INTRODUCTION
Over the past three decades, Acinetobacter baumannii has emerged as one of the most important pathogens for healthcare institutions around the world [Reference Peleg, Seifert and Paterson1–Reference Falagas3]. This organism is particularly associated with ventilator-associated pneumonia, bacteraemia and urinary tract infection, especially in intensive-care units (ICUs) [Reference Peleg, Seifert and Paterson1–Reference Falagas3]. The ability of A. baumannii to survive under a wide range of environmental conditions and to persist on surfaces for extended periods of time make it a frequent cause of nosocomial outbreaks [Reference Peleg, Seifert and Paterson1, Reference Dijkshoorn, Nemec and Seifert2], and many such incidents have been reported in recent years [Reference Perez4]. Additionally, the isolates responsible for these outbreaks are commonly resistant to multiple antimicrobials, including carbapenems.
The increasing rates of carbapenem-resistant A. baumannii (CRAB) isolates represents a major clinical and public-health concern since these drugs are the most potent therapy available against these organisms [Reference Zavascki5]. Polymyxins B and E (colistin) are frequently the only therapeutic option for these isolates [Reference Zavascki6, Reference Zavascki and Li7]. Multidrug-resistant (MDR) A. baumannii, including CRAB, colonization or infection tends to occur in patients with serious underlying diseases who are exposed to antimicrobial agents, have prolonged stay in an ICU, and are under mechanical ventilation [Reference Peleg, Seifert and Paterson1–Reference Perez4]. Given the severity of illness in these patients crude mortality rates for MDR A. baumannii are usually high [Reference Falagas3] and the attributable mortality of these infections regardless of the severity of underlying illness has proved very difficult to determine [Reference Falagas3].
Several studies have described CRAB outbreaks worldwide and some have investigated factors associated with mortality due to CRAB [Reference Peleg, Seifert and Paterson1–Reference Zavascki5]. However, none have focused on identifying specific risk factors for mortality in the context of an outbreak. Thus, the aim of the current study was to assess risk factors for 30-day mortality in patients with CRAB infection or colonization during an outbreak in an ICU from a tertiary-care hospital.
METHODS
Study design
A cohort study was performed at a general 22-bed ICU of the Ernesto Dornelles Hospital, a 300-bed tertiary-care hospital in Porto Alegre, Southern Brazil. The study period was from March 2006 (recovery of the index case) to December 2008. Patients with positive culture for CRAB were included in the study if the isolate was recovered during the ICU admission or within 72 h of discharge from the ICU. They were excluded if the CRAB was recovered <48 h after ICU admission. Only the first isolate of each patient was considered. All patients were followed up to death or to 30 days after onset of infection.
Data were collected from patients' medical charts and hospital epidemiology and infection control service records. The study was approved by the ethics committee of the Federal University of Rio Grande do Sul. Written informed consent was not required as the study was retrospective.
Variables and definitions
The primary outcome was 30-day mortality. Potential risk factors assessed were: age; sex; baseline diseases (malignancy; AIDS; neurological, pulmonary, digestive tract, renal or cardiac diseases; and diabetes), APACHE II score [Reference Knaus8] at admission to the ICU and at onset of infection (defined as the day of culture collection that resulted in the growth of CRAB); arterial oxygen pressure/inspired oxygen fraction (PaO2/FiO2) at onset of infection; previous surgery; presence of mechanical ventilation at onset of infection; haemodialysis; polymicrobial infection (defined as the recovery of another organism at the same time from the same site of CRAB); presence of concomitant infection on the day of CRAB recovery (defined as the presence of an infection by another organism at a site distinct from that affected by CRAB); length of ICU stay before CRAB recovery; classification of the CRAB isolate as infecting or colonizing according to Centers for Disease and Control (CDC) criteria [Reference Garner9]; presentation with septic shock (systolic blood pressure <90 mmHg or a reduction by ⩾40 mmHg from baseline in the absence of other causes for hypotension persisting despite adequate fluid resuscitation, or need for vasopressor agents to maintain normal blood pressure levels) [Reference Bone10]; administration of appropriate therapy, defined as the use of an antimicrobial to which the isolate exhibited in-vitro susceptibility (polymyxin B and tigecycline were not tested for all isolates, but were considered appropriate therapy since all isolates tested were susceptible); time to initiate the therapy; and antimicrobial association (treatment with more than one antimicrobial agent).
Microbiology and molecular typing
Isolates were identified by the Vitek system (bioMérieux, France). Susceptibility was determined by the disk diffusion method and the results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) criteria [11]. A sample of 27 isolates were tested for susceptibility to polymyxin B by agar dilution method and for tigecycline by E-test® (Slowna, Sweden). Minimum inhibitory concentrations (MICs) of imipenem and meropenem were also determined by E-test. The 27 isolates were also tested by multiplex PCR for bla OXA-23, bla OXA-24, bla OXA-58 and bla OXA-51 genes as described previously [Reference Woodford12].
Twenty-four of these selected isolates were genotyped by pulsed-field gel electrophoresis (PFGE) using the restriction endonuclease ApaI. Analysis of PFGE patterns was performed by visual inspection using the criteria of Tenover et al. [Reference Tenover13].
Statistical analysis
All statistical analyses were performed using SPSS for Windows, version 13.0 (SPSS Inc., USA). Bivariate analyses were performed separately for each of the variables. P values were calculated using χ2 or Fisher's exact tests for categorical variables and Student's t test for continuous variables. Variables for which the P value was in the bivariate analysis were included one by one in a Cox regression model according to their P value. Risk factors were checked for confounding and collinearity. A P value of ⩽0·10 was set as the limit for acceptance or removal of the new terms in the model. Proportional hazards assumption was graphically checked inspecting the log[–log(S)] plot. All tests were two-tailed and P⩽0·05 was considered significant.
RESULTS
During the study period, 2918 patients were admitted to the ICU (annual mean 973 admissions), resulting in a total of 19 042 patient-days (annual mean 6347 patient-days). Of the 2918 patients, 72 (2·5%) yielded at least one positive culture for CRAB. Six of these patients were excluded because the isolate was recovered within 48 h of ICU admission, resulting in 66 patients being included in the study. The isolates recovered from these 66 patients represented 60·0% of all Acinetobacter spp. isolates recovered during the study period (n=110). The proportion of carbapenem resistance in Acinetobacter spp. from the ICU increased from 29·4% (10/34) in 2006, to 68·6% (24/35) in 2007 to 78·0% (32/41) in 2008. The overall incidence rate of CRAB was 3·78/1000 patient-days (1·49 in 2006, 4·32 in 2007, 5·45 in 2008).
The overall in-hospital mortality of patients with CRAB was 69·7% (46/66) and the 30-day mortality was 47·0% (31/66). The main characteristics of the patients are summarized in Table 1. The bivariate analyses of risk factors for 30-day mortality in the 66 patients are shown in Table 2. Considering the results of bivariate analysis, the following variables were included one by one in the Cox multivariate model: septic shock, APACHE II score at onset of infection, mechanical ventilation, bacteraemia, appropriate therapy, time to initiate appropriate therapy, and APACHE II score at admission. All variables remaining in the final multivariate model are shown in Table 3. Mortality rates of the 24 patients who received appropriate therapy compared to the 42 who did not receive appropriate therapy are shown in Figure 1.
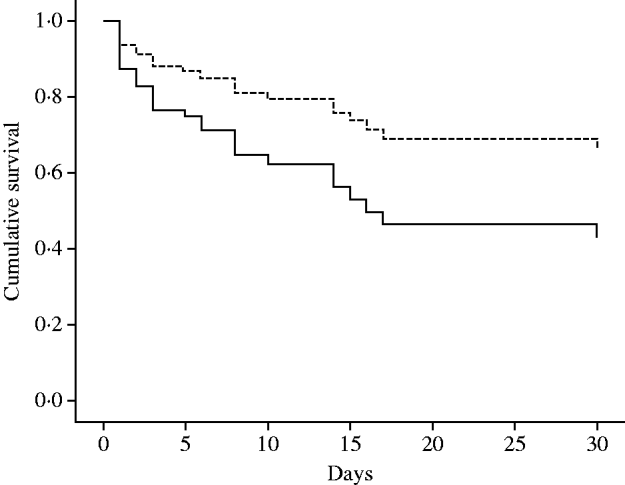
Fig. 1. Adjusted survival curves of patients according to administration of appropriate therapy (P value for this variable=0·09). Mortality rates of the 24 patients who received appropriate therapy (- - -)=76/1000 patient-days. Mortality rates of the 42 patients who did not receive appropriate therapy (——)=255/1000 patient-days. Survival curves adjusted for APACHE II score at onset of infection and presentation with septic shock.
Table 1. Characteristics of 66 patients with carbapenem-resistant Acinetobacter baumannii (CRAB)
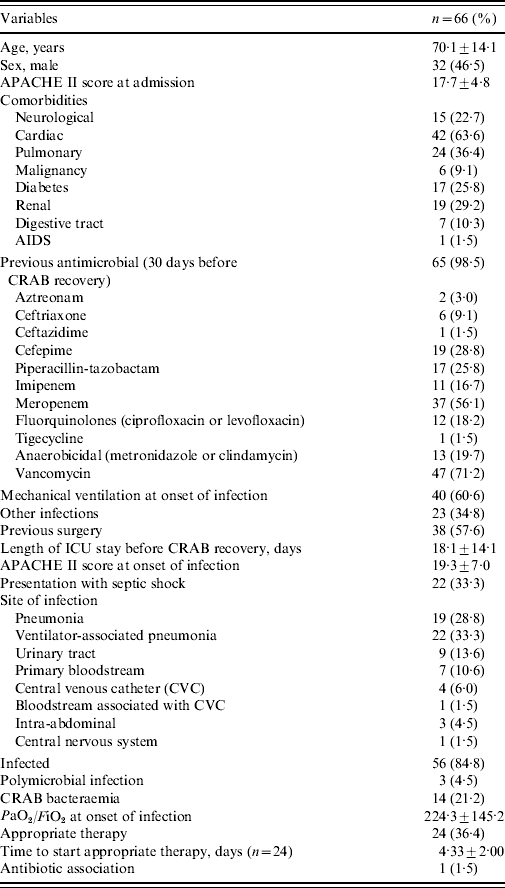
PaO2/FiO2, arterial oxygen pressure/inspired oxygen fraction.
All variables are n (%) or median±standard deviation.
Table 2. Bivariate analysis of risk factors for 30-day mortality in the 66 patients with carbapenem-resistant Acinetobacter baumannii (CRAB)
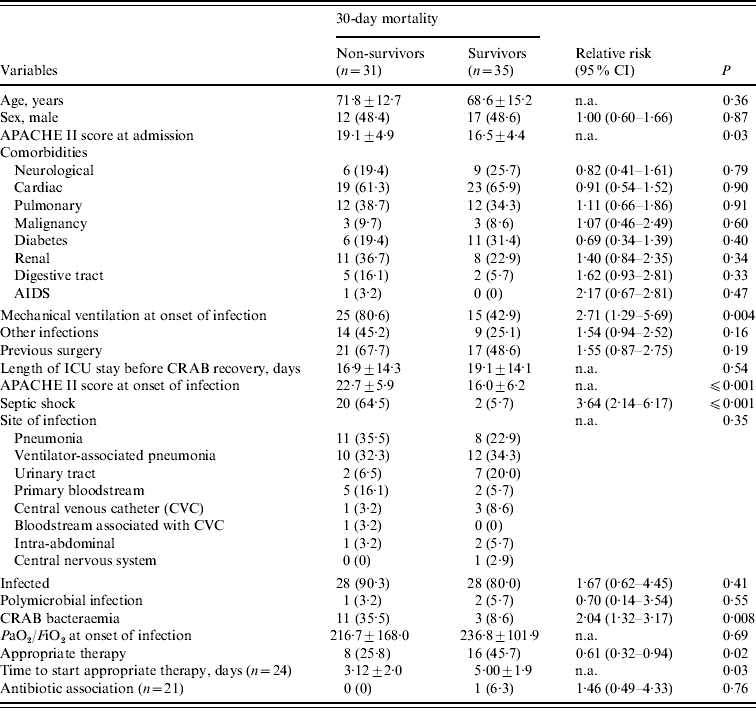
CI, Confidence interval; n.a., not applicable; PaO2/FiO2, arterial oxygen pressure/inspired oxygen fraction.
All variables are n (%) or median±standard deviation.
Table 3. Multivariate analysis of factors associated with 30-day mortality

aHR, Adjusted hazard ratio; CI, confidence interval.
Patients who received appropriate therapy were more frequently treated with polymyxin B (70·8%, 17/24), followed by ampicillin-sulbactam (16·7%, 4/24) and tigecycline (12·5%, 3/24). Of these patients, 30-day mortality was 35·3% (6/17) for those treated with polymyxin B, 25·0% (1/4) for those treated with ampicillin-sulbactam and 33·3% (1/3) for those treated with tigecycline (P=0·86). The 24 patients started therapy after a mean of 4·4 days (range 1–9 days) from CRAB recovery. All patients treated with polymyxin B received 50 mg/day by continuous intravenous infusion, except one who received 100 mg/day.
All 27 CRAB isolates proved to be OXA-23 producers, and all of them were also positive for bla OXA-51. The MIC50 and MIC90 of imipenem and meropenem were both >32 mg/l. All tested isolates were susceptible to polymyxin B (both MIC50 and MIC90=0·5 mg/l, range <0·25–0·5 mg/l) and tigecycline (both MIC50 and MIC90=1·5 mg/l, range 0·125–2·0 mg/l). The amplified product of one bla OXA-23-positive isolate was sequenced and confirmed as OXA-23 by a BLAST homology search.
Of the 24 isolates submitted to molecular typing, 13 (54·2%) represented a major clone (A) of which 11 had identical profiles and two were closely related. Nine further isolates represented a second clone (B), and the remaining two isolates were distinct.
DISCUSSION
This study was undertaken to evaluate risk factors for mortality in patients infected or colonized with CRAB. Our findings showed high overall in-hospital mortality (69·7%) and 30-day mortality (47·0%). The presence of septic shock and the APACHE II score at onset of infection were both independently associated with 30-day mortality in a multivariate Cox regression model. Administration of appropriate therapy was not statistically significant in the final model, but a clear trend to lower 30-day mortality rates in such patients was observed.
Our findings allow some conclusions. First, severity of infection presentation represented by septic shock at onset of infection, and severity of baseline condition represented by APACHE II score at onset of infection, are both the main predictors of 30-day mortality in ICU patients with CRAB, which is in accord with other studies assessing mortality by infections due to A. baumannii [Reference Sunenshine14–Reference Livermore17]. Second, we could not unequivocally show the effect of appropriate therapy on 30-day mortality in ICU patients. Although 30-day mortality rates were decreased in those who received appropriate therapy, this variable was not statistically significant in the final multivariate model (adjusted hazard ratio 0·48, P=0·09). Even though we attempted to control for disease severity using the APACHE II score, residual confounding may explain this non-significant result. Additionally, we believe that the magnitude of the effect of therapy on outcome was probably affected by the delay in administration of the first dose after the onset of infection and by the administration of polymyxin B (most patients were treated with this drug) in dosages lower than those currently recommended [Reference Zavascki5, Reference Zavascki6]. Importantly, the high mortality rates found in our study, which may be partially explained by the severity of the patients' infection, are possibly related to this low proportion of patients (36·4%) who received appropriate therapy. This latter finding as well as the delay in starting therapy may have occurred owing to a potential underestimation of the pathogenic role of CRAB.
Interestingly, time to initiate therapy in the 24 patients who received appropriate therapy was significantly lower in patients who died within 30 days in bivariate analysis, which is in contrast to many findings demonstrating that the earlier appropriate therapy is employed the lower the mortality [Reference Erbay15, Reference Metan, Sariguzel and Sumerkan16, Reference Falagas and Rafailidis18–Reference Apisarnthanarak and Mundy20]. Nevertheless, this variable was not significantly associated with outcome in the multivariate model and this may be easily explained by the fact that patients who presented with septic shock at onset of infection received appropriate treatment significantly earlier than those who had no such a presentation (3·0±1·6 days vs. 4·9±2·0 days, respectively; P=0·03).
Interestingly, our study did not show a significant difference in 30-day mortality between patients classified as colonized by CRAB, according to CDC criteria, and those infected by those organisms. Although it might be due to lack of statistical power, it is possible that some patients could have been misclassified using these criteria, since we do not believe that a real colonization would have the same impact as active infections in the patients' outcomes.
Of patients who received appropriate therapy, there was no significant difference in 30-day mortality according to the antimicrobial used. Most patients were treated with polymyxin B, and 16/17 patients treated with this drug had CRAB pneumonia or ventilator-associated pneumonia (data not shown). It has been shown when using the currently recommended dosages of polymyxin B that peak free-plasma concentration are around 2 mg/l or even lower [Reference Zavascki21]. The low MICs for polymyxin B found in the tested isolates of our study could partially explain the relatively good responses observed for patients treated with this drug, considering the low dose regimens administered.
Although not all isolates could be recovered for molecular evaluation, all tested isolates proved to be OXA-23 producers which is a major resistance mechanism to carbapenems in A. baumannii isolates [Reference Zavascki5, Reference Mugnier22, Reference Higgins23] and has been associated with many CRAB outbreaks worldwide, including in Brazil [Reference Dalla-Costa24–Reference Martins26]. Additionally, all isolates had the OXA-51 gene, which has been shown to be intrinsic to A. baumannii [Reference Turton27].
Our study has some limitations that should be noted. First, there are the limitations that are characteristic of studies using a retrospective design. However, most of our variables were objective and not affected by subjective evaluation. A possible single exception, classification as infected or colonized, was assessed prospectively by the infection control team of this hospital for surveillance purposes. Additionally, not all isolates were tested for polymyxin B and tigecycline, and all were considered susceptible to these agents. Nonetheless, isolates from distinct clones were tested, most were from patients who had received these drugs (data not shown) and all presented MICs within the susceptible range for polymyxin B and all had MICs ⩽2 mg/l for tigecycline. It should also be noted that E-test for tigecycline, used in our study, may overestimate the MIC of A. baumannii isolates [Reference Casal28].
Another limitation of our study, which did not affect our main findings, is that not all isolates had their genetic basis of resistance determined and not all were submitted to molecular typing. The outbreak in the institution of this study occurred in parallel with a large city-wide outbreak after a long period of stable carbapenem susceptibility rates [Reference Martins26, Reference Zavascki29], and although the great majority of the isolates recovered from distinct institutions of our city were also OXA-23 producers, in a few isolates no carbapenemase was detected [Reference Martins26]. Thus, it is possible that another resistance mechanism might be responsible for the carbapenem-resistant phenotype of the remaining isolates, considering the characteristic of the isolates recovered from other institutions in our city. The same consideration can be applied to species-level identification as not all isolates were tested for bla OXA-51, therefore it is possible that some isolates belonged to other species of the A. calcoaceticus–A. baumannii complex.
In summary, our study showed that severity of baseline condition and severity of infection presentation were major risk factors for mortality in patients with CRAB infections at an ICU during an outbreak caused by these organisms. Patients who received appropriate treatment tended to have lower mortality rates, despite delay in administration and low-dosage regimens. Appropriate therapy may be the only modifiable variable able to improve outcome of these patients.
ACKNOWLEDGEMENTS
A.P.Z. is a Research Fellow from the National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil (301829/2008-0).
DECLARATION OF INTEREST
None.






