Introduction
Spatial distribution and movement patterns of wild ungulates are strongly dependent on the spatial and temporal heterogeneity of biotic and abiotic resources (Bailey et al., Reference Bailey, Gross and Laca1996; Fryxell et al., Reference Fryxell, Wilmshurst and Sinclair2004). In most African ecosystems, feeding, drinking and resting places are subject to high seasonal variability and can be spatially segregated at certain times of the year. In this context, animals adopt space-use, movement and activity strategies that allow them to minimize detrimental effects of the main limiting factors to reach, at different scales, suitable trade-offs between several constraints and needs that must be addressed simultaneously (Godvik et al., Reference Godvik, Loe and Vik2009; Massé and Côté, Reference Massé and Côté2009).
Ungulates, like the vast majority of higher vertebrates, do not move erratically through the environment, but restrict their movements to sites much smaller in size than their locomotion capabilities allow, and which they mostly exploit over a long period. Based on this, Burt (Reference Burt1943) conceptualized the concept of home range as ‘the area traversed by the individual in its normal activities of food gathering, mating, and caring for young’. The home range is thus the spatial result of the movements and behaviours that an individual (or a social group) expresses at different spatiotemporal scales to survive and reproduce, in other words to maximize its (their) selective value. The home range is a central concept in ecology because it materializes a link between the movement of an animal and the resources necessary for its survival and reproduction.
In this chapter, we present the current state of knowledge on the space-use and feeding ecology of the African buffalo. This chapter also introduces some behavioural traits required for the comprehensiveness of this chapter, and which are further developed in other chapters. Most of what we know today about the behavioural ecology of savanna buffalo comes from studies focused on the Cape buffalo. In contrast, the ecology of the West and Central African savanna buffalo remains thus far poorly investigated. For this reason, and because several behavioural traits are similar within savanna buffalo subspecies, we present all savanna subspecies in one section, while the forest buffalo is the subject of a separate section.
Habitat
African buffalo live in a wide range of habitats, from open grasslands to rainforests, including all intermediate vegetation types: scrublands, woodlands and deciduous forests. African buffalo persist in semi-arid environments, as long as surface water is available within 20–40 km, year round (Cornélis et al., Reference Cornélis, Melletti, Korte, Melletti and Burton2014).
Biomass or production of savanna ecosystems, such as buffalo populations, is positively correlated with mean annual rainfall and the soil quality (Coe et al., Reference Coe, Cumming and Phillipson1976; Sinclair, Reference Sinclair1977; Grange and Duncan, Reference Grange and Duncan2006; Winnie et al., Reference Winnie, Cross and Getz2008). In similar water regime conditions, the nutrient content of vegetation and primary production are much lower on poor than on rich soils (Breman and De Wit, Reference Breman and De Wit1983; Le Houérou, Reference Le Houérou2008). The low biomass of wild ungulates in savanna ecosystems of West Africa (compared to those of volcanic areas in East Africa) mainly results from poorer soil conditions (Bell, Reference Bell, Huntley and Walker1982; East, Reference East1984; Fritz, Reference Fritz1997).
Savanna Buffalo
Savanna buffalo are mainly found in habitats with a high herbaceous biomass. In eastern and southern Africa, suitable grasses for Cape buffalo are found in several types of woodland, such as mopane (Colophospermum mopane), miombo (Brachystegia spp.), acacia (Acacia spp.) and Baikiaea spp. In West and Central Africa, savanna buffalo live in a variety of habitats ranging from typical Sahelian shrub savannas (Combretum spp., Terminalia spp, Acacia spp.) to Sudanian woodlands (e.g. Isoberlinia doka, Daniellia oliveri, Burkea africana).
Forest Buffalo
Forest buffalo are forest-dwellers, inhabiting rainforests with grassy glades, watercourse areas and mosaics of equatorial forests and savannas. This subspecies is absent (or present at very low densities) in continuous forests (Blake, Reference Blake2002; Melletti, Penteriani and Boitani, Reference Melletti, Penteriani and Boitani2007; Melletti, Reference Melletti2008).
Suitable habitats for forest buffalo are mosaics of forest with equatorial savannas or clearings, which consist of grassy vegetation and shrubs (Reitsma, Reference Reitsma1988; Blake, Reference Blake2002). Blake (Reference Blake2002) at Noubale–Ndoki National Park (NP) found high buffalo abundance close to open grassy areas with low abundance in the closed canopy forest, suggesting it is unsuitable for forest buffalo. A significant relationship between buffalo and natural forest clearings was also reported at Dzanga–Ndoki NP, where clearings were the centre of buffalo home ranges. In addition, no signs of buffalo presence were recorded over 500 m from clearings (Melletti, Penteriani and Boitani, Reference Melletti, Penteriani and Boitani2007; Melletti Reference Melletti2008). In Cameroon, buffalo rarely penetrated into the forest more than 300 m from logging roads, which were the main feeding sites for buffalo (Bekhuis et al., Reference Bekhuis, Jong and Prins2008).
Behaviour
Savanna Buffalo
African savanna buffalo are gregarious animals living in herds, and core members include adult females, subadults, juveniles and calves. Young females are known to maintain post-weaning bonds with their mother until the birth of their first calf, and probably longer. In contrast, young males gradually become independent, and are likely to form subadult male groups within the mixed herd. At the age of about 4–5 years (occasionally earlier), the males temporarily leave the herd to form bachelor groups of 2–20 individuals (sometimes more). The elder males (from about 10 years) sometimes permanently leave the herd, but this behaviour is not systematic (Sinclair, Reference Sinclair1977; Prins, Reference Prins and Beekman1996). Adult males competing for females leave the herd once their body condition decreases. Bachelor groups spend more time foraging and recover physical condition by foraging on patches of habitat too small and sometimes too risky for the herd, such as dense riparian woodland (Prins, Reference Prins and Beekman1996; Turner et al., Reference Turner, Jolles and Owen-Smith2005; see Chapter 6 for more details).
Savanna buffalo herd size varies across their distribution from as few as 20 to as many as 2000 individuals in the floodplains of eastern and southern Africa (Sinclair, Reference Sinclair1977; Prins, Reference Prins1989, Reference Prins and Beekman1996). In West Africa (WAP Regional Park), the mean herd size was about 45 individuals when excluding bachelor males, and the largest herds were estimated to contain about 150 individuals (Cornélis et al., Reference Cornélis, Benhamou and Janeau2011). Similar figures were reported for Central African savanna buffalo, except in floodplain areas such as Zakouma NP (Chad), where herds up to 800 individuals were observed (D. Cornélis, personal observation).
Forest Buffalo
Forest buffalo form small and stable herds of 3–25 individuals (Melletti, Penteriani, Mirabile and Boitani, Reference Melletti, Penteriani, Mirabile and Boitani2007; Korte, Reference Korte2008b; Melletti, Reference Melletti2008). Herds generally comprise several adult females with their young and one or two bulls (Dalimier, Reference Dalimier1955; Blake, Reference Blake2002; Melletti, Penteriani and Boitani, Reference Melletti, Penteriani and Boitani2007). In Lopé National Park, Gabon, the mean group size for the 18 herds was 12 ± 2 (range of means = 3–24; Korte, Reference Korte2008a). Larger herds were shown to contain a higher proportion of open habitat within their home range.
Although observations of solitary individuals (males and females) were reported, there is no evidence of the occurrence of sexual segregation in herds. Males apparently access profitable resources all year long and do not leave the mixed herds to form bachelor groups.
Home Range and Movements
The African buffalo is generally considered a sedentary species. In buffalo, home range (HR) size and movements (either seasonal or daily) are related to the spatiotemporal distribution of key resources. As pointed out previously, space use tends to be largely constrained by access to water in most study locations.
Savanna Buffalo Subspecies
Savanna buffalo herds exhibit HRs that generally range between 50 and 350 km2 (see Table 6.1 in Chapter 6). The larger HRs are generally observed in areas where resources are spatially segregated, which forces herds to undertake seasonal movements (see below). In contrast, males living in bachelor groups use smaller patches of habitat and form smaller HRs (0.5 and 4 km2; Grimsdell, Reference Grimsdell1969; Sinclair, Reference Sinclair1977; Taylor, Reference Taylor1985; Naidoo et al., Reference Naidoo, Du Preez and Stuart-Hill2012a).
In some systems, the HR size of mixed herds increases during the rainy season and decreases during the dry season (Naidoo et al., Reference Naidoo, Preez and Stuart-Hill2012b; Roug et al., Reference Roug, Muse and Clifford2020). During the rainy season, forage and water are abundant across the landscape and herds can move freely to find productive areas, whereas water availability often limits movement during the dry season, constraining buffalo to areas close to permanent water sources. In other systems that either have abundant natural surface water or extensive man-made water networks, buffalo HR sizes will increase during the dry season as they search for optimal foraging grounds (Ryan et al., Reference Ryan2006).
Some buffalo populations migrate between seasonal HRs (Cornélis et al., Reference Cornélis, Benhamou and Janeau2011; Bennitt et al., Reference Bennitt, Bonyongo and Harris2016; Sianga et al., Reference Sianga, Fynn and Bonyongo2017), although the exact migration pattern is not always consistent across years (Roug et al., Reference Roug, Muse and Clifford2020). Some buffalo populations were shown to undertake partial migration, meaning that within a group (or subpopulation), some will be migrant and others resident (Cornélis et al., Reference Cornélis, Benhamou and Janeau2011). Some groups exhibiting intermediate or partially migratory behaviour were qualified as ‘expanders’ (Naidoo et al., Reference Naidoo, Preez and Stuart-Hill2012b).
Naidoo et al. (Reference Naidoo, Du Preez and Stuart-Hill2014) report several seasonal movements of buffalo from the Kavango–Zambezi Transfrontier Conservation Area (KAZA TFCA) that are the longest documented distances for this species. A female collared on the Kwando River floodplains in Namibia during the dry season moved 87 km west along the Caprivi Strip before returning to her dry season range, a round trip journey of >170 km. The following wet season, she again moved ~87 km south into Botswana, before returning to the same dry season range for a different >170 km journey.
The most frequent pattern of buffalo seasonal movements is the use of extensive upland woodland and savanna systems during the wet season, which support more abundant high-quality grasses. Once grasses and waterholes in wooded savannas have dried out, buffalo move to lowland habitats, such as extensive wetlands, riparian areas and lake shore grasslands where sufficient soil moisture for plant growth and green forage persists into the dry season (Vesey-FitzGerald, Reference Vesey-FitzGerald1960; Taylor, Reference Taylor1985; Prins, Reference Prins and Beekman1996; Cornélis et al., Reference Cornélis, Benhamou and Janeau2011; Sianga et al., Reference Sianga, Fynn and Bonyongo2017). During drought years, buffalo resort to eating more productive and less digestible forage in the form of tall robust grasses and sedges and even reeds. They will resort to browsing in the absence of sufficient grass (Jarman and Jarman, Reference Jarman and Jarman1973; Stark, Reference Stark1986).
For example, in Ruaha National Park (Tanzania), Cape buffalo showed strong association with habitats near dry season water sources, whereas they were distributed widely across the park during the wet season (Roug et al., Reference Roug, Muse and Clifford2020). As cool drier seasons advance, herds may move progressively towards localized concentrations of remaining suitable grazing resources and water supplies, with increasing herd size.
In W Regional Park (Burkina Faso, Benin, Niger), the movements of seven West African savanna buffalo breeding herds were monitored using GPS collars (Cornélis et al., Reference Cornélis, Benhamou and Janeau2011). In the dry season, herds were shown to range close (within 5.3 ± 2.0 km, mean ± SD) to segments of permanent rivers. At the onset of the monsoon rains, all herds but one (which had year-round access to suitable resources along the Niger River) performed a larger (35 ± 10 km) south-west movement in response to a large-scale directional gradient of primary production. Furthermore, the establishment of wet season HRs appears conditioned by a threshold (~10 per cent) in the availability of perennial grasses, underlining the key role played by this resource for buffalo.
This was also observed for wet season ranges of buffalo in northern Botswana, where buffalo favoured areas >15 km from permanent water that have the highest cover of high-quality perennial grasses (Sianga et al., Reference Sianga, Fynn and Bonyongo2017). This is likely because leafy perennial grasses facilitate maximum intake for a large-bodied herbivore that relies on a tongue-sweep strategy to increase bite size.
Several recent studies based on GPS tracking have highlighted the occurrence of one-way movements over long distances, akin to dispersion. In the KAZA TFCA, two GPS-tracked females (among 30 females and five males tracked) undertook a long-range (~200 km) dispersal from the Kwando and Kavango Rivers in Namibia towards the Okavango Delta (Naidoo et al., Reference Naidoo, Du Preez and Stuart-Hill2014). In the Great Limpopo TFCA, a total of 66 GPS collars were deployed during 2008–2013 on females (47 adults, 19 subadults; Caron et al., Reference Caron, Cornélis and Foggin2016). Among this sample, three subadults were reported to leave the HR of their herd and disperse over 90 km.
Perhaps the most remarkable long-range movements came from female adult animals tagged in Mahango National Park, Namibia that exited via a break in the park fence in 2011. Numerous sightings of these animals suggest they moved as a group from north-eastern Botswana into Khaudum National Park. From there, the animals split into two groups, with one group observed in Angola near the Kavango River, around 250 km from where they had been ear-tagged. Another ear-tagged animal was eventually shot in an agricultural area in the Otjozondjupa Region in central Namibia. This location was a staggering 500 km from where she was tagged, far outside what is considered current buffalo range in Namibia (Martin, Reference Martin2002).
Although the occurrence of large-amplitude dispersions is probably inherent to the spatial behaviour of some buffalo, it cannot be excluded at this stage of knowledge that some of these movements result from disorientation due to the anaesthesia required to fit the GPS collars.
Forest Buffalo Subspecies
HR data are limited for forest buffalo because there are few sites where direct observation is possible due to the forest habitat and the animal’s elusive nature (Blake, Reference Blake2002; Melletti, Penteriani, Mirabile and Boitani, Reference Melletti, Penteriani, Mirabile and Boitani2007; Korte, Reference Korte2008b). Melletti, Penteriani, Mirabile and Boitani (Reference Melletti, Penteriani and Boitani2007) report an HR of 8 km2 for a herd of 24 buffalo at Danzga-Ndoki NP (Central African Republic). Based on seven radio-collared adult female forest buffalo at Lopé National Park, Gabon, over a two-year period (2002–2004), HRs of female forest buffalo averaged 4.6 km2 with little HR range overlap (Korte, Reference Korte2008b). Space use within HRs varies with season, with a preference for marshes during the wet season (September through February) and for forest in the dry season (March through August).
Forest buffalo HRs are thus much smaller than those of the savanna subspecies. It is likely that this pattern, common to all studies, results from the spatial arrangement of suitable resources in dense tropical forest, but also to less pronounced seasonality of the environment.
Activity Patterns
African buffalo display a large array of activity modes, including feeding, resting/ruminating, relocating between foraging areas, vigilance, wallowing and drinking. The relative proportions of these activity modes are mainly driven by spatiotemporal changes in the quality and availability of resources, interspecific competition, weather conditions and predation pressure (Sinclair, Reference Sinclair1977; Prins, Reference Prins and Beekman1996; Ryan and Jordaan, Reference Ryan, Knechtel and Getz2005; Owen-Smith et al., Reference Owen-Smith, Fryxell and Merrill2010).
Savanna Buffalo Subspecies
Daily Movements
Previous investigations on the daily movement rates of buffalo herds (based on short-term sampling, radiotelemetry or path retracement) reported contrasted results. Taylor (Reference Taylor and Walker1989) reported mean distances moved over a 24-hour period of <4 km compared to >6 km elsewhere (Sinclair, Reference Sinclair1977; Conybeare, Reference Conybaere1980). These authors reported that proximity of both food and water influence movements, and that bachelor males tend to move much less than herds. In South Africa (Kruger NP), buffalo herds were estimated to move 3.5 km per day during the dry season and 3.1 km during the wet season (Ryan and Jordaan, Reference Ryan, Knechtel and Getz2005). In contrast, in Cameroon (Benoué NP), Stark (Reference Stark1986) estimated a buffalo herd to travel on average 7.2 km per day during the rainy season and 5.6 km during the dry season.
More recently, Cornélis et al. (Reference Cornélis, Benhamou and Janeau2011) showed that GPS-tracked adult female savanna buffalo (n = 7) in West Africa (W Regional Park) travelled on average 6.5 ± 0.5 km (mean ± SD) per day at an annual scale. Analyses showed no significant differences between herds. In contrast, analyses at a subannual scale globally emphasized interesting trends in movement speed. Daily speed peaked around the dry–wet season transition (8.4 ± 0.5 km), either during the late dry season phases or during the early wet season phases. Minimum daily values (4.8 ± 0.4 km) were observed in most herds during the phases corresponding to the late wet season or just after. The daily movement speed thus followed in all herds a decreasing pattern between dry–wet and wet–dry season transitions.
Similar patterns were reported in Ruaha NP, where daily movements of GPS-tracked herds averaged 4.6 km with the longest distances (mean 6.9 km) travelled during November at the end of the dry season and beginning of the wet season. The shortest daily distances (mean 3.6 km) travelled occurred in the wet season in April–June (Roug et al., Reference Roug, Muse and Clifford2020).
Grazing and Ruminating
Ruminant ungulates such as buffalo spend a large proportion of their time feeding, and must additionally allocate time to ruminating, which results in an overall total time (feeding + ruminating) of 70–80 per cent (Beekman and Prins, Reference Beekman and Prins1989; Prins, Reference Prins and Beekman1996). In savanna buffalo, most studies report feeding time accounting for 35–45 per cent of the 24-hour activity budget (Grimsdell and Field, Reference Grimsdell and Field1976; Sinclair, Reference Sinclair1977; Mloszewski, Reference Mloszewski1983; Stark, Reference Stark1986; Prins, Reference Prins and Beekman1996; Winterbach and Bothma, Reference Winterbach and Bothma1998; Ryan and Jordaan, Reference Ryan, Knechtel and Getz2005; Bennitt, Reference Bennitt2012).
Grazing most often takes place in the early morning and late afternoon, and during the first half of the night, suggesting that buffalo cease feeding during the hottest part of the day and during the coolest part of the night for thermoregulation purposes. Note that an exception to this feeding pattern was observed at Lake Manyara NP (Tanzania), with the main grazing bout occurring between 10:00 and 14:00 hours (Prins, Reference Prins and Beekman1996). In most studies, buffalo herds appear to spend an equal or greater proportion of time feeding at night than during the day (Sinclair, Reference Sinclair1977; Taylor, Reference Taylor1985; Prins and Lason, Reference Prins, Sinclair, Knight, Kingdon and Hoffmann1989; Ryan and Jordaan, Reference Ryan, Knechtel and Getz2005). At a seasonal scale, most authors reported a trend towards more time spent feeding in the dry than the wet season in response to lower vegetation quality and quantity and decreased intake rates. Other modes of activity such as resting, rumination and vigilance are not mutually exclusive, thus making it hard to individualize ratios and to compare across studies.
Most studies reported average rumination times of around 30–35 per cent of the 24-hour activity budget (Sinclair, Reference Sinclair1977; Prins, Reference Prins and Beekman1996; Winterbach and Bothma, Reference Winterbach and Bothma1998; Ryan and Jordaan, Reference Ryan, Knechtel and Getz2005). Seasonal trends in rumination time are the subject of debate; Sinclair (Reference Sinclair1977) reported that buffalo spend more time ruminating during the dry season, whereas Beekman and Prins (Reference Beekman and Prins1989) found the opposite.
Thermoregulation
Buffalo generally cease feeding during the hottest part of the day and during the coolest part of the night for thermoregulation purposes. During the hottest hours of the day, mixed herds generally seek shade (Sinclair, Reference Sinclair1977). Contrary to females and young, males generally wallow during the hottest time of the day for periods lasting up to three hours. This practice is more effective in temperature regulation than the use of shade (Sinclair, Reference Sinclair1977).
In West Africa (WAP Regional Park), the daily activity patterns of buffalo herds were explored using biorhythm indices derived from GPS location data and activity sensors (Cornélis et al., Reference Cornélis, Benhamou and Janeau2011). At a daily scale, herds were equally active during night and day, and were mostly crepuscular, with two main active periods per day (dawn and dusk). Buffalo rested during the hottest hours of the day, and the duration of the resting bout was particularly marked during the hot dry season (Figure 5.1; Cornélis et al., Reference Cornélis, Melletti, Korte, Melletti and Burton2014).
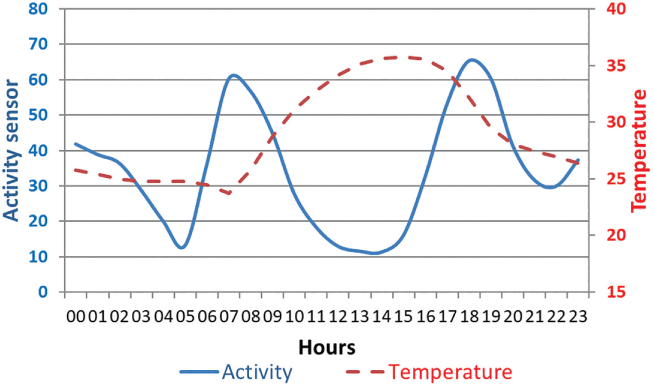
Figure 5.1 Average daily activity of a buffalo cow tracked in the WAP transfrontier conservation area (Burkina Faso, Benin, Niger) from March 2007 to February 2008 (using temperature and activity sensors embedded in the GPS collar). The first peak of activity started at dawn and the second finished after dusk, both lasting on average about 3–4 hours.
Water Resources and Drinking Activity
In many savanna regions of Africa, pronounced seasonal variability in rainfall results in wildlife being restricted to floodplains and other habitats adjacent to permanent surface water in the dry season. Nearly 100 per cent of the biomass density of large water-dependent grazing herbivores has been found within a 15 km radius from surface water (Western, Reference Western1975). Nevertheless, while rarely quantified, ephemeral water sources far from permanent surface water also allow wildlife to exploit forage and other resources further afield, influencing their distribution, abundance and movement (Naidoo et al., Reference Naidoo, Brennan and Shapiro2020; Rumiano et al., Reference Rumiano, Gaucherel and Degenne2021).
African buffalo are water-dependent: they can sweat profusely (Taylor, Reference Taylor1970), the water content of their faeces is high (about 80 per cent: Prins, Reference Prins and Beekman1996) and their body water turnover rate is high (King, Reference King1979). They have to drink at least every two days, taking about 45 litres daily, and they are not able to survive on the moisture content of their food (Prins, Reference Prins and Beekman1996).
Previous studies on drinking reported contrasted hourly patterns. In Hwange NP (Zimbabwe), equipped with pumped artificial surface water supplies, Weir and Davidson (Reference Weir and Davidson1965) found during the dry season that buffalo drank in the late afternoon (16:30–20:00) with a shorter peak early in the morning (08:30–10:30). In Kruger NP (South Africa), Ryan and Jordaan (Reference Ryan, Knechtel and Getz2005) found two main periods of diurnal drinking (the early morning and mid-morning) and their observations suggest more time devoted to drinking during the day than at night. In contrast, Winterbach and Bothma (Reference Winterbach and Bothma1998) found in Willem Pretorius game reserve (South Africa) that buffalo drank in the early afternoon. Grimsdell and Field (Reference Grimsdell and Field1976) also found that herds in Rwenzori NP (Uganda) drank in the mid-morning. In one study, buffalo were shown to modulate hourly drinking patterns according to the risk of predation by preferentially avoiding waterholes during high-risk hours of the day (dawn and dusk), when lions were likely to be in their vicinity (Valeix et al., Reference Valeix, Fritz and Loveridge2009).
A detailed analysis of drinking behaviour was reported by Taylor (Reference Taylor and Walker1989) in Matusadona NP (Zimbabwe), where Lake Kariba provides permanent water supplies for buffalo, in addition to seasonal rivers and pans that hold water in pools. During the hot dry season, herds drank once during the daytime, either around midday or between 16:00 and 19:00. The average drinking time took 20 minutes. In the wet season buffalo herds were a greater distance away from permanent water (mean 2.8 km) than during the dry season, when the mean distance from permanent water was 1.3 km. The converse held for seasonal water supplies. Buffalo herds were closer to these (1.5 km) in the wet season but further away in the dry season (2.4 km). This is to be expected because buffalo disperse during the wet season when abundant food and water resources are widespread, but concentrate on the lakeshore during the dry season where the only readily available food and water resources are present (Taylor, Reference Taylor and Walker1989).
For bachelor males, over 50 per cent of drinking bouts occurred between 10:00 and 14:00 with a peak from 11:00 to 12:00. Males drank throughout the day from before 06:00 until 20:00 and in the early morning hours at 02:00–03:00 (Taylor, Reference Taylor and Walker1989). During the wet season an individual may drink 2–3 times from the lake’s edge, but usually once in the morning and once in the afternoon. During the cooler months, drinking during the day usually occurred only once, either late morning or at midday. As the hot season progressed, the number of drinking bouts increased to two or three in the morning and afternoon. Drinking bouts for individual males were, on average, of 1–3 minutes’ duration.
Overall, however, in the literature reviewed, there appears to be no clear seasonal trends in drinking frequency among savanna buffalo.
Forest Buffalo Subspecies
Much less is known about the movement and activity of forest buffalo compared to savanna populations. In Dzanga–Ndoki NP (Central African Republic), a study of a single buffalo herd showed that daily distances travelled by buffalo were generally very short (i.e. 500–1500 m) and were mainly restrained to clearings and surrounding areas (Melletti, Reference Melletti2008). The maximum distance travelled during a 24-hour tracking period was about 4000 m. Like savanna buffalo, forest buffalo wallow in the late afternoon; however, they spend more time in wallows during the dry season (Melletti, Penteriani, Mirabile and Boitani, Reference Melletti, Penteriani, Mirabile and Boitani2007; Korte, Reference Korte2008a).
Feeding Ecology
Buffalo are ruminants, essentially feeding on grass and roughage. This species is capable of subsisting on pastures too coarse and too tall for most other herbivores (Bothma, Reference Bothma and du Bothma2002), and as a ‘bulk grazer’ they ingest around 2.2 per cent of their body mass daily. This represents on average 6.5 kg for a forest buffalo of 300 kg, 11 kg for a West or Central African buffalo of 500 kg and 15 kg for a Cape buffalo of 700 kg.
Buffalo are very efficient grazers (their adapted dentition and a mobile tongue allowing the ingestion of high quantities of grass in a short amount of time). Optimal feeding conditions for buffalo prevail when the grass forms swards and leaf heights reach and exceed 10 cm, as in flood plains or in forest glades (Prins, Reference Prins and Beekman1996). However, buffalo cannot cut pastures as short as other species. The African buffalo thus occupies an important niche, opening up habitats that are preferred by short-grass grazers (Prins, Reference Prins and Beekman1996; Estes, Reference Estes1991). Their primary competitors are cattle Bos taurus, African elephant Loxodonta africana, plains zebra Equus quagga and wildebeest Connochaetes taurinus (Sinclair, Reference Sinclair1977; de Boer and Prins, Reference de Boer and Prins1990; Plumptre and Harris, Reference Plumptre and Harris1995; Prins, Reference Prins and Beekman1996).
Savanna (Cape, West and Central African) Buffalo
In savanna buffalo, optimal dietary conditions occur during the rainy season, while the end of the long dry season is a period of food scarcity (both in quantity and quality; Prins and Sinclair, Reference Prins2013). Detailed diet studies conducted in eastern and southern Africa indicated that buffalo are resource limited and constrained by a minimum dietary protein concentration of 7–8 per cent to make fermentation in the rumen possible (Sinclair, Reference Sinclair1977; Prins, Reference Prins and Beekman1996). In such a situation, buffalo thus face a trade-off of quality and quantity in obtaining an appropriate protein-to-fibre ratio in their diet (Redfern et al., Reference Redfern, Ryan and Getz2006; Ryan et al., Reference Ryan and Jordaan2012). During the dry season, savanna buffalo are generally forced to become more selective, and to partially switch their diet to browse (see below). Despite this, buffalo were reported to live at or below nitrogen requirements for prolonged periods, accounting for visibly losing body condition (Prins, Reference Prins1989; Ryan, Reference Ryan, Cross and Winnie2006; Ryan et al., Reference Ryan and Jordaan2012). In natural ungulate communities, the regulation of populations is driven by two processes: a control by available food resources (‘bottom-up regulation’) on the one hand, and a control by predation (‘top down’ regulation) on the other (Hunter and Price, Reference Hunter and Price1992). In the case of savanna buffalo, most authors agree that resources play a dominant role in the regulation of populations (Sinclair et al,. Reference Sinclair, Mduma and Brashares2003; Hopcraft et al., Reference Hopcraft, Olff and Sinclair2010).
There is moderate consensus in feeding studies on Cape buffalo about the preferred grass species. Usually species that are avoided contain low nutritious quality or the presence of aromatic oils (Prins, Reference Prins and Beekman1996). During the dry season, usual food resources are of poor quality, due to lignifications and high standing biomass. During this period, floodplain species (e.g. Leersia hexandra) and riverine forest species (e.g. Setaria sphacelata) become important. In areas with upwelling groundwater, species such as Cyperus laevigatus may also form a mainstay in the dry season (Prins and Sinclair, Reference Prins2013). Buffalo prefer grasses such as Cynodon dactylon, but may also eat broad-leaved grasses, such as Panicum maximum. Sinclair, in the Serengeti, reported that buffalo preferred soft, nutritious grass such as Digitaria macroblephora. Although there is little information on seasonal changes on diet, Sinclair (Reference Sinclair1977) analysed stomach contents and showed that Cape buffalo select more grass leaf at the end of the rainy season. Finally, Taylor (Reference Taylor1985) found that grasslands dominated by Panicum repens were the best habitat on the shores of Lake Kariba (Matusadona NP, Zimbabwe). In Cape buffalo, the diet of the different age/sex classes does not appear to differ and dry matter food intake ranges between 1.2 per cent and 3.5 per cent of body mass, similarly to other ruminants, but subject to food quality (Sinclair, Reference Sinclair1977; Prins and Beekman, Reference Prins and Lason1989).
Few studies investigated the feeding ecology of West and Central African buffalo. In West Africa (WAP Regional Park), habitat selection analysis emphasized the importance of perennial grasses (e.g. Andropogon gayanus; Cornélis et al., Reference Cornélis, Benhamou and Janeau2011). In this study, a gradient in primary production appeared to determine large-scale movements of herds at the onset of the wet season, but its action clearly was modulated by the proportion of perennials available. Buffalo herds were shown to establish early wet season HRs at locations where this proportion reached a 9 per cent threshold. At Benoué NP (Cameroon), Stark (Reference Stark1986) similarly reported a very high proportion of grasses in the diet, particularly Andropogon gayanus, which represented 51 per cent of the volume ingested in the dry season, versus 40 per cent in the wet season. At Zakouma NP (Chad), Gillet (Reference Gillet1969) noted a preference for Setaria anceps (particularly new shoots after fire) and Andropogon gayanus, but also Vetiveria nigritana. In the dry season, the unburned straws of Echinochloa obtusiflora appeared to be preferred.
Savanna buffalo are primarily grazers, but partially switch to browse when grasses become tall and lignified (Field, Reference Field1972; Leuthold, Reference Leuthold1972; Sinclair, Reference Sinclair1977; Mloszewski, Reference Mloszewski1983; Hashim, Reference Hashim1987; Prins, Reference Prins and Beekman1996). Shrub and tree leaves have been shown to contain higher protein (nitrogen) content than dry grasses during the dry season (Kone et al., Reference Kone, Richard and Guerin1990; Prins, Reference Prins and Beekman1996). As mentioned above, the contribution of nitrogen from browse facilitates the fermentation of fibrous grass in the rumen, which they eat in high quantities during the dry season. According to Estes (Reference Estes1991), the browse can represent up to 5 per cent of the total diet, but higher figures were reported in several sites (e.g. 26 per cent in dry season in Cameroon; Taylor, Reference Taylor1985). A wide range of species of shrubs and trees are consumed throughout African savanna, including Grewia spp., Heeria spp., Colophospermum mopane, Combretum spp., Capparis spp. and Piliostigma spp. (Pienaar, Reference Pienaar1969; Jarman, Reference Jarman1971; De Graaf et al., Reference De Graaf, Schultz and van der Waet1973; Taylor and Walker, Reference Taylor and Walker1978; Ryan, Reference Ryan, Cross and Winnie2006). In drier habitats in the Eastern Cape, buffalo appear to be adapted to eat woody species because grassy vegetation is scarce. In this particular habitat, during the dry season, up to 33 per cent of their diet comprises species such as Acacia sp., Plumbago sp. and Grewia sp.
In some areas buffalo can maintain or create ‘grazing lawns’ if the feeding interval is short enough (Prins, Reference Prins and Beekman1996). Evidence of this ‘returning’ behaviour has also been described for buffalo in Kruger NP and Klaserie Private Nature Reserve in South Africa (Bar-David et al., Reference Bar-David, Bar-David and Cross2009). They may also create favourable lawns in conjunction with other large herbivores such as elephants at Lake Rukwa, Tanzania (Vesey-FitzGerald, Reference Vesey-FitzGerald1960) and Benoué NP in central Cameroon (H. H. T. Prins, personal observation).
Geophagy has been reported at several sites, where clay or substrates rich in iron may explain this preference. On Mount Kenya, geophagy is reported in the bamboo belt (2100–3000 m), where clay soils are rich in iron and aluminium (Grimshaw et al., Reference Grimshaw, Cordeiro and Foley1995). This rare activity is carried out mainly by solitary individuals on Mount Kenya (Mahaney, Reference Mahaney1987). In Lent Valley in Kilimanjaro, buffalo enter into caves rich in sodium bicarbonate and chew off the soda deposits.
Forest Buffalo
Few data exist on the feeding ecology of forest buffalo because direct observations are rarely feasible in rainforest habitats. Blake (Reference Blake2002) in Noubale–Ndoki NP and Melletti (Reference Melletti2008) in Dzanga–Ndoki NP observed buffalo feeding mainly on Poaceae and Cyperaceae within clearings, in particular on Rhyncospora corymbosa, Kyllinga sp. and Cyperus sp. Blake (Reference Blake2002) also recorded several species of Marantaceae, including Marantochloa purpurea, M. cordifolia, M. filipes and Halopegia azurea. In addition, species of the Commelinaceae family such as Commelina diffusa and Palisota brachythyrsa and a species of algae (Spirogyra sp.) were recorded. Melletti (Reference Melletti2008) also found indirect signs of feeding activity on Commelina sp and Palisota sp. in the understory of Gilbertodendrum dewevrei mono-dominant forest.
Bekhuis et al. (Reference Bekhuis, Jong and Prins2008) used micro-histological faecal analysis in a two-month study to determine the diet of forest buffalo at Campo-Ma’an NP in southern Cameroon. They found that the most important part of the diet was composed of graminoids (43 per cent, with Leptochloa caerulescens representing 15 per cent of the total diet), non-graminoid monocots (21 per cent, mainly Commelinaceae such as Palisota spp.), dicotyledoneous plants (33 per cent, mainly leaves) and cryptogamous plants (3 per cent). The composition of the diet suggests that the buffalo fed mainly along logging roads and river banks (Bekhuis et al., Reference Bekhuis, Jong and Prins2008). Using a similar method at Lopé NP (Gabon), Lustenhouwer (Reference Lustenhouwer2008) and van der Hoek et al. (Reference van der Hoek, Lustenhouwer, Jeffery and van Hooft2013) found that the majority of plants consumed by forest buffalo were monocotyledons, primarily grasses (Poaceae) and sedges (Cyperaceae), with a low portion of dicotyledonous plants in the diet. In the same study area, van der Hoek et al. (Reference van der Hoek, Lustenhouwer, Jeffery and van Hooft2013) emphasized the importance of savanna habitat, noting that controlled burning is a key tool for maintaining open areas.
Thus far, no evidence of the existence of grazing lawns was found in the different studies focused on the feeding ecology of forest buffalo, even in areas of high density such as Lopé NP (L. Korte and M. Melletti, personal observations).
Conclusion
The African buffalo is endowed with an amazing adaptability, which probably explains why this species is one of the most successful large African mammals in terms of geographical distribution, abundance and biomass. On an evolutionary scale, African buffalo have adapted their phenotype across their distribution range, which covers almost all natural ecosystems south of the Sahara. For example, the mass ratio between the large Cape buffalo and the small forest buffalo is about 2.7 to 1. With the exception of the elephant, few species have an equivalent mass range across their distribution area. The African buffalo has also managed to adapt its behaviour to the different ecosystems in which it lives. For example, in dense tropical forests where food resources are scarce and aggregated, the African buffalo forms small herds of 5–20 animals. In contrast, in the rich pastures of the savanna floodplains, the African buffalo can gather in herds of up to 2000 animals.
The space-use behaviour of buffalo herds also differs strongly according to the spatial arrangement of resources. In areas where buffalo access profitable resources all year long, the herds settle in home ranges of a few square kilometres. In contrast, in areas where resources are spatially segregated, buffalo occupy home ranges of several hundreds of square kilometres. In some regions, buffalo undertake seasonal movements of several dozen kilometres. Such migrations may be only partial within a population, meaning that it is not undertaken by all herds.
At a seasonal and daily scale, buffalo are also able to modulate their social behaviour within the herd according to the availability of resources. The herd splits into subgroups and reforms within the contours of a long-lasting (multiannual) home range, according to mechanisms that remain largely unexplored (Chapter 6). Finally, within their seasonal home range, we also saw that buffalo can regulate their diet and activity patterns according to the quality and quantity of food resources, as well as according to abiotic factors such as temperature.
Thus, despite reported regional declines in buffalo population numbers due, inter alia, to climate change, habitat fragmentation, livestock development and diseases, the species may be flexible enough to adapt to the wide range of challenges it faces and will face in the coming decades.


