Introduction
Jumping to conclusions (JTC) is a reasoning bias characterised by a tendency to seek less information before making a decision (Fine, Gardner, Craigie, & Gold, Reference Fine, Gardner, Craigie and Gold2007). It has been reported in patients with a first episode of psychosis (FEP) (Langdon, Still, Connors, Ward, & Catts, Reference Langdon, Still, Connors, Ward and Catts2014), in chronic psychosis (Moritz, Van Quaquebeke, & Lincoln, Reference Moritz, Van Quaquebeke and Lincoln2012), in relatives of patients with psychosis (Van Dael et al., Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006), in people at clinical high-risk for psychosis (CHR) (Rausch et al., Reference Rausch, Eisenacher, Elkin, Englisch, Kayser, Striepens and Wagner2016), and in healthy volunteers with delusional beliefs (Linney, Peters, & Ayton, Reference Linney, Peters and Ayton1998). Some studies have found JTC to be particularly associated with delusions (Freeman, Pugh, & Garety, Reference Freeman, Pugh and Garety2008; Jolley et al., Reference Jolley, Thompson, Hurley, Medin, Butler, Bebbington and Garety2014) or proneness to delusions (Broome et al., Reference Broome, Johns, Valli, Woolley, Tabraham, Brett and McGuire2007), but this has not been reported consistently in all studies (Catalan et al., Reference Catalan, Simons, Bustamante, Olazabal, Ruiz, Gonzalez de Artaza and Gonzalez-Torres2015; So et al., Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington and Garety2012). Among people at CHR, there is a diversity of potential clinical outcomes. While a minority of individuals will go on to transition to psychosis, among those that do not, the severity of psychotic symptoms may either increase or decrease, and level of functioning may worsen or improve (Fusar-Poli et al., Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton, Valmaggia and McGuire2012; Michel, Ruhrmann, Schimmelmann, Klosterkötter, & Schultze-Lutter, Reference Michel, Ruhrmann, Schimmelmann, Klosterkötter and Schultze-Lutter2018). The presence of a reasoning bias in CHR participants is of particular interest because it might influenceany of these outcomes that may occur. In particular, because JTC has been associated with psychosis and delusions (So, Siu, Wong, Chan, and Garety, Reference So, Siu, Wong, Chan and Garety2016), JTC might increase the likelihood of attenuated psychotic symptoms persisting or progressing into a psychotic disorder. Only one previous study has investigated the relationship between JTC and clinical outcomes in CHR participants. Winton-Brown et al. (Reference Winton-Brown, Broome, Allen, Valli, Howes, Garety and McGuire2015) followed up a relatively small CHR sample (N = 23) and did not find a relationship between JTC and later transition to psychosis. However, JTC has been associated with adverse clinical outcomes in patients with psychosis. Rodriguez et al. (Reference Rodriguez, Ajnakina, Stilo, Mondelli, Marques, Trotta and Murray2018) reported that JTC at baseline was associated with a higher risk of compulsory admissions to hospital and police interventions at follow-up.
The present study aimed to examine the JTC bias at baseline in a large CHR sample, and to investigate its relationship with clinical outcomes. JTC was assessed in CHR participants and controls at baseline and after 1 and 2 years. Outcomes were assessed in terms of transition to psychosis, the severity of psychotic symptoms, and level of functioning. We tested the hypothesis that within the CHR sample, the presence of a JTC bias would be associated with adverse clinical outcomes.
Methods
Sample
Participants were recruited from 11 centers from July 2010 to August 2015, as part of the European Union Gene–Environment Interactions (EU–GEI) study (European Network of National Networks studying Gene-Environment Interactions in et al., Reference van Os, Rutten, Myin-Germeys, Delespaul, Viechtbauer and Mirjanic2014). The overall design of the study was naturalistic, longitudinal, and prospective, consisting of a baseline and two follow-up time points. CHR participants (N = 303) were recruited from local clinical early detection services, while healthy controls (HC; N = 57) were recruited from the same geographical areas. Inclusion criteria for all participants were: being aged 15–35; being able to consent, and having adequate language skills local to each center. In addition, CHR participants had to meet high risk for psychosis criteria defined according to the Comprehensive Assessment of At-Risk Mental States scale (CAARMS) (Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005). The exclusion criteria for CHR participants were: having had prior experience of a psychotic episode of more than 1-week as determined by the CAARMS (Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005) and Structural Clinical Interview for DSM Disorders (SCID) (Wittchen, Zauding, & Fydrich, Reference Wittchen, Zauding and Fydrich1997); previous treatment with an antipsychotic for a psychotic episode; and IQ < 60. Exclusion criteria for HC included meeting criteria for CHR, and report a personal or (first-degree) family history of psychosis.
Procedures
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Medical Ethics Committees approved all procedures involving human subjects at each participating site and approved the study protocol. Written informed consent was obtained from all participants. All participants were assessed at baseline and after 12 and 24 months. If a CHR subject transitioned to psychosis, a follow-up assessment was made as soon as possible after transition, 1 year after transition, and 2-years after transition.
Instruments
Detailed socio-demographic characteristics were assessed using the modified Medical Research Council socio-demographic Schedule (European Network of National Networks studying Gene-Environment Interactions in et al., Reference van Os, Rutten, Myin-Germeys, Delespaul, Viechtbauer and Mirjanic2014; Mallett, Reference Mallett1997).
The CAARMS was used to measure subclinical psychotic symptoms (Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005). CAARMS is a semi-structured interview that assesses psychotic symptoms and a range of other psychopathological symptoms occurring in emerging psychotic disorder. Individuals were classified as CHR for psychosis if they met at least one of the following risk criteria: (i) Vulnerability Group (a first-degree relative with a psychotic disorder or personal diagnosis of schizotypal personality disorder, in combination with a significant drop in functioning); (ii) Attenuated Psychotic Symptoms (psychotic symptoms sub-threshold either in intensity or frequency); (iii) Brief Limited Psychotic Symptoms (a recent brief psychotic episode that resolved spontaneously within 1 week).
The Scale for Assessment of Negative Symptoms (SANS) (Andreasen, Reference Andreasen1982), the Montgomery-Asberg Depression Scale (MADRS) (Montgomery & Asberg, Reference Montgomery and Asberg1979), and the Young Mania Rating Scale (Young, Biggs, Ziegler, & Meyer, Reference Young, Biggs, Ziegler and Meyer1978) were used to assess negative and affective symptoms.
The Structured Clinical Interview for DSM-IV Disorders (SCID) (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams1995) was used to establish the presence of other psychiatric disorders and to exclude the presence of current psychotic disorders.
The disability subscale of the Global Assessment of Functioning (GAF disability) scale (APA, 2000) was used to assess functional outcome. The GAF scoring system is based on the severity of symptoms and level of functioning for individuals under mental health care. As the GAF score decreases, symptoms and the severity of a mental health illness are more severe. The total GAF score was not used because it can reflect symptom severity as well as the level of functioning.
CAARMS, SCID, and GAF measures were repeated at each follow-up time point. Transition to psychosis was defined according to the CAARMS criteria (Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005). A short version of the WAIS-III test (Velthorst et al., Reference Velthorst, Levine, Henquet, de Haan, van Os, Myin-Germeys and Reichenberg2013) was used to assess IQ.
A computerised version of the ‘beads’ task (Garety, Hemsley, & Wessely, Reference Garety, Hemsley and Wessely1991) was used to assess probabilistic reasoning. Participants were shown two jars of coloured beads in equal but opposite ratios (60 red: 40 blue; 60 blue: 40 red). The jars were hidden and, at the start of the task, a sequence of beads from one of the two jars was displayed to the participants. After each bead, participants were asked if they were ready to decide which jar the beads were being drawn from or if they would like to see another bead before making a decision. In the present study, we examined two variables: (i) ‘Draws to decision’ (DTD), the number of draws made before making a decision; and (ii) ‘JTC/no JTC’, where JTC is operationally defined as reaching a decision after two or fewer beads (Garety et al., Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler and Dudley2005; Rodriguez et al., Reference Rodriguez, Ajnakina, Stilo, Mondelli, Marques, Trotta and Murray2018; So et al., Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington and Garety2012; So & Kwok, Reference So and Kwok2015). This threshold is thought to reflect a clear expression of a reasoning bias and is useful in discriminating between clinical and non-clinical groups (Garety et al., Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler and Dudley2005; Van Dael et al., Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006). We used the most difficult version of the task (60:40), as this has been found to be sensitive to detecting effects in relation to variation in levels of psychotic symptoms (Lincoln, Ziegler, Mehl, & W, Reference Lincoln, Ziegler, Mehl and Rief2010; So et al., Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington and Garety2012).
Statistical analyses
One-way analyses of variance (ANOVA) were used to examine differences in continuous variables at baseline, 1-year, and 2-year follow up. For categorical variables, chi-square tests and Fisher´s exact test, when indicated, were performed. To analyse the stability of JTC rates across follow-ups, we performed a McNemar´s test.
Cross-sectional associations between JTC and the psychotic symptoms at baseline were analysed using hierarchical logistic regression models. The number of beads requested by a participant yielded a continuous outcome variable with a range of 0–20. This variable was positively skewed, and we followed standard procedure to categorize JTC reasoning bias in a binary category requesting two or fewer beads before making a decision or more than two (JTC/no JTC). In order to assess whether any association with delusional symptoms was independent of other positive, negative or affective symptoms, subsequent analyses were performed in which all symptom domains assessed with the CAARMS were entered simultaneously in the model.
The relationship between JTC and transition to psychosis within 2 years was analysed using the Cox regression method adjusting by gender, age, ethnicity (White, Black, Asian, Mixed, North African, and others), site, and IQ.
Examining the relationship between JTC at baseline and functional outcome, at follow-up in the CHR group involved data with a multilevel structure because multiple observations were nested within participants (level 2), and participants were nested within sites (level 3). Consequently, we used a three-level mixed model with fixed and random effects to analyse and control for the within-subject level of clustering and clustering within-sites. An unstructured covariance pattern model was used to model the dependency of the repeated observations of the same subject while study site was included as a random factor to account for the dependency of the subjects within site (Brown & Prescott, Reference Brown and Prescott2015). The GAF disability scale at follow-up was used as the dependent variable while the JTC (as categorical variable yes/no) was used as independent variable. Subsequently, we estimated the effect of JTC on the GAF scale. The following a priori-selected confounders were included in the model: transition, age, gender, IQ, and ethnicity. The model included the interaction between JTC at baseline and its evolution across different time points.
Finally, we assessed the accuracy of the model to predict unseen cases of the same underlying population (internal validation) using repeated 10-fold cross-validation (Hastie, Tibshirani, & Friedman, Reference Hastie, Tibshirani and Friedman2009). In 10-fold cross-validation, the single available dataset is randomly divided into 10 folds of equal size. In turn, each fold is used as the unseen data (test set) with the remaining n-1 folds pooled together as the training set (Stahl & Pickles, Reference Stahl and Pickles2018). Prediction accuracy is estimated by comparing observed and precited GAF scores and quantified as explained variance (r 2). We repeated this procedure 200 times to get a stable estimate and report the average over the 200 10-folds runs.
Because mixed-effects models with incomplete cases are difficult to assess in repeated cross-validation, we estimated the prediction accuracy for time 2 and 3 separately and included study site as a categorical factor. Because the number of missing predictors was relatively small (N = 11 out of 201 available observations for each time point), a complete case analysis was performed. Because of the large number of study sites relative to sample size, we rerun the cross-validation without study site.
All the analyses were performed with STATA 15 package (StataCorp., 2017). Except for the cross-validation, which was performed in R using the package glmnet (Friedman, Hastie, & Tibshirani, Reference Friedman, Hastie and Tibshirani2010).
Results
In total, 411 individuals were assessed at baseline (i.e. 344 CHR and 67 HC). A total of 10 HC and 41 CHR participants were excluded, as they did not complete the ‘beads’ task at baseline. The non-included sample did not differ significantly from the included sample in terms of gender, age, IQ, ethnicity, or GAF scores (online Supplementary Table S1). The demographic and clinical features of the groups are shown in Table 1. At baseline, IQ and GAF disability scores were higher in the HC than the CHR group. Relative to the total sample at baseline (N = 360), 173 participants (45.3%) completed the task at 1-year follow-up, and 134 (37.2%) did so at 2-year follow-up. At follow up, there were no significant differences between the remaining participants and drop-outs in terms of ethnicity, gender, IQ, or GAF disability score. However, they differed in age at both, 1 year [non-missing 23.8 (s.d. = 4.8) v. missing 21.8 (s.d. = 4.7), p < 0.05], and 2 years [non-missing 23.2 (s.d. = 4.6), and missing 21.7 (s.d. = 4.8), p < 0.05].
Table 1. Socio-demographic and clinical characteristics of the sample
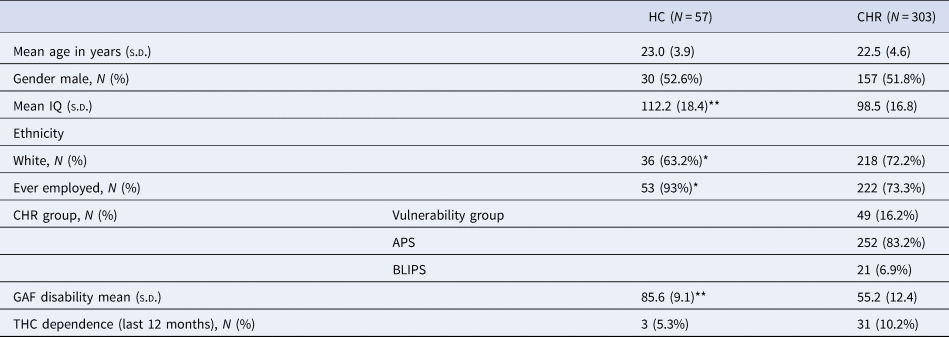
HC, healthly controls; CHR, clinical high-risk for psychosis; IQ, intelligence quotient; THC cannabis.
*p < 0.05;**p < 0.001.
In total, 8 CHR participants (5.9%) were taking antipsychotic medication at baseline, 69 were taking antidepressants (22.8%) and 17 sedatives (5.6%, including benzodiazepines). Only one HC (1.7%) used antidepressants and another one (1.7%) used sedatives.
To analyse longitudinal changes in JTC status within the CHR group subsequent to baseline, we used the last available ‘JTC/no JTC’ measure for the dropouts cases [average follow-up time = 21.2 months (s.d. = 0.5)]. A total of 15 CHR (23.4%) who were classified as ‘JTC’ at baseline changed to ‘no-JTC’, at 1-year follow-up, while 4 (1.7%) classified as ‘no-JTC’ at baseline were classed as ‘JTC’ at 1-year follow up (p = 0.02). There were no significant changes in ‘JTC/no JTC’ status between 1-year and 2-year follow-up (p = 0.14). There were no significant changes in ‘JTC/no JTC’ status in HC group.
Relationship between JTC and transition to psychosis
After 2-years, 58 CHR individuals (16.1%) had made a transition to psychosis (CHR-T), while 245 had not (CHR-NT). There were no statistically significant differences between CHR-T and CHR-NT or between these subgroups and HC in the percentage of JTC rates at baseline, or 1-year follow-up. However, the rate of JTC in the CHR-T group was significantly higher than in the other groups at 2-year follow-up (χ2 = 8.13, df = 2; p = 0.012) (Fig. 1). At this time point, the CHR-T participants had already developed psychosis; the mean days between the transition and the last ‘beads’ task assessment was 795 days (s.d. = 85.3). The number of DTD by the group is detailed in online Supplementary Table S2.
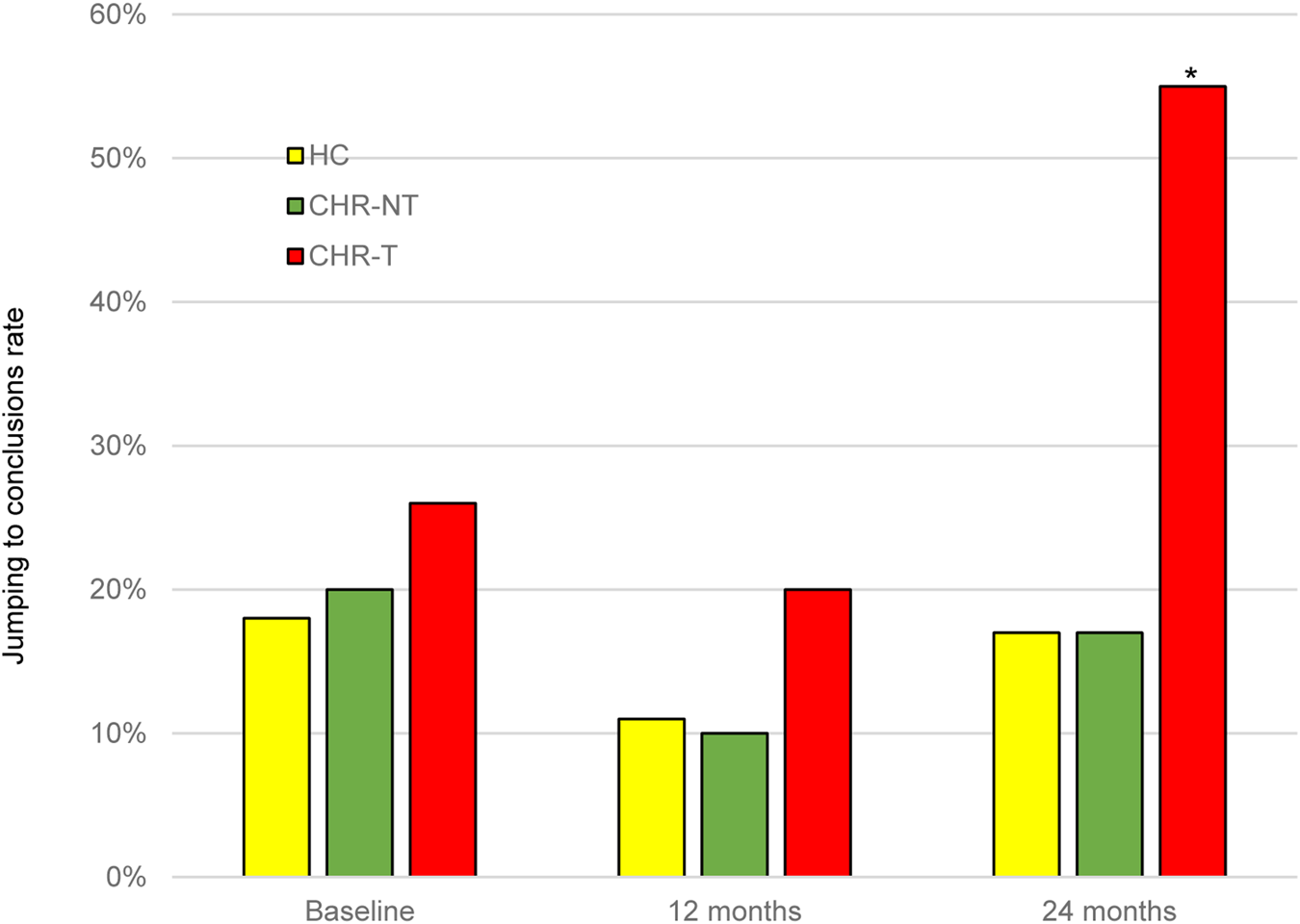
Fig. 1. Level of JTC bias at baseline and 1- and 2-year follow-up. *significant level p < 0.05. JTC, jumping to conclusions; HC, healthy controls; CHR-T, clinical high-risk subjects who made a transition to psychosis; CHR-NT, clinical high-risk subjects who did not make transition to psychosis.
The Cox regression revealed no significant association between JTC and transition to psychosis in the CHR group (all p's > 0.05). Within the CHR group, there were no significant or trend-level associations between ‘beads’ task performance and scores on the CAARMS subscales (positive, negative and general psychopathology), the SANS, the Young Rating Mania score or the MADRS. A descriptive summary of the symptoms, according to JTC group, is detailed in online Supplementary Table S3.
Relationship between JTC and functional outcome
Within the CHR group, there was a significant difference in GAF disability scores at both 1-year (Z = −2.3, p = 0.02), and 2-year (Z = −2.0, p = 0.04) follow-up between the ‘JTC’ group and ‘no-JTC’ group. This difference was not present at baseline. At both time points, the tendency to JTC was associated with a lower level of functioning (Fig. 2).
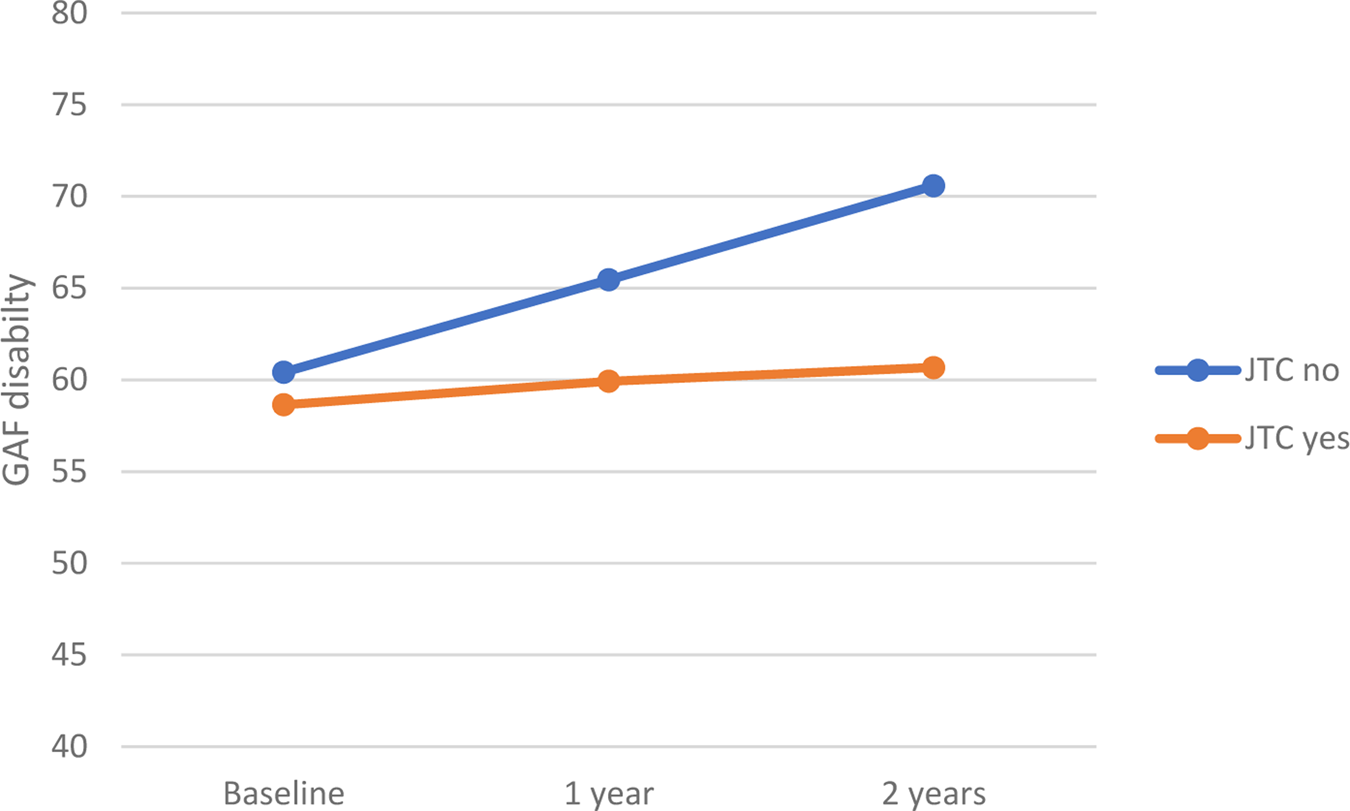
Fig. 2. Relationship between JTC and level of functioning in CHR subjects. GAF means during the follow-up period. JTC, jumping to conclusions; GAF, Global Assessment of Functioning.
There was a significant association between JTC and IQ in the whole sample (p < 0.001); however, the effect size of this association was small (R 2 = 0.06).
In the CHR group, there was an association between JTC at baseline and the GAF disability score at follow-up. At 2-year follow-up, the CHR sub-group with ‘JTC’ reasoning bias at baseline scored 5.3 points less [95% CI (−8.5 to −2.1), p = 0.001, adjusted] than the CHR sub-group with ‘no-JTC’ at baseline (Table 2).
Table 2. Relation between JTC at baseline and GAF disability evolution at follow-up after adjusting by other variables in CHR population
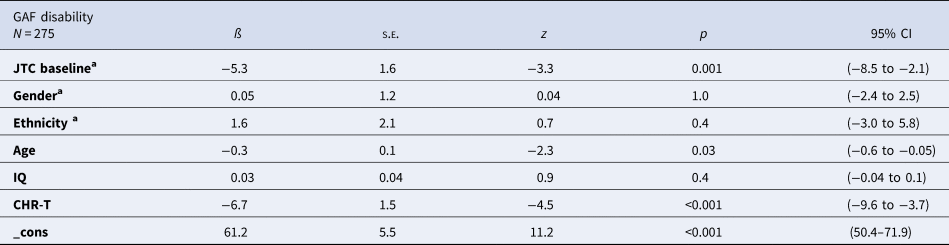
IQ, intelligence quotient; CHR-T, CHR subjects who made a transition to psychosis; s.e., standard error; 95% CI, 95% confidence interval of Beta.
a Reference group: ‘no JTC’ at baseline. White ethnicity. Male gender.
Repeated 10-fold cross-validation of two linear regressions predicting GAF disability, including site as a fixed factor, showed that the ‘JTC’ at baseline model predicted 26% of the variance of GAF score at time 2 and 24.7% at time 3. Removing site as predictor further increased the prediction accuracy to 30.5% (1-year follow-up) and 30.6% (2-years follow-up).
Discussion
This study sought to examine reasoning bias in the CHR population and its relationship with clinical outcomes. To our knowledge, this is the largest study of JTC in a CHR sample to date. We found that there were no differences between CHR and HC at baseline, but that a significant reasoning bias emerged in CHR-T participants when they were reassessed 2 years after transition. Within the CHR sample, there were no differences at baseline between participants who did or did not transition to psychosis, but those who developed psychosis showed a greater reasoning bias at 2-year follow-up. Similarly, within the CHR sample, the tendency to JTC was not associated with the level of functioning at baseline, but it was significantly associated with lower functioning at 1-year and 2-year follow-up. However, we did not find a significant relationship between JTC and positive psychotic symptomatology in the CHR sample.
Previous studies have reported a higher prevalence of JTC in CHR participants than in HC (Broome et al., Reference Broome, Johns, Valli, Woolley, Tabraham, Brett and McGuire2007; Rausch et al., Reference Rausch, Eisenacher, Elkin, Englisch, Kayser, Striepens and Wagner2016). However, in the present study, this difference was only present at 2-year follow-up and only in those who made transition to psychosis. Methodological differences can partially explain this discrepancy. Rausch et al. (Reference Rausch, Eisenacher, Elkin, Englisch, Kayser, Striepens and Wagner2016) used the SIPS and SPI-A to define the CHR state in a sample of 188 subjects, analysing the differences between CHR subgroups (basic symptoms v. at-risk mental state status). The SIPS semi-structured interview (McGlashan, Walsh, & Woods, Reference McGlashan, Walsh and Woods2010) and the CAARMS (Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005) present some differences in the measures of frequency and severity of the psychotic symptoms. The SIP-A (Schultze-Lutter, Addington, Ruhrmann, & Klosterkötter, Reference Schultze-Lutter, Addington, Ruhrmann and Klosterkötter2007) specifically focuses on the detection of basic symptoms. We cannot exclude that these differences might have an impact on the comparability of the results. In addition, although Broome et al. (Reference Broome, Johns, Valli, Woolley, Tabraham, Brett and McGuire2007) used the CAARMS to define CHR status, the sample analysed was smaller (N = 35) compared to the present one. The larger sample in the current study might have increased the statistical power to detect the possible existing differences. None of these studies analysed the relation between JTC and the transition to psychosis.
In the present study, the presence of a reasoning bias in CHR-T participants at follow up but not at baseline (prior transition to psychosis), suggests that JTC may be linked to change in clinical status subsequent to baseline. This would be consistent with our finding that within the CHR sample, JTC was associated with a low level of functioning at follow up, and with the onset of psychosis. We did not find evidence that JTC was linked to the severity of psychotic symptoms, as has been reported in some previous studies in the CHR population (Winton-Brown et al., Reference Winton-Brown, Broome, Allen, Valli, Howes, Garety and McGuire2015) and schizophrenia (Dudley, Taylor, Wickham, & Hutton, Reference Dudley, Taylor, Wickham and Hutton2016). However, in a recent meta-analysis, a link between JTC and the psychotic symptom was not found (So et al., Reference So, Siu, Wong, Chan and Garety2016).
To the best of our knowledge, only one previous study has examined whether, in CHR individuals, JTC is associated with subsequent transition to psychosis (Winton-Brown et al., Reference Winton-Brown, Broome, Allen, Valli, Howes, Garety and McGuire2015). This study reported no relationship between JTC at baseline and subsequent transition to psychosis. While this negative result might have been related to the small CHR sample in that study (n = 23), this is much less likely to have been a factor in the present study, which had a large CHR sample at baseline, of whom 58 CHR made a subsequent transition to psychosis. In the current work, the presence of a reasoning bias after the onset of psychosis suggests that this may be specific to the emergence of frank psychosis, as opposed to the CHR state. However, we also found an association with poor functioning, a measure that is independent of transition status. An association with transition might also reflect a relationship with the severity of psychotic symptoms. However, our analyses found no evidence that JTC was linked to symptom severity. Finally, because the final follow-up assessment in the CHR-T participants was performed after they had transitioned to psychosis, it is possible that the findings were related to the effects of treatment initiated after psychosis onset. However, So et al. (So, Garety, Peters, & Kapur, Reference So, Garety, Peters and Kapur2010) did not find that antipsychotic treatment influences JTC bias.
In our sample, the proportion of the CHR participants for whom the status changed from `JTC´ to `no-JTC´ after 1-year follow-up was 23.4%. This suggests that this group is prone to change. Ormrod et al. (Reference Ormrod, Shaftoe, Cavanagh, Freeston, Turkington, Price and Dudley2012) investigated the progression of JTC in FEP patients. When tested over two-time points around 8 months apart from baseline, there was a degree of instability in JTC over time. However, in people with more long-standing psychosis, the decision-making style, in terms of JTC rates, does not seem to change (Peters & Garety, Reference Peters and Garety2006; So et al., Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington and Garety2012). It is possible that people in the early stages of psychosis are more likely to improve this trait (Catalan et al., Reference Catalan, Simons, Bustamante, Olazabal, Ruiz, Gonzalez de Artaza and Gonzalez-Torres2015).
CHR participants with a JTC bias failed to show an improvement in the level of functioning over the 2 years subsequent to baseline that was evident in the subgroup who did not have a JTC bias at baseline (Fig. 2). This suggests that in this population, the presence of a reasoning bias is associated with a relatively poor prognosis, in terms of functional outcome. Moreover, the cross-validation demonstrated that baseline JTC reasoning bias predicted GAF disability (30.6% explained variance) at 2-year follow-up (predicted values correlate by 0.55). Although the prediction finding is modest for it to be used in a prediction tool, the internal validation shows that the model is useful for understanding the factors that influence the evolution of the GAF disability scale. This finding could be used in future research to develop a productive tool.
Previous studies described a link between JTC and functional outcomes. For example, Rodriguez et al. (Reference Rodriguez, Ajnakina, Stilo, Mondelli, Marques, Trotta and Murray2018) showed that, in a FEP sample, JTC was related to a higher risk of subsequent compulsory admission and police intervention, both related to a worse social functioning. Dudley et al. (Reference Dudley, Daley, Nicholson, Shaftoe, Spencer, Cavanagh and Freeston2013) found that patients with psychosis whose JTC bias improved over 2-year follow up had a better clinical outcome than patients with a persistent JTC bias. Accordingly, a prospective study in a general population sample with affective dysregulation reported that the subsequent onset of psychosis was more likely when a JTC bias was present (Rauschenberg et al., Reference Rauschenberg, Reininghaus, Ten Have, de Graaf, van Dorsselaer, Simons and van Os2020).
It has been suggested that JTC bias is related to the decision-making process (Turner et al., Reference Turner, MacBeth, Larkin, Moritz, Livingstone, Campbell and Hutton2018), and the decision-making process based on weak evidence might be linked with a worse functioning outcome in the long term. However, the formation of delusions needs other concurrent factors, such as the necessity of closure (McKay, Langdon, & Coltheart, Reference McKay, Langdon and Coltheart2007) and overconfidence in errors (Moritz et al., Reference Moritz, Goritz, Balzan, Gaweda, Kulagin and Andreou2017).
In our sample, the level of positive psychotic symptoms was not associated with the rate of JTC reasoning bias, suggesting that JTC could reflect a more general vulnerability to disorders with psychosis proneness rather than to specific psychotic symptoms. The low psychotic symptoms level in our sample could, at least in part, explain the lack of association between JTC and psychotic symptoms. Similarly, in a recent meta-analysis So et al. (Reference So, Siu, Wong, Chan and Garety2016) could not demonstrate a definitive relationship between JTC and psychotic symptoms. Nevertheless, as Bentall (Reference Bentall1999) stated, the assumption behind the dichotomous trait-state distinction is that abnormalities are either present before the emergence of symptoms (in which case they might play a causal role) or covary with symptoms (in which case they may be either part of the symptom picture or epiphenomena). Thus, this is not the only possible relationship between JTC and psychotic symptoms. It might be that cognitive performance underpinning JTC in psychotic patients and in psychosis-proneness populations can be normal under optimal environmental circumstances and become pathological if these circumstances become unfavourable.
Our data highlight the relationship between the presence of JTC and worse functioning outcomes. Since some therapeutic strategies have demonstrated to be useful in the treatment of JTC (Moritz et al., Reference Moritz, Andreou, Schneider, Wittekind, Menon, Balzan and Woodward2014; Roberts et al., Reference Roberts, Combs, Willoughby, Mintz, Gibson, Rupp and Penn2014; Waller, Freeman, Jolley, Dunn, & Garety, Reference Waller, Freeman, Jolley, Dunn and Garety2011), it would be recommendable incorporating these strategies in the early treatment of CHR population. For example, recently Turner et al. (Reference Turner, MacBeth, Larkin, Moritz, Livingstone, Campbell and Hutton2018) reported the benefits of a specific intervention derived from the meta-cognitive treatment manual developed by Moritz et al. (Moritz, Woodward, & Burlon, Reference Moritz, Woodward and Burlon2007) in reducing JTC bias.
Strengths and limitations
Strengths of the present study include the large size of the CHR sample and the availability of data from repeated assessments at 1 and 2 years from baseline. Although there was substantial attrition over the follow-up period, having a large sample at baseline ensured that there were still sufficient subjects to detect significant longitudinal effects. Because at baseline most of the CHR sample was either antipsychotic naïve or had been minimally treated, the results are unlikely to be related to the effects of antipsychotic treatment. Because of evidence that the association between psychotic symptoms and JTC might no longer be significant when general intelligence is taken into account (European Network of National Networks studying Gene-Environment Interactions in et al., Reference van Os, Rutten, Myin-Germeys, Delespaul, Viechtbauer and Mirjanic2014; So et al., Reference So, Freeman, Dunn, Kapur, Kuipers, Bebbington and Garety2012; Tripoli et al., Reference Tripoli, Quattrone, Ferraro, Gayer-Anderson, Rodriguez, La Cascia and Di Forti2020), we included a measure of IQ in our analyses. This indicated that the associations between JTC and transition and a poor level of functioning at follow-up were not attributable to an effect of IQ, or other potentially confounding variables such as age, gender, and ethnicity.
This study presents also some limitations. The size of the HC group did not match that of the CHR sample, which might have reduced our power to detect statistically significant associations within the HC group. In addition, the follow-up dropout rate could have influenced the strength of the conclusions, since patients that chose to participate in the study might have presented with a less severe psychotic symptomatology.
Conclusions
This study suggests that the presence of a reasoning bias in people at CHR for psychosis is associated with adverse clinical outcomes, such as a low level of functioning. This finding together with findings around the efficacy of treatment approaches targeting JTC (Garety et al., Reference Garety, Waller, Emsley, Jolley, Kuipers, Bebbington and Freeman2015; So et al., Reference So, Chan, Chong, Wong, Lo, Chung and Chan2015) may show promise in enhancing treatment responses in CHR population.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720003396
Acknowledgements
We would like to thank all participants who took part in the study. This work was supported by the European Network of National Schizophrenia Networks Studying Gene–Environment Interactions (EU-GEI) Project is funded by grant agreement HEALTH-F2-20010–241909; [Project EU-GEI] from the European Community´s Seventh Framework Programme. Additional support was provided by a Medical Research Council Fellowship to M Kempton (grant MR/J008915/1). N Barrantes-Vidal received additional support from the Ministerio de Ciencia, Innovación e Universidades (PSI2017-87512-C2-1-R) and the Generalitat de Catalunya (2017SGR1612 and ICREA Academia Award). The study received financial support by French Health Ministry (PHRC, AOM-07-118, ‘Influence of cannabis psychopathological outcome in At-risk mental state’ (ICAAR study)) et de la Fondation pour la Recherche Médicale (fellowship OG). Sainte-Anne Hospital promoted the study. D Stahl was part-funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London Maudsley Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. C Pantelis was supported by an Australian National Health & Medical Research Council (NHMRC) Senior Principal Research Fellowship (1105825).
Conflict of interest
None.
Appendix
EU-GEI High Risk Study Group Author list
Philip McGuire1, Lucia R. Valmaggia2, Mathilde Antoniades1, Maria Calem1, Matilda Azis1, Sara Pisani1, Lieuwe de Haan3, Mark van der Gaag4,5, Eva Velthorst3,6, Tamar C. Kraan3, Daniella S. van Dam3, Nadine Burger5, Barnaby Nelson7, Patrick McGorry7, G Paul Amminger7, Christos Pantelis8, Athena Politis7, Joanne Goodall7, Anita Riecher-Rössler9, Stefan Borgwardt9, Charlotte Rapp9, Sarah Ittig9, Erich Studerus9, Renata Smieskova9, Rodrigo Bressan10, Ary Gadelha10, Elisa Brietzke11, Graccielle Asevedo10, Elson Asevedo10, Andre Zugman10, Neus Barrantes-Vidal12, Tecelli Domínguez-Martínez13, Anna Racioppi14, Thomas R. Kwapil15, Manel Monsonet14, Lídia Hinojosa14, Mathilde Kazes16,17, Claire Daban16,17, Julie Bourgin16,17, Marion Plaze16,17, Célia Jantac16,17, Marie-Odile Krebs16,17, Dorte Nordholm18, Lasse Randers18, Kristine Krakauer18, Louise Birkedal Glenthøj18, Birte Glenthøj18, Merete Nordentoft18, Stephan Ruhrmann19, Dominika Gebhard19, Julia Arnhold20, Joachim Klosterkötter19, Gabriele Sachs21, Iris Lasser21, Bernadette Winklbaur21, Philippe A Delespaul,22,23, Bart P. Rutten22, Jim van Os1,24
Department of Psychosis Studies 1Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King's College London, De Crespigny Park, Denmark 458 Hill, London, United Kingdom SE5 8AF; 2Department of Psychology, Institute of Psychiatry, Psychology & Neuroscience, King's College London, De Crespigny Park, Denmark Hill, 456 London, United Kingdom SE5 8AF; 3AMC, Academic Psychiatric Centre, Department Early Psychosis, Meibergdreef 5, 1105 AZ Amsterdam, The Netherlands; 4VU University, Faculty of Behavioural and Movement Sciences, Department of Clinical Psychology and EMGO+ Institute for Health and Care Research, van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; 5Parnassia Psychiatric Institute, Department of Psychosis Research, Zoutkeetsingel 40, 2512 HN The Hague, The Netherlands; 6Icahn School of Medicine at Mount Sinai, department of Psychiatry, 1425 Madison Ave, New York, NY 10029; 7Centre for Youth Mental Health, University of Melbourne, 35 Poplar Road (Locked Bag 10), Parkville, Victoria 485 3052, Australia; Orygen, Parkville, Victoria, Australia; 8Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne & Melbourne Health, Carlton South, Victoria, Australia; 9Medical Faculty, University of Basel, Basel, Switzerland; 10LiNC - Lab Interdisciplinar Neurociências Clínicas, Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP; 11Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP; 12Departament de Psicologia Clínica i de la Salut (Universitat Autònoma de Barcelona), Fundació Sanitària Sant Pere Claver (Spain), Spanish Mental Health Research Network (CIBERSAM); 13CONACYT-Dirección de Investigaciones Epidemiológicas y Psicosociales, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz (México); 14Departament de Psicologia Clínica i de la Salut (Universitat Autònoma de Barcelona); 15Department of Psychology, University of Illinois at Urbana-Champaign (USA); 16INSERM, IPNP UMR S1266, Laboratoire de Physiopathologie des Maladies Psychiatriques, Université Paris Descartes, Université de Paris, CNRS, GDR3557-Institut de Psychiatrie Paris, France; 17Faculté de Médecine Paris Descartes, GHU Paris - Sainte-Anne, Service Hospitalo-Universitaire, Paris, France; 18Mental Health Center Copenhagen and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS, Mental Health Center Glostrup, Mental Health Services in the Capital Region of Copenhagen, University of Copenhagen; 19Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany; 20Psyberlin, Berlin, Germany; 21Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria; 22Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, South Limburg 463 Mental Health Research and Teaching Network, Maastricht University Medical Centre, P.O. Box 616, 6200 MD 464 Maastricht, The Netherlands; 23Mondriaan Mental health Trust, P.O. Box 4436 CX Heerlen, The Netherlands; 24Department of Psychiatry, UMC Utrecht Brain Center, Utrecht University Medical Centre, 3584 CG Utrecht, The Netherlands.







