adapted to the programmatic context with consideration for both operational and public health implications.
Acknowledgements
Financial support: This work was supported by MSF Operational Center Paris. Conflict of interest: None. Authorship: E.G., L.K.K., M.S.E., K.P. and S.I. conceived and designed the experiment. E.G. analysed the data. All authors contributed to the interpretation of the data and preparation of the manuscript. Ethics of human subject participation: The data analyses were exempted from review by the MSF Ethical Review Board given the use of de-identified, routine programme data.






