Introduction
The first 1000 d, defined as pregnancy and the first 2 years of life, represent a period of increased nutritional demands that can have lasting health and development consequences if not met. Historically, the use of supplements has been viewed as a relatively quick and easy way to address deficiencies in vitamins and minerals. However, there is increasing concern about the health risks associated with supplement use, and global momentum to find sustainable and affordable food system solutions to micronutrient deficiencies(1). Aquatic foods, long recognised as an excellent source of protein and essential fatty acids, have gained recent attention as a possible dietary option to promote to reduce micronutrient deficiencies, although the focus has thus far been on fish and fish products(Reference Byrd, Shieh, Mork, Pincus, O’Meara and Atkins2). Molluscs and crustaceans, which are defined in this review as all edible shellfish, have unique nutrient profiles that differ from fish. For example, clams, mussels and oysters are remarkably rich in iron and zinc and have a more diverse nutrient profile in comparison with most aquatic and terrestrial animal-source foods(Reference Golden, Koehn, Shepon, Passarelli, Free and Viana3–5). Moreover, one global modelling study associated country-level intake of molluscs and crustaceans with reduced prevalence of childhood anaemia(Reference Iannotti, Blackmore, Cohn, Chen, Gyimah and Chapnick6). Thus, mollusc and crustacean consumption has the potential to address micronutrient deficiencies that are prevalent in pregnancy and early childhood while simultaneously providing high-quality proteins and essential fatty acids.
However, in areas where shellfish allergies are prevalent and water pollution is a problem, encouraging mollusc and crustacean consumption during the first 1000 d presents a dilemma. There is limited, recent evidence on the global prevalence of shellfish allergies in children. The most recent review from 2016 reports prevalence ranging from 0% to 10% across all age groups worldwide(Reference Moonesinghe, Mackenzie, Venter, Kilburn, Turner and Weir7), and another review from the same year reports that about 0·5–2·5% of the food allergies globally may be attributed to shellfish(Reference Khora8). In some countries in Asia, a region where aquatic food consumption is widespread, shellfish is the leading risk factor for anaphylaxis in older children and adults(Reference Loh and Tang9).
Globally, there are also concerns of environmental contamination of molluscs and crustaceans. Mercury and other heavy metals such as cadmium or lead have long been an issue, as they can bioaccumulate in aquatic foods and lead to a multitude of adverse outcomes, including reproductive harm, neurological diseases and cognitive impairment(Reference Al osman, Yang and Massey10). The concern of contamination has expanded to include pesticides and industrial compounds, many of which have been banned by the 2001 Stockholm Convention on Persistent Organic Pollutants, and more recently microplastics(Reference Fiedler, Kallenborn, de Boer and Sydnes11,12) . Exposure is most concerning in the first 1000 d when there is brain and organ development and rapid physical growth.
Evidence to date on health-related outcomes associated with mollusc and crustacean consumption comes largely from observational studies examining distinct nutrition or environmental contaminant outcomes. There is a need to synthesise this literature to better comprehend the trade-off risks particularly for nutritionally vulnerable periods of life. Therefore, we aimed to conduct a scoping review examining how mollusc and crustacean consumption in the first 1000 d relates to maternal and child nutrient status, health and development.
Methods
This review was conducted and is reported in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement(Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann and Mulrow13,Reference Moher, Shamseer, Clarke, Ghersi, Liberati and Petticrew14) . The protocol for this review is registered with the University of York’s International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022320454.
Eligibility criteria
We included peer-reviewed articles reporting evidence from observational studies and interventional trials that assessed molluscs/crustaceans as a dietary exposure during pregnancy, lactation, and childhood (up to 2 years of age) in apparently healthy cohorts. In some articles, the aims were not specifically related to the first 1000 d. However, we included such articles when they included stratified analyses for our populations of interest, or when a significant proportion of the study cohort fell within our population of interest. Eligibility was also specific to consumption during the first 1000 d, regardless of when the outcomes were measured. In some cases, outcomes measured were outside of the first 1000-d window. However, if the study retrospectively assessed mollusc/crustacean consumption during the first 1000 d, we considered such studies eligible. Other studies were excluded if they studied outcomes during the first 1000 d, but retrospectively assessed periconceptional mollusc/crustacean intake (prior to pregnancy).
We further excluded studies when mollusc/crustacean intake was not assessed. In some studies, consumption of different aquatic animal foods, including molluscs and crustaceans, was assessed as exposures but outcomes were not specifically assessed in relation to mollusc/crustacean intake. Additional exclusion criteria for articles were as follows: without human subjects; without health or neurobehavioral developmental outcomes; describing outcomes for populations that were not apparently healthy (for example, populations with pre-existing allergies); not available in English; and presented as background or review papers. There were no restrictions set for the geographic location of the study. However, as environmental contamination of shellfish can fluctuate with changing policies and development, we limited our search to articles published from the year 2000 to reflect current risks and benefits.
Search strategy
We conducted our search via three electronic databases (Scopus, Medline via PUBMED, and Global Health via EBSCO). Prior to the search, the research team compiled a comprehensive list of keywords based on a pre-defined PICO framework that aligned with the review’s aims. For the population, key terms included different synonyms or phrases characteristic of the first 1000 d, including ‘child’, ‘infant’, ‘preschool’, ‘pregnancy’ and ‘lactation.’ For the intervention or exposure, we identified a comprehensive list of edible mollusc/crustacean species and included them with basic keywords like ‘shellfish’ as well as different names of classes within the Mollusca phylum group. No search terms were developed for the comparator, given the likely heterogeneity in comparators across the different studies. For the outcomes, we identified broader MESH terms pertaining to maternal and child health as well as nutrition outcomes. We also included additional keywords based on the existing literature and some keystone papers associating aquatic animal foods with different outcomes. Terms here included markers or indicators for different heavy metal contaminants, trace and macro minerals, anthropometric outcomes, allergies and hypersensitivity. The full electronic search strategy is detailed in Supplementary Table 1. The team implemented the final search across the three databases on 23 March 2022.
Study selection
Records obtained from the databases were merged and imported to the Zotero citation management system, where duplicates were identified and removed. Subsequently, records were imported to the web-based software, Rayyan, where additional duplicates were eliminated(Reference Ouzzani, Hammady, Fedorowicz and Elmagarmid15). Two reviewers (E.A.G. and E.K.) independently screened titles and abstracts of included records in Rayyan to determine eligibility for a full text review. Conflicts were then resolved by a third reviewer (B.M.O. or L.L.I.). Next, the reviewers (E.A.G. and E.K.) conducted a full text review to further determine eligibility for data extraction. Disagreements resulting from this stage were resolved through group consensus.
Data extraction
We developed a data extraction form to collect data on the following: (1) article information (first author surname; year of publication); (2) study details (study design; study aims; population description – cohort/sample description; sample size, lactating women; pregnant women; or children within the first 1000 d); (3) geographic details (country of study; World Bank income classification as a low- or middle-income country (LMIC)); (4) exposures and outcomes (molluscs/crustaceans studied; indicators/biomarkers measured; study findings). Although the studies may have reported a range of outcomes, we only reported specific indicators that were assessed in relation to shellfish consumption. Two reviewers (E.K. or E.A.G.) extracted the data, and a third reviewer (B.M.O.) subsequently validated the information collected in the data extraction form.
Results
A total of forty-six articles were included in this review (Supplementary Table 2). We identified 7307 articles from PubMed, Scopus and EBSCO Global Health. After removing duplicates and screening titles and abstracts for eligibility, 325 articles were assessed for inclusion by full text review. Primary reasons articles were excluded after full text review included: consumption did not occur within the first 1000 d (n = 61); consumption was reported in a manner that grouped mollusc and crustacean consumption with other seafood (e.g. total seafood consumption) (n = 65); the study did not include human participants (n = 46); the study was performed in a population with a specified health condition (n = 30); and the article was not in English (n = 59). See Fig. 1 for the study flow diagram.
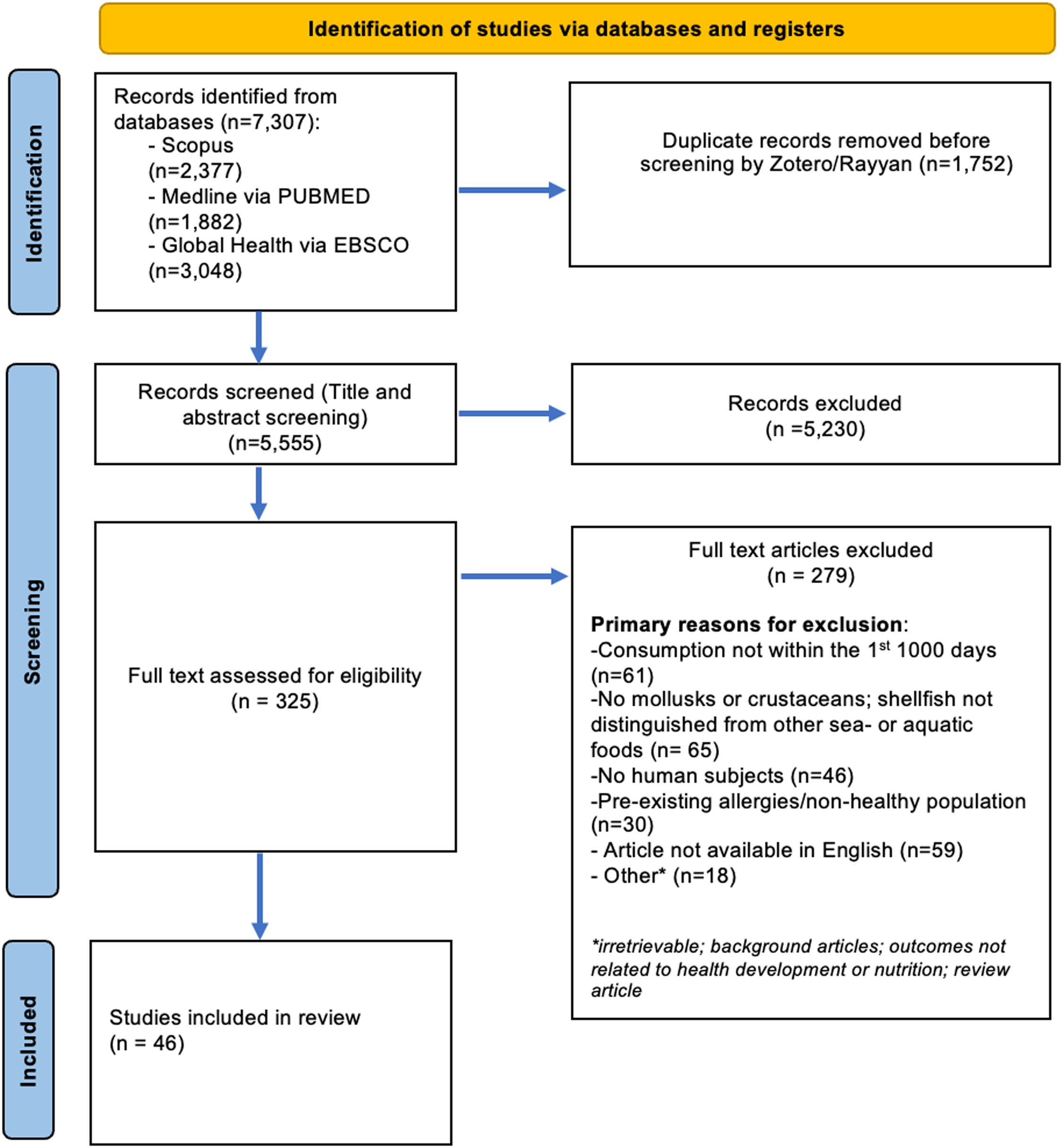
Fig. 1. Flow diagram of selection of studies for inclusion in the scoping review.
Approximately 44% of the articles (n = 20) reported on pregnant women only, 15% reported on children only (n = 7), 30% reported on pregnant women–child dyads (n = 14) and 11% reported on lactating women (n = 5) (Fig. 2). Crustacean consumption was estimated in twenty-seven studies, with four out of these studies mentioning only crustaceans as part of their surveys, either in terms of all crustaceans, or in terms of shrimp, crab, lobster or prawn. Mollusc consumption was reported in twenty-five studies, with two out of these studies mentioning only molluscs as part of their surveys. Studies estimated total mollusc consumption or intake of specific molluscs, such as clams, mussels, cockles, scallops, oysters, squid, cuttlefish, snails and octopus. The majority of studies (n = 40) mentioned total shellfish consumption as a dietary exposure of interest, with twenty-three studies examining both molluscs and crustaceans and seventeen studies reporting shellfish exposures without specifying the category. In terms of reported outcomes, the number of studies reporting toxicological outcomes (heavy metals and other contaminants) (n = 23) was substantially greater than any other outcome in our review (Fig. 3). Most of the studies (n = 36) were conducted in high-income countries, primarily in the United States, Europe and Asia. No studies were conducted in Africa (Fig. 4).

Fig. 2. Number of studies published related to maternal and child health and mollusc and crustacean consumption by study population.

Fig. 3. Number of studies published related to maternal and child health and mollusc and crustacean consumption by outcome.

Fig. 4. Number of studies published related to maternal and child health and mollusc and crustacean consumption by country.
Nutritional status
Iodine, fatty acids, molybdenum and selenium were the only nutrients examined in relation to shellfish consumption identified in this review. Three studies examined iodine (urinary iodine, n = 2; iodine intake, n = 1)(Reference Refaat and Azzeh16–Reference Melero, Runkle, Garcia de la Torre, De Miguel, Valerio and Del Valle18), two studies examined fatty acids(Reference Julvez, Fernández-Barrés, Gignac, López-Vicente, Bustamante and Garcia-Esteban19,Reference Brantsæter, Englund-Ögge, Haugen, Birgisdottir, Knutsen and Sengpiel20) , one study examined urinary molybdenum and one study examined selenium in breastmilk(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21). All iodine studies were conducted among large cohorts of pregnant women(Reference Refaat and Azzeh16–Reference Melero, Runkle, Garcia de la Torre, De Miguel, Valerio and Del Valle18). Consuming shellfish increased the probability of meeting the iodine RDA for pregnant women(Reference Melero, Runkle, Garcia de la Torre, De Miguel, Valerio and Del Valle18). Shellfish consumption increased urinary iodine concentration in one study(Reference Refaat and Azzeh16) and showed a non-significant trend for increased urinary iodine concentration in a second study(Reference Alvarez-Pedrerol, Ribas-Fitó, García-Esteban, Rodriguez, Soriano and Guxens17). For fatty acids, maternal shellfish intake during the first trimester was significantly correlated with cord blood eicosapentaenoic acid concentrations, but not docosahexaenoic acid concentrations(Reference Julvez, Fernández-Barrés, Gignac, López-Vicente, Bustamante and Garcia-Esteban19). Shellfish consumption during pregnancy contributed to 2·2% of long chain n-3 polyunsaturated fatty acid intake from food in the Norwegian Mother and Child Cohort, a study that included over 60 000 pregnant women(Reference Brantsæter, Englund-Ögge, Haugen, Birgisdottir, Knutsen and Sengpiel20). There was no significant association of shellfish intake with breast milk selenium concentration in lactating women nor urinary molybdenum in pregnant women(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21).
Summary
Overall, the present literature in relation to nutritional status suggests that shellfish consumption may be positively associated with iodine and fatty acid status but not selenium or molybdenum status. No other nutritional status indicators were examined in relation to shellfish consumption.
Maternal health and birth outcomes
Five studies measured the association of shellfish consumption during pregnancy with birth weight with mixed results(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22–Reference Mendez, Plana, Guxens, Foradada Morillo, Albareda and Garcia-Esteban26). Total shellfish consumption was associated with lower birth weight in two studies(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23) , a small cohort in Italy and the Generation R study in the Netherlands. However, total shellfish consumption was associated with higher birth weight in the large Norwegian Mother and Child Cohort study (n = 62 099 mother–infant dyads)(Reference Brantsæter, Birgisdottir, Meltzer, Kvalem, Alexander and Magnus24), and no association in a large cohort in the United States(Reference Mohanty, Thompson, Burbacher, Siscovick, Williams and Enquobahrie25). The fifth study was conducted in Spain and observed an association of shellfish consumption with lower birth weight but reported results by shellfish category and not just total consumption. Specifically, that study of 592 mother–infant dyads reported that infants born to women with high crustacean intake and high intake of other shellfish (clams, mussels, oysters, squid, cuttlefish, octopus) had a mean birth weight that was 115 g and 91 g less than women with low intake of crustaceans and other shellfish, respectively(Reference Mendez, Plana, Guxens, Foradada Morillo, Albareda and Garcia-Esteban26). Four of these studies also measured birth length and head circumference(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22–Reference Mohanty, Thompson, Burbacher, Siscovick, Williams and Enquobahrie25). No association was found with birth length. Three of the studies found no association with head circumference(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Brantsæter, Birgisdottir, Meltzer, Kvalem, Alexander and Magnus24,Reference Mohanty, Thompson, Burbacher, Siscovick, Williams and Enquobahrie25) while one reported a non-significant trend of higher shellfish consumption with lower head circumference (r = −0·17, P = 0·08)(Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23). One study reported a higher mean ponderal index among female infants born to women with high shellfish intake (>1 serving per week) versus women with low shellfish intake (<0·2 servings per month) (mean difference 1·1 kg/m3, 95% confidence interval (CI) 0·3–1·9 kg/m3) but not among male infants (mean difference 0·2 kg, 95 % CI −0·7 to 1·2 kg/m3)(Reference Mohanty, Thompson, Burbacher, Siscovick, Williams and Enquobahrie25). One study measured foetal growth in the second and third trimester and reported no significant associations with shellfish consumption(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22).
Three studies examined small-for-gestational-age (SGA), also with mixed results(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Mendez, Plana, Guxens, Foradada Morillo, Albareda and Garcia-Esteban26,Reference Amezcua-Prieto, Martínez-Galiano, Salcedo-Bellido, Olmedo-Requena, Bueno-Cavanillas and Delgado-Rodríguez27) . The Generation R study in the Netherlands reported no association of SGA with maternal shellfish consumption(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22), a study in Spain (n = 592 women) reported higher risk of SGA(Reference Mendez, Plana, Guxens, Foradada Morillo, Albareda and Garcia-Esteban26), while another study in Spain (n = 518 women) reported lower risk of SGA(Reference Amezcua-Prieto, Martínez-Galiano, Salcedo-Bellido, Olmedo-Requena, Bueno-Cavanillas and Delgado-Rodríguez27). The study that reported higher SGA risk found that maternal intake of crustacean consumption >1 serving per week was associated with increased risk of SGA (odds ratio (OR) 2·56, 95% CI 1·11–5·89) but there was no association with other shellfish intake(Reference Mendez, Plana, Guxens, Foradada Morillo, Albareda and Garcia-Esteban26). The study reporting lower SGA risk found that compared with pregnant women that never ate shellfish, pregnant women that ate bivalve molluscs (>1 serving per week) had a 75% reduced risk of SGA (OR 0·25, 95% CI 0·08–0·76) and pregnant women that ate a serving of cephalopods one to three times a month had a 38% reduced risk of SGA (OR 0·62, 95% CI 0·44–0·87), but there was no association of SGA with crustacean consumption(Reference Amezcua-Prieto, Martínez-Galiano, Salcedo-Bellido, Olmedo-Requena, Bueno-Cavanillas and Delgado-Rodríguez27).
The relationship between shellfish consumption and angiogenic factors that influence foetal growth (placental growth factor (PIGF) and soluble Flt-1 (sFlt-1)) was assessed in the Generation R cohort and showed non-significant associations(Reference Bautista Niño, Tielemans, Schalekamp-Timmermans, Steenweg-de Graaff, Hofman and Tiemeier28).
Four studies examined the association between maternal shellfish consumption and preterm birth(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29,Reference Wang, Fu, Deng, Zhu, Zhang and Zhang30) . Three of the studies (a small cohort in Italy, a large cohort in the United States, and the Generation R study in the Netherlands) found no association(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) , and one study conducted in 10 179 pregnant women in China reported a reduced risk of preterm birth (OR 0·45, 95% CI 0·26–0·76)(Reference Wang, Fu, Deng, Zhu, Zhang and Zhang30). The cohort study in Italy also examined gestational duration and reported no association(Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23).
Four studies examined the association between shellfish consumption and maternal health(Reference Refaat and Azzeh16,Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21,Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) . Maternal health outcomes examined included gestational diabetes(Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) , pregnancy-induced/gestational hypertension(Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) and pre-eclampsia(Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29). No evidence of an association was found between shellfish consumption and these outcomes(Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) . In the study that explored shellfish intake in relation to urinary iodine(Reference Refaat and Azzeh16), the researchers also explored a range of outcomes related to thyroid hormones during pregnancy. They reported significant negative associations between shellfish consumption in pregnant women and isolated hypothyroxinaemia (OR 0·496, 95% CI 0·056–0·819) (P = 0·02) as well as hyperthyroidism (OR 0·323, 95% CI 0·097–0·978)(Reference Refaat and Azzeh16). Associations for subclinical and overt hypothyroidism were, however, non-significant(Reference Refaat and Azzeh16). One of the studies assessing heavy metal concentrations in breast milk samples found non-significant associations between the shellfish consumption and the activity of glutathione S-transferase – an enzyme that can convert lipid-soluble toxins into water-soluble forms for excretion(Reference Gropper and Smith31) – in breastmilk(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21).
Summary
In sum, the association between shellfish consumption and birth anthropometry is inconclusive with the limited number of studies reporting both positive and negative associations with birth weight, length and head circumference. The current research suggests that shellfish consumption may reduce the risk of preterm birth, although several studies reported no association. Shellfish consumption has not been demonstrated to be associated with any primary maternal health outcomes (gestational diabetes, pregnancy-induced hypertension, pre-eclampsia), although research is limited in this area.
Child health
Child allergies and hypersensitivity were explored in nine studies, primarily in relation to shellfish consumption during pregnancy. In a large Taiwan-based cohort of children under the age of 3 years (n = 813), the respective prevalence of mollusc, shrimp and crab allergies were less than 1%(Reference Wu, Tsai, Huang, Chang, Lin and Huang32). A similar study from Singapore reported similar findings for the prevalence of shellfish allergy at ages 12, 18, 24, 36 and 48 months and found non-significant associations between the timing of shellfish introduction and the development of food allergy(Reference Tham, Lee, Chan, Loo, Toh and Goh33). A US-based study also measured shellfish allergy, reporting a prevalence of less than 1% for physician-diagnosed shellfish, crustacean and mollusc allergy among children aged 0–2 years(Reference Wang, Warren, Gupta and Davis34). In a French study, consumption of shellfish at least once a month during pregnancy was associated with increased risk of food allergy in children at age 2 years (adjusted OR (aOR) 1·62, 95% CI 1·11–2·36), but not with wheezing or eczema(Reference Pelé, Bajeux, Gendron, Monfort, Rouget and Multigner35). One study from England reported non-significant findings in the relationship between shellfish consumption during pregnancy at varying doses and atopy as well as allergic disease at ages 3 and 10 years(Reference Moonesinghe, Patil, Dean, Arshad, Glasbey and Grundy36). In a study conducted in the Netherlands, consuming 1–13 g of shellfish per week during pregnancy was also found to increase the risk of childhood wheezing (aOR 1·20, 95% CI 1·04–1·40) and eczema (aOR 1·18, 95% CI 1·01–1·37)(Reference Leermakers, Sonnenschein-van der Voort, Heppe, de Jongste, Moll and Franco37).
Four of the nine studies on child allergies evaluated allergy and hypersensitivity using biomarkers of immune response. In another Taiwanese cohort, the researchers found a non-significant association between shellfish restriction up to age 12 months and IgE sensitisation (OR 1·17, 95% CI 0·67–2·04)(Reference Hua, Yao, Chen, Tsai, Liao and Lai38). Similar findings were reported for atopic dermatitis in this cohort (OR 1·06, 95% CI: 0·49–2·29)(Reference Hua, Yao, Chen, Tsai, Liao and Lai38). A study from the United States also found non-significant relationships between average shellfish intake during the first and second trimester and allergy biomarkers in early childhood(Reference Maslova, Rifas-Shiman, Oken, Platts-Mills and Gold39). One study from China explored the relationship between prenatal shellfish intake and cord blood IgE levels as well as genes implicated in the pathways for interleukins 4 and 13(Reference Chen, Lee, Wu, Chen, Wei and Tseng40). Generally, the researchers found no associations with the outcomes of interest. They did, however, find a gene-specific relationship for interleukin 13 (IL13); for the IL13 gene variant rs20541, statistically significant relationships were found for infants with the GG genotype(Reference Chen, Lee, Wu, Chen, Wei and Tseng40).
Two studies examined the association between maternal shellfish consumption and child cognition(Reference Julvez, Fernández-Barrés, Gignac, López-Vicente, Bustamante and Garcia-Esteban19,Reference Vecchione, Vigna, Whitman, Kauffman, Braun and Chen41) . One study additionally measured autism spectrum disorder-related traits(Reference Vecchione, Vigna, Whitman, Kauffman, Braun and Chen41), and the other study also examined parent-reported ADHD(Reference Julvez, Fernández-Barrés, Gignac, López-Vicente, Bustamante and Garcia-Esteban19). Neither outcome was associated with maternal shellfish consumption.
Summary
Overall, child allergy has been the primary focus of child health research related to shellfish consumption. In studies that examined prevalence of shellfish allergy in children, it was consistently reported as less than 1%. There is some evidence that shellfish consumption during pregnancy may be associated with an increased risk of shellfish allergy among the offspring, although there were several studies that reported no significant association. Whether shellfish consumption is related to child cognition and development cannot be determined with the limited literature, although currently no associations have been reported.
Heavy metals
Mercury concentrations in blood, hair, breastmilk or cord blood were measured in ten studies among pregnant women, lactating women and children(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21,Reference Basu, Tutino, Zhang, Cantonwine, Goodrich and Somers42–Reference Wu, Yan, Xu, Wu, Li and Zhou50) . Overall, shellfish consumption was associated with higher mercury concentrations in blood with four studies reporting higher blood mercury concentrations in association with total shellfish consumption(Reference Basu, Tutino, Zhang, Cantonwine, Goodrich and Somers42,Reference Gao, Li, Wang, Yan, Zhou and Yan44,Reference Golding, Steer, Hibbeln, Emmett, Lowery and Jones46,Reference Jain47) . A study that recruited pregnant women from a major city in each region of China reported maternal shellfish consumption during pregnancy increased the risk of foetal mercury exposure as measured in cord blood by two-fold (OR 2·21, 95% CI: 0·21–1·37)(Reference Wu, Yan, Xu, Wu, Li and Zhou50). Two studies measured mercury in breastmilk(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21,Reference Vollset, Iszatt, Enger, Gjengedal and Eggesbø49) . A study in Norway examined breastmilk mercury concentrations among 300 women according to intake of crab, shrimp or mussels/scallops and showed increased intake of each category was associated with higher mercury concentrations(Reference Vollset, Iszatt, Enger, Gjengedal and Eggesbø49). A smaller study in Mexico reported no significant association with total shellfish consumption and breastmilk mercury concentrations(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21). Three studies examined shellfish consumption and hair mercury concentrations and reported no significant associations(Reference Bentzen, Castellini, Gaxiola-Robles, Zenteno-Savín, Méndez-Rodríguez and O’Hara43,Reference Gaxiola-Robles, Bentzen, Zenteno-Savín, Labrada-Martagón, Castellini and Celis45,Reference Miyashita, Sasaki, Ikeno, Araki, Ito and Kajiwara48) .
A total of three studies measured arsenic status with mixed results(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21,Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51,Reference Soler-Blasco, Murcia, Lozano, Sarzo, Esplugues and Vioque52) . In a study of lactating women in Mexico, arsenic concentrations in breastmilk were lower among women that ate shellfish more than once a month as compared with women that either ate shellfish less than once a month or not at all(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21). However, two studies of pregnant women (in Spain and the United States) both reported higher urinary arsenic concentrations with shellfish consumption(Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51,Reference Soler-Blasco, Murcia, Lozano, Sarzo, Esplugues and Vioque52) .
Three studies measured cadmium status(Reference Vollset, Iszatt, Enger, Gjengedal and Eggesbø49,Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51,Reference González, Calderón, Rúbies, Timoner, Castell and Domingo53) . A study in Spain reported that squid was responsible for 28% of the cadmium intake of infants and 38% of the cadmium intake of children(Reference González, Calderón, Rúbies, Timoner, Castell and Domingo53). A study in the United States of 558 pregnant women reported no association between shellfish consumption and urinary cadmium(Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51). A study in Norway of 300 lactating women showed a consistent trend of lower cadmium concentrations in breastmilk with shrimp intake, crab intake and mussel/scallop intake(Reference Vollset, Iszatt, Enger, Gjengedal and Eggesbø49).
Only one study examined lead in relation to shellfish consumption(Reference Saoudi, Dereumeaux, Goria, Berat, Brunel and Pecheux54). The large cohort study was conducted in France and showed that cord blood lead concentrations were lower among infants born to women who did not consume any shellfish compared with infants born to women who consumed shellfish(Reference Saoudi, Dereumeaux, Goria, Berat, Brunel and Pecheux54).
Summary
To summarise, mercury was the most-studied heavy metal and most, but not all, studies reported that higher shellfish consumption was associated with higher concentrations of mercury in the body. Associations with other heavy metals examined (arsenic, cadmium and lead) are inconclusive due to limited research and mixed results.
Other contaminants
Polychlorinate biphenyls (PCBs) are carcinogenic compounds used in the production of industrial and consumer products. PCBs have been banned internationally since 2001 by the Stockholm Convention on Persistent Organic Pollutants. Despite the ban, PCBs continue to affect human health due to their long half-life. Three studies have measured PCB status in relation to shellfish consumption(Reference Miyashita, Sasaki, Ikeno, Araki, Ito and Kajiwara48,Reference Cao, Yan, Yu, Tian, Zhao and Liu55,Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56) . A large cohort study of 1017 mother–infant dyads in China measured PCBs in cord blood(Reference Cao, Yan, Yu, Tian, Zhao and Liu55), a small study of 39 lactating women in Italy measured PCBs in breastmilk(Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56), and a third study of 367 pregnant women in Japan measured PCBs in blood(Reference Miyashita, Sasaki, Ikeno, Araki, Ito and Kajiwara48). No association between shellfish consumption and PCBs was found in any of the studies(Reference Miyashita, Sasaki, Ikeno, Araki, Ito and Kajiwara48,Reference Cao, Yan, Yu, Tian, Zhao and Liu55,Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56) .
Polybrominated diphenyl ethers (PBDEs) are compounds found in flame retardants and a wide array of other products, including plastics, electronics, furnishing, etc. Three studies examined whether shellfish may also be a source of PBDEs with mixed results(Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56–Reference Wang, Wang, Chen, Chao, Tsou and Shy58). A small study of lactating women in Italy reported no association between shellfish consumption and PBDE concentrations in breastmilk(Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56). A study in Spain of 541 mother–infant dyads demonstrated higher PBDE cord blood concentration incrementally among infants born to women consuming shellfish(Reference Costa, Lopez-Espinosa, Vizcaino, Murcia, Iñiguez and Navarrete-Muñoz57). In contrast, a small study of twenty lactating women in Taiwan reported lower PBDE breastmilk concentrations for women that ate shellfish nine or more times per month(Reference Wang, Wang, Chen, Chao, Tsou and Shy58).
Bisphenol A (BPA) is an industrial compound found in polycarbonate plastics and epoxy resins(Reference Staples, Dome, Klecka, Oblock and Harris59). In China, a study of 506 pregnant women reported that those who always consumed shellfish had significantly higher urinary BPA concentrations than those that seldom consumed shellfish(Reference Zhao, Wang, Pan, Shi, Wang and Tian60).
Organochlorine pesticides (OCPs) are pesticides that have been extensively used in the past but, due to concerns around a long half-life and neurotoxicant and carcinogenic effects, have increasingly been banned in countries(Reference Keswani, Dilnashin, Birla, Roy, Tyagi and Singh61). A large cohort study that enrolled pregnant women in China examined whether shellfish consumption was associated with indicators of OCP exposure in cord blood(Reference Cao, Yan, Yu, Tian, Zhao and Liu55). The three OCPs included in the study, all of which have been banned globally by the Stockholm Convention on Persistent Organic Pollutants, were (1) hexachlorobenzene (HCB), (2) beta-hexachlorocyclohexane (β-HCH) and (3) dichlorodiphenyltrichloroethane (DDT). No association was reported with HCB for shellfish consumption(Reference Cao, Yan, Yu, Tian, Zhao and Liu55). Higher shrimp intake was associated with higher cord blood concentrations of β-HCH, but no significant associations with crab intake or a group of other shellfish (oyster, clam, snail and scallop analysed together)(Reference Cao, Yan, Yu, Tian, Zhao and Liu55). No association was reported with DDT for shrimp or crab intake; however, higher intake of the group of other shellfish was associated with higher cord blood DDT concentrations(Reference Cao, Yan, Yu, Tian, Zhao and Liu55). As oyster, clam, snail and scallop were analysed as a group, it is unclear which specific mollusc is associated with higher DDT concentrations.
Polycyclic aromatic hydrocarbons (PAHs) are chemical compounds that may be carcinogenic and are produced from the burning of coal, oil, gas, wood, garbage and tobacco and cooking meat and other foods at high heat(Reference Ifegwu and Anyakora62). Benzo[a]pyrene (BaP) is the most-studied PAH. A study of 657 pregnant women in Spain found that, out of 24 food groups analysed, shellfish ranked as one of the top three food group predictors for BaP dietary intake and total PAH dietary intake(Reference Duarte-Salles, Mendez, Pessoa, Guxens, Aguilera and Kogevinas63).
Dioxins are a group of carcinogenic compounds produced during industrial processes and some natural processes, such as volcanic eruptions(Reference Tavakoly Sany, Hashim, Salleh, Rezayi, Karlen and Razavizadeh64). Most human exposure is due to contaminated food, primarily meat, dairy, fish and shellfish(Reference Tavakoly Sany, Hashim, Salleh, Rezayi, Karlen and Razavizadeh64). A study of 140 lactating women in Vietnam reported that women that consumed marine crab and shrimp had significantly higher concentrations of dioxins in breastmilk than women who did not consume these shellfish(Reference Anh, Nishijo, Tai, Maruzeni, Morikawa and Anh65). No other shellfish intake was examined in this study.
Perfluoroalkyl substances (PFAS) are chemical compounds that resist heat, oil, water and stains and are used as coatings on a variety of products, including furniture, non-stick cookware and food packaging(Reference Xiao66). PFAS have a long half-life and bioaccumulate in aquatic foods, including shellfish(Reference Giffard, Gitlin, Rardin, Petali, Chen and Romano67). A large cohort study of pregnant women in Spain found that women with high shellfish intake, defined as 0·8 servings per week or more, had higher PFAS plasma concentrations than women with the lowest intake(Reference Manzano-Salgado, Casas, Lopez-Espinosa, Ballester, Martinez and Ibarluzea68).
Summary
Overall, research of how shellfish consumption relates to contaminants other than heavy metals is relatively new; however, the current literature consistently suggests that higher shellfish consumptions is associated with higher concentrations of the selected contaminants (PCBs, PBDEs, BPA, OCPs, PAHs, dioxins, PFAS) studied in women and infants.
Discussion
This review aimed to summarise the impacts of mollusc and crustacean consumption on maternal and child health and child development outcomes. Our review found that, although the literature is limited, there are some interesting trends that are emerging. Of note, shellfish consumption was consistently associated with higher concentrations of mercury in blood. Other heavy metals either had mixed results or just a single study presenting results. Research examining chemical and pesticide concentrations in humans supports shellfish as a possible source of exposure. The limited research on nutritional biomarker status suggests an association between shellfish consumption and iodine status and potentially also for fatty acids, but very few nutrients have been studied. Overall, preterm birth was not associated with shellfish consumption, but newborn anthropometry had more mixed results, with several studies reporting that higher shellfish consumption was associated with a lower birth weight. There was mixed evidence regarding shellfish consumption during pregnancy and early life on prospective risk of shellfish allergy in offspring. Lastly, the two studies that examined child development and the four studies that examined maternal health outcomes reported no significant associations.
Despite shellfish serving as a nutrient-dense food that could help address the increased nutrient needs in the first 1000 d, there were surprisingly few studies that have examined specific nutrient intake or status related to shellfish consumption, with only four nutrients investigated (iodine, fatty acids, selenium and molybdenum). Some shellfish such as oysters, clams and crab are noted for high zinc and iron concentrations(4,5) . As both zinc and iron deficiencies are common in the first 1000 d, it would be beneficial for future research to examine whether shellfish consumption is associated with zinc and iron status among pregnant women and children. Likewise, maternal health and child development has to date been understudied in relation to mollusc and crustacean consumption. Given the positive associations between shellfish consumption and iodine status, there is particular support for further examination of child development outcomes, as iodine status during pregnancy and early childhood impacts cognitive development(Reference Bath69,Reference Bath, Steer, Golding, Emmett and Rayman70) .
When considering the forty-six studies included in this review, it was striking that none of these studies has been conducted in Africa. This is particularly important to note for a number of reasons. First, improving nutrition in the first 1000 d is increasingly identified as a public health goal for many countries in Africa, with improved nutrition as a key component(Reference Pentecost and Ross71,Reference Wrottesley, Lamper and Pisa72) . Shellfish have the potential to serve as a food source for key micronutrient deficiencies that are common in Africa, such as iron and zinc, while also serving as an excellent source of protein(4,5) . Second, many countries in Africa struggle with environmental policy regulation and enforcement to ensure that the waters where shellfish harvesting occurs do not exceed health hazard indexes for heavy metals and other pollutants. Indeed, there have been studies in African countries that have confirmed contamination of shellfish with heavy metals and other compounds associated with adverse health outcomes(Reference Gbogbo, Otoo, Asomaning and Huago73,Reference El Nemr, El-Said, Ragab, Khaled and El-Sikaily74) . Clearly, there is a compelling need to evaluate the potential trade-offs in terms of risks and benefits for shellfish nutrition in Africa.
It is also critical to note that, while the majority of the studies focused on measuring biomarkers of environmental exposures related to shellfish consumption, there was wide heterogeneity in terms of outcomes being measured. Among the twenty-seven studies that measured exposure to heavy metals or other pollutants, there were eleven different heavy metals and other pollutants that were assessed. Mercury was the only contaminant that had a strong evidence base. Arsenic(Reference Gaxiola-Robles, Labrada-Martagón, Celis de la Rosa A de, Acosta-Vargas, Méndez-Rodríguez and Zenteno-Savín21,Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51,Reference Soler-Blasco, Murcia, Lozano, Sarzo, Esplugues and Vioque52) , cadmium(Reference Vollset, Iszatt, Enger, Gjengedal and Eggesbø49,Reference Osorio-Yáñez, Gelaye, Enquobahrie, Qiu and Williams51,Reference González, Calderón, Rúbies, Timoner, Castell and Domingo53) , PCB(Reference Miyashita, Sasaki, Ikeno, Araki, Ito and Kajiwara48,Reference Cao, Yan, Yu, Tian, Zhao and Liu55,Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56) and PBDE(Reference Ingelido, Ballard, Dellatte, di Domenico, Ferri and Fulgenzi56–Reference Wang, Wang, Chen, Chao, Tsou and Shy58) each had three studies reporting results. The other six outcomes were supported by just a single study each. This is problematic as the issue of publication bias makes it more likely that there will be a significant association reported when only a single study has published on the topic. All six of the studies do, in fact, report a significant association, so the overall results related to environmental exposures should be interpreted with caution. However, it is critical that additional research be conducted to clarify the safety of mollusc and crustacean consumption. They are a part of many dietary patterns globally, and maintaining them as a safe food source is supportive of different cultures.
Whether shellfish consumption should be encouraged during pregnancy is still inconclusive based on our findings from this review related to preterm birth, low birth weight and SGA. Of the four studies that examined preterm birth or duration of gestation, three found no significant association(Reference Heppe, Steegers, Timmermans, Breeijen den, Tiemeier and Hofman22,Reference Le Donne, Alibrandi, Vita, Zanghì, Triolo and Benvenga23,Reference Mohanty, Siscovick, Williams, Thompson, Burbacher and Enquobahrie29) . These studies were based in Italy, the United States and the Netherlands, representing a wide range of shellfish consumption typically present in the diet. The one study that reported a significant reduction in preterm birth with higher shellfish consumption was conducted in China and had over 10 000 study participants(Reference Wang, Fu, Deng, Zhu, Zhang and Zhang30). This association may be due to shellfish serving as a source of omega-3 long-chain polyunsaturated fatty acids (LCPUFAs), which have been shown to reduce risk of preterm birth in randomised clinical trials(Reference Middleton, Gomersall, Gould, Shepherd, Olsen and Makrides75). It is unclear why the association would only be evident in China but additional research in this area is warranted. The mixed findings for low birth weight and SGA similarly are lacking any clear explanation for differing results based on geography or study size. Randomised controlled trials have shown omega-3 LCPUFA supplements during pregnancy reduce the risk of low birth weight but have little effect on SGA(Reference Middleton, Gomersall, Gould, Shepherd, Olsen and Makrides75).
There are several strengths of this review. The primary strength is that it covered a broad spectrum of health and development indicators, including measures related both to adverse and beneficial health outcomes, to ensure this review is most useful to those working in policy and programmatic action. Further, our review was conducted according to best practices for reviews, including pre-registering our protocol, reviewing abstracts by two separate reviewers and a third when there was discrepancy, and following PRISMA guidelines for reporting. However, there are some limitations to note when interpreting the results of this review. As no studies were conducted in Africa, there is limited generalisability to that region. In addition, all studies were cross-sectional studies or cohort studies, and there were no randomised controlled trials present in the literature. There was a heterogeneity in measures of outcomes, which created difficulty in summarising the literature. Further, several outcomes that may be associated with shellfish consumption were either absent from the literature or understudied; thus, a lack of a reported association in this review should be understood in that context. For example, while our review found multiple articles that measured environmental contaminants in breastmilk, we found no articles that reported on shellfish allergens in breastmilk. Research on shellfish allergen detection in breastmilk and other understudied outcomes would be beneficial.
Conclusion
To our knowledge, this is the first comprehensive review of evidence focused on molluscs and crustaceans in human nutrition. We found both health benefits and risks in association with the aquatic animals consumed during the first 1000 d of life, but importantly, major gaps in the literature – including a notable lack of studies conducted in LMICs in Africa among nutritionally vulnerable populations. Studies largely came from high-income countries, with little to no evidence from nutritionally vulnerable populations. We identified minimal evidence on critical limiting nutrients such iron and zinc, despite shellfish concentrations in these important nutrients. The findings for mercury and other contaminants were concerning and merit attention by governments around the world to ensure the safety of aquatic foods. Molluscs and crustaceans hold potential for enhancing diet diversity and confronting a range of nutrient deficiencies, but there is a need to evaluate contextual factors and generate more evidence to understand the trade-off health risks and benefits, particularly for LMICs in Africa.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954422424000064.
Data sharing
Data are available on request from the corresponding author.
Financial support
None.
Competing interests
None.
Authorship
B.M.O. and L.I. conceptualised and designed the study; E.A.G. and E.K. conducted the literature search; B.M.O., E.A.G. and L.I. drafted the manuscript; B.M.O., E.A.G., K.R. and L.I. contributed to interpretation and revision of the manuscript; all authors reviewed the final manuscript.







