The hepatic hormone hepcidin regulates serum Fe concentrations and Fe homoeostasis(Reference Sangkhae and Nemeth1). Hepatic hepcidin synthesis is stimulated by high intracellular and extracellular Fe concentrations and inflammation and is inhibited by hypoxia and erythropoietic drive(Reference Sangkhae and Nemeth1). We recently assessed the strengths of these opposing signals to regulate serum hepcidin (SHep) by inducing acute inflammation using a vaccine model in young women with and without Fe-deficiency anaemia(Reference Stoffel, Lazrak and Bellitir2). There was no increase in SHep in anaemic women despite an acute increase in IL-6, suggesting Fe homoeostasis prioritises correction of Fe-deficiency anaemia over Fe sequestration during mild inflammation. However, previous studies are equivocal: some show inflammation is a strong inducer of hepcidin but this effect is blunted by Fe deficiency (ID) and/or enhanced erythropoiesis(Reference Huang, Constante and Layoun3–Reference Cherian, Forbes and Cook6), other studies suggest inflammatory regulators dominate over Fe stores.
Overweight/obesity (OW/OB) is a chronic low-grade inflammatory disorder, and C-reactive protein (CRP), α-1 glycoprotein (AGP) and IL-6 concentrations are higher than in normal-weight (NW) subjects(Reference Cepeda-Lopez, Melse-Boonstra and Zimmermann7). OW/OB women absorb Fe poorly, have hypoferraemia and are at higher risk for ID compared with NW individuals(Reference Cepeda-Lopez, Aeberli and Zimmermann8). The disordered Fe metabolism in OW/OB is likely mediated by higher SHep caused by adipocyte-associated inflammation. In a prospective study, inflammation and SHep were decreased in OW/OB women during weight loss after bariatric surgery, and this predicted improved Fe absorption in Fe-deficient subjects(Reference Cepeda-Lopez, Allende-Labastida and Melse-Boonstra9). IL-6 is needed for the induction of hepcidin during inflammation(Reference Nemeth, Rivera and Gabayan10). The non-steroidal anti-inflammatory drug ibuprofen reduces IL-6 production through inhibition of cyclo-oxygenase COX-1 and COX-2(Reference Rainsford11).
There is a lack of prospective experimental data assessing whether low-grade inflammation increases SHep and reduces oral Fe absorption in Fe-deficient OW/OB women. Therefore, our study aim was to test whether treating low-grade inflammation in Fe-depleted OW/OB women would lower SHep and improve Fe absorption. As a model for chronic low-grade inflammation, we studied OW/OB women and compared them with NW controls and measured changes in SHep and Fe absorption before and after treatment with 3 × 600 mg ibuprofen daily for 14 d. We used stable Fe isotope techniques to quantify erythrocyte Fe incorporation of dietary Fe given with and without ascorbic acid, an enhancer of non-heme Fe absorption. Our hypotheses were: (1) in NW women without inflammation, ibuprofen treatment would have no effect on SHep, Fe metabolism or the enhancing effect of ascorbic acid (AA) on Fe absorption and (2) in contrast, in OW/OB women with inflammation, (a) ibuprofen treatment would reduce inflammation and SHep; (b) the decrease in SHep would reverse hypoferraemia and improve Fe absorption and (c) AA would increase Fe absorption to a greater extent after ibuprofen treatment than before.
Methods
Subjects
Subjects were recruited from the students and staff of the University of Monterrey, Mexico. We recruited young women because they are a group at high risk of ID. We included twenty NW and sixteen OW/OB White and Hispanic women into the study. Inclusion criteria for the study were as follows: (1) female, (2) 18–45 years of age, (3) either NW with a BMI between 18·5 and 24·9 kg/m2 or OW/OB with a BMI between 29 and 40 kg/m2, (4) no chronic illness and no significant medical conditions that could influence Fe or inflammatory status other than OW/OB, (5) non-smoking, and (6) non-pregnant and/or planning a pregnancy, (7) no Fe supplement intake within 2 weeks before study start and during the study and (8) no regular use of medication (except oral contraceptives). Written informed consent was obtained from all women. The ethics committees of the ETH Zurich, Switzerland, and the Ethics Committee of the University of Monterrey, Mexico, approved the protocol and it was registered at clinicaltrials.gov (NCT 02745925).
Sample size calculation
To detect a 30 % difference in Fe absorption within groups (NW and OW/OB), based on a log mean fractional Fe absorption (FIA) of 1·28 (NW) and 1·10 (OW/OB) and with an sd of 0·23 (from recent studies by our group), a power of 80 % and an α-error probability of 5 %, the required sample size was thirteen subjects per group. Considering the need for compliance with ibuprofen intake, we anticipated a dropout rate of about 30 % and thus aimed to recruit seventeen women per group.
Study design
At screening, we measured body weight (kg) to the nearest 0·1 kg on a digital scale and height (m) to the nearest 1·0 cm with the use of a stadiometer(Reference Gibson12). BMI was calculated and participants were either assigned to the NW or the OW/OB study group. Participants were asked to not eat any meat or legumes in the 2 d before each test meal administration. On the first visit (day 1) subjects came in fasting at 08.00 hours ± 1 h, venepuncture was done and an Fe-isotope-labelled standardised test meal was consumed under standardised conditions and close supervision (Fig. 1). Afterwards, subjects were instructed not to eat or drink for a 3-h period. The procedures were repeated on the next day (day 2): subjects received an identical labelled meal with the addition of AA. The AA was added at a molar ratio of AA:Fe of 2:1. Participants received the meals with and without AA in a random order. We labelled the test meals with 6 mg 57Fe (without AA) or 58Fe (with AA) as ferrous sulphate (FeSO4) added directly into the test meals immediately before consumption. The test meal consisted of a white flour bread roll (approximately 90 g) with butter (15 g) and honey (approximately 30 g) spread on top. The subjects were given 300 ml of still water with the meal. Fourteen days after the second test meal was consumed (day 16), a second fasting venepuncture (at 08.00 hours ± 1 h) was done for the analyses of Hb and erythrocyte isotopic composition, as well as for Fe status, inflammation and hepcidin determination. Then, participants received twenty-one capsules containing 600 mg of ibuprofen (Actron Ibuprofen, Bayer) and they were instructed to take one capsule every 8 h (three times per day) for 7 d. On day 23, participants returned any remaining ibuprofen capsules and they answered standardised questions about their health. Participants received another twenty-one ibuprofen capsules for the next week. On day 30, a third fasting venepuncture (at 08.00 hours ± 1 h) was done for the analysis of Hb and erythrocyte isotopic composition as well as for Fe status, inflammation and hepcidin determination. On days 30 and 31, participants received a second round of labelled test meals with and without AA, as described above. Participants were given another twenty-one ibuprofen capsules, after 1 week, compliance and health status were assessed, and twenty-one ibuprofen capsules were given for the last study week. On day 45, a final fasting venepuncture (at 08.00 hours ± 1 h) was done for the analysis of Hb and erythrocyte isotopic composition as well as for Fe status, inflammation and hepcidin determination. Participants received a form where they entered the date and time of each tablet intake. At each study visit, participants returned this form together with the remaining ibuprofen capsules.
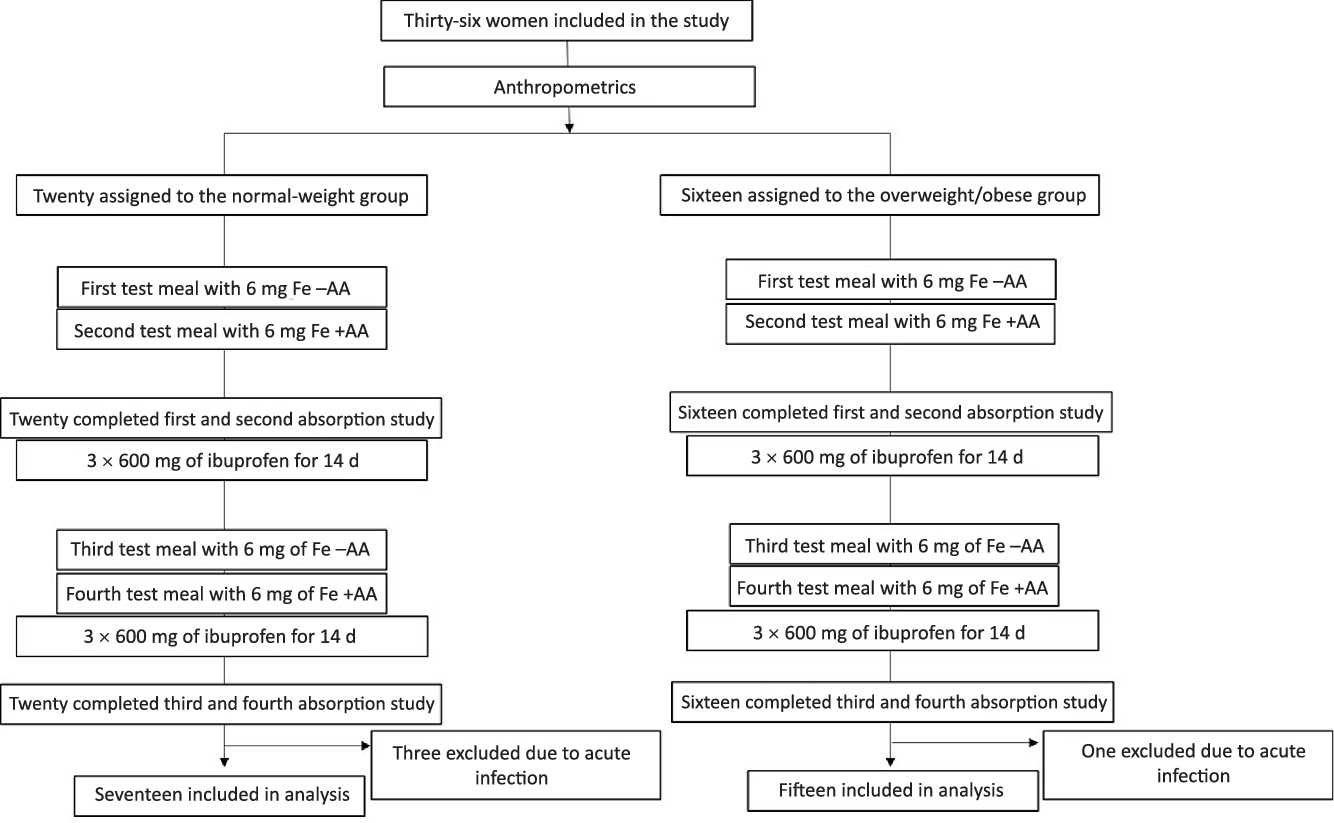
Fig. 1. Study design. Test meals with and without ascorbic acid (AA) were given in random order.
Preparation of isotopically labelled iron
Isotopic-labelled 57Fe- and 58Fe-FeSO4 were prepared from isotopically enriched elemental Fe by dissolution in diluted sulphuric acid(Reference Cercamondi, Egli and Ahouandjinou13). The isotopic composition of the FeSO4 tracer was measured by multicollector-inductively coupled plasma mass spectrometer (MC-ICP-MS); the Fe concentration in the tracer solution was determined by inverse isotopic dilution MC-ICP-MS. The solutions were stored in polytetrafluoroethylene containers and flushed with argon to keep the Fe in the +2 oxidation state.
Laboratory analysis
Hb was measured using a Coulter Counter (HemoCue AB) with three-level quality control material on the day of each blood collection. Serum Fe and total Fe-binding capacity (TIBC) were measured using colorimetry. Measurements were used to calculate transferrin saturation as (serum Fe/TIBC) × 100. We measured serum transferrin receptor, serum ferritin (SF), and high-sensitive CRP and AGP using a multiplex immunoassay(Reference Erhardt, Estes and Pfeiffer14), SHep using immunoassay (DRG Instruments GmbH) and IL-6 using immunoassay (R&D Systems Inc.). Body Fe stores (BIS) were calculated using the TfR:SF ratio as described by Cook et al. (Reference Cook, Flowers and Skikne15). We defined Fe depletion as SF ≤ 45 µg/l, ID as SF < 15 µg/l and anaemia as Hb < 120 g/l(16,17) .
For isotope analysis, whole blood was mineralised by microwave digestion, Fe was separated by anion-exchange chromatography and isotopic analysis was performed with the use of inductively coupled plasma MS (Neptune; Thermo-Finnigan)(Reference Walczyk, Davidsson and Zavaleta18). The calculation of the amount of isotopic label present in the blood of the subject was based on the shift of the isotopic ratios in the blood after red cell incorporation of the absorbed isotopic label, and this was used to estimate FIA(Reference Walczyk, Davidsson and Zavaleta18), assuming an 80 % incorporation of absorbed Fe into the erythrocytes(Reference Hosain, Marsaglia and Noyes19).
Statistical analysis
Statistical analyses were conducted with the use of SPSS (IBM SPSS statistics, version 22). SF and TfR were adjusted for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) regression correction method(Reference Namaste, Rohner and Huang20). FIA was adjusted for a SF level of 25 µg/l(Reference Cook, Dassenko and Lynch21). Data were checked for normal distribution by Shapiro–Wilk testing and for the presence of outliers (defined as +/−3 sd from the mean). Non-normally distributed data were logarithmically transformed for statistical analyses. Data were expressed as mean values and standard deviations (for normally distributed data) or as medians and interquartile ranges (IQR) (for non-normally distributed data even after log transformation). Non-parametric tests were applied for data that remained non-normally distributed after log transformation. Comparisons of the treatment effect between groups (NW and OW/OB) were done using linear mixed model analysis with post hoc Bonferroni correction. Group and treatment were defined as fixed effects. For the linear mixed model on SHep, we included BIS, transferrin saturation and BMI as covariates. For the linear mixed model on FIA, we included AA as an additional fixed effect. We conducted linear multiple regression analysis on SHep, IL-6, serum Fe, transferrin saturation and FIA with AA and FIA without AA. Known parameters reflecting Fe status (BIS), inflammation (IL-6) or Fe status and inflammation (SHep) were chosen as predictors in the regression models. If the dependent variable was not normally distributed, log-transformed data were used. Differences were considered significant at P < 0·05.
Results
We began recruiting NW women on 25 April 2016 and completed the NW study group on 23 June 2016. We began recruiting OW/OB women on 26 May 2018 and we completed the OW/OB group on 13 October 2018. Sixty women were screened for the study, twenty-four of them were not included because they declined to provide consent. Data from three NW women were not included into data analyses because they had inflammation (an elevated CRP > 5 mg/l or elevated AGP > 1 g/l) at baseline. Data from one OW/OB woman were not included into data analyses because she had a sharply elevated CRP after ibuprofen treatment, likely due to an upper respiratory tract infection. Thus, analyses were performed on data from thirty-two women: seventeen in the NW group and fifteen in the OW/OB group (Fig. 1). All women complied with the ibuprofen treatment and all completed the protocol. Compliance with treatment was 87 %. Median age and mean BMI and waist circumference of the NW and OW/OB participants were 26 (IQR 20, 35) and 32 (IQR 28, 41) years (P < 0·05), 21·4 (sd 1·7) and 33·5 (sd 3·3) kg/m2 (P < 0·001), and 73 (sd 5) and 104 (sd 8) cm (P < 0·001). Also, 50 and 90 % of the NW and OW/OB women had depleted BIS. Anaemia and ID prevalence were 5·9 % (for both) in the NW group and 13·3 and 20·0 % in the OW/OB group, respectively. Fe and inflammatory status by group are shown in Table 1.
Table 1. Iron and inflammatory variables at baseline and after 14 d treatment with ibuprofen in healthy normal-weight and overweight/obese women with chronic inflammation*
(Medians and interquartile ranges (IQR); mean values and standard deviations)
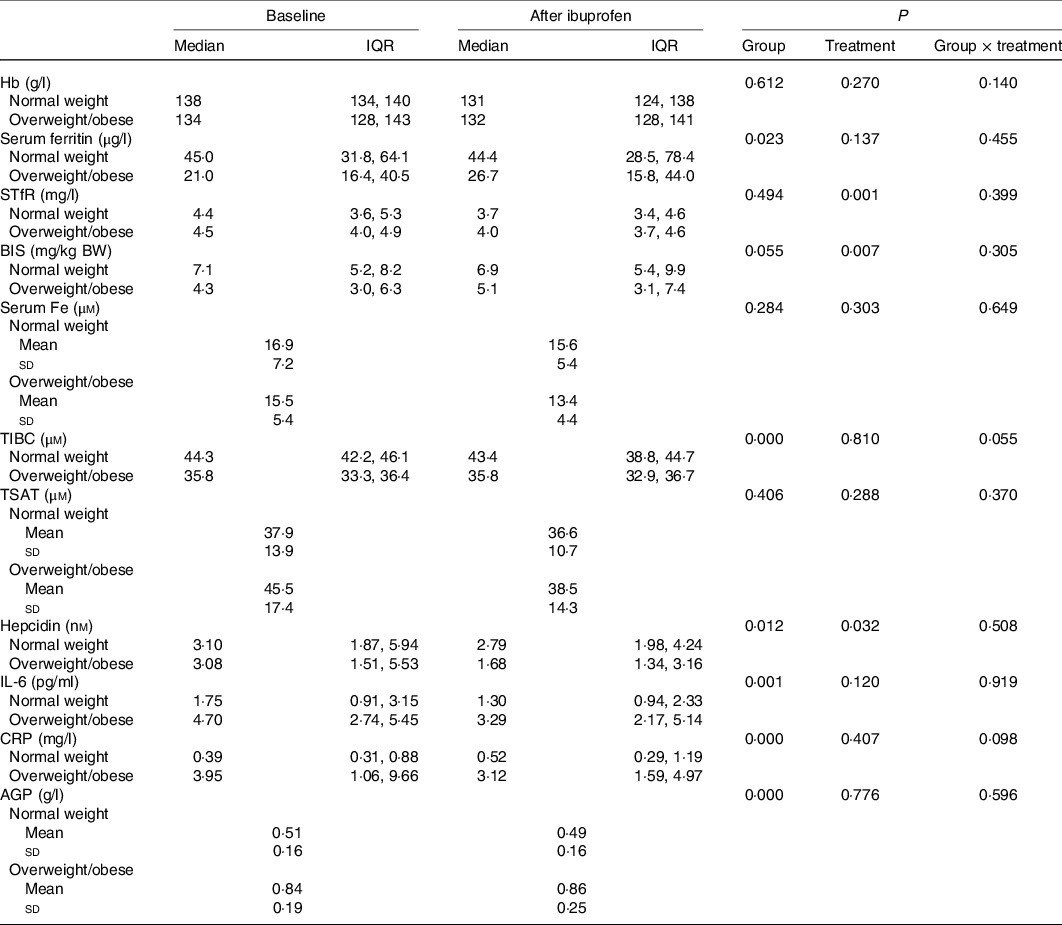
sTfR, serum transferrin receptor; BIS, body Fe stores; BW, body weight; TIBC, total Fe-binding capacity; TSAT, transferrin saturation; CRP, high-sensitive C-reactive protein; AGP, α-1 glycoprotein.
* Comparisons of the treatment effect between groups (normal weight and overweight/obese) were done by using linear mixed model analysis with post hoc Bonferroni correction. Group and treatment were defined as fixed effects. Model on hepcidin is corrected for BIS, TSAT and BMI.
There were significant group effects on SHep, SF, TIBC, IL-6, CRP and AGP (all P < 0·05) and a borderline significant group effect on BIS (P = 0·055). There were significant treatment effects on serum transferrin receptor, BIS and SHep (all P < 0·05). In the OW/OB group, SHep decreased by 45 % from 3·08 (IQR 1·51, 5·53) to 1·68 (IQR 1·34, 3·16) nm after ibuprofen treatment, with no effect on serum Fe (Fig. 2(a) and (b)). Although CRP and IL-6 decreased from 3·95 (IQR 1·06, 9·66) to 3·12 (IQR 1·59, 4·97) mg/l (P = 0·407) and from 4·7 (IQR 2·74, 5·45) to 3·29 (IQR 2·17, 5·14) pg/ml (P = 0·120) in the OW/OB group, there were no significant treatment effects on these two parameters (Fig. 3(a) and (b)). There were no significant group by treatment interactions; there were two borderline significant groups by treatment interactions on TIBC (P = 0·055) and on CRP (P = 0·098). There was a significant AA effect on FIA (P < 0·001): AA increased FIA by 100 and 50 % in the NW and OW/OB groups at baseline. After ibuprofen intake, FIA was increased by AA by 150 and 120 % in the NW and OW/OB group (Fig. 4(a) and (b)). There were no treatment or group effects on FIA (Table 2).
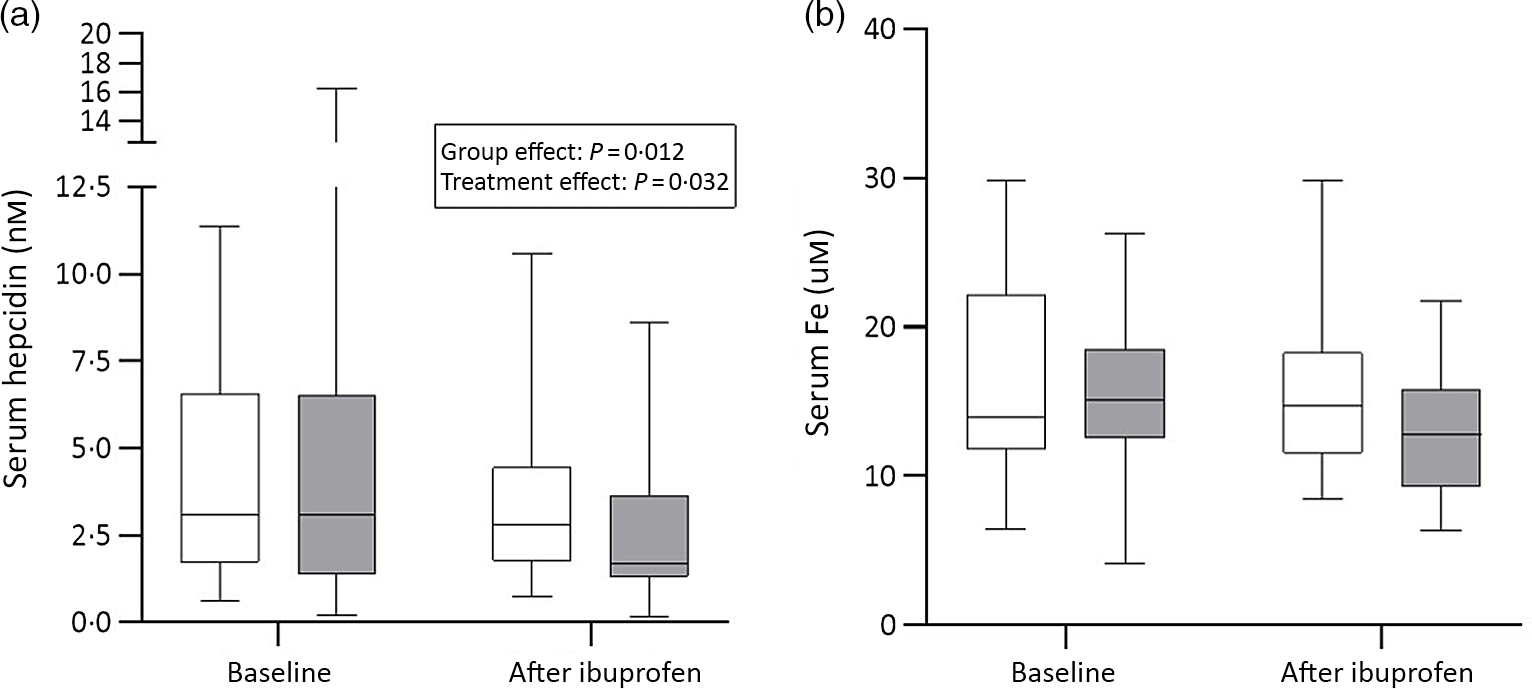
Fig. 2. Serum hepcidin and serum iron response to ibuprofen treatment (3 × 600 mg/d) for 14 d. (a) Serum hepcidin and (b) serum iron concentrations before and after ibuprofen intake for 14 d in normal-weight (n 17) and overweight/obese (n 15) women. Boxes indicate medians and interquartile ranges and whiskers describe the range of the data (minimum to maximum). ![]() , Normal-weight;
, Normal-weight; ![]() , overweight/obese.
, overweight/obese.
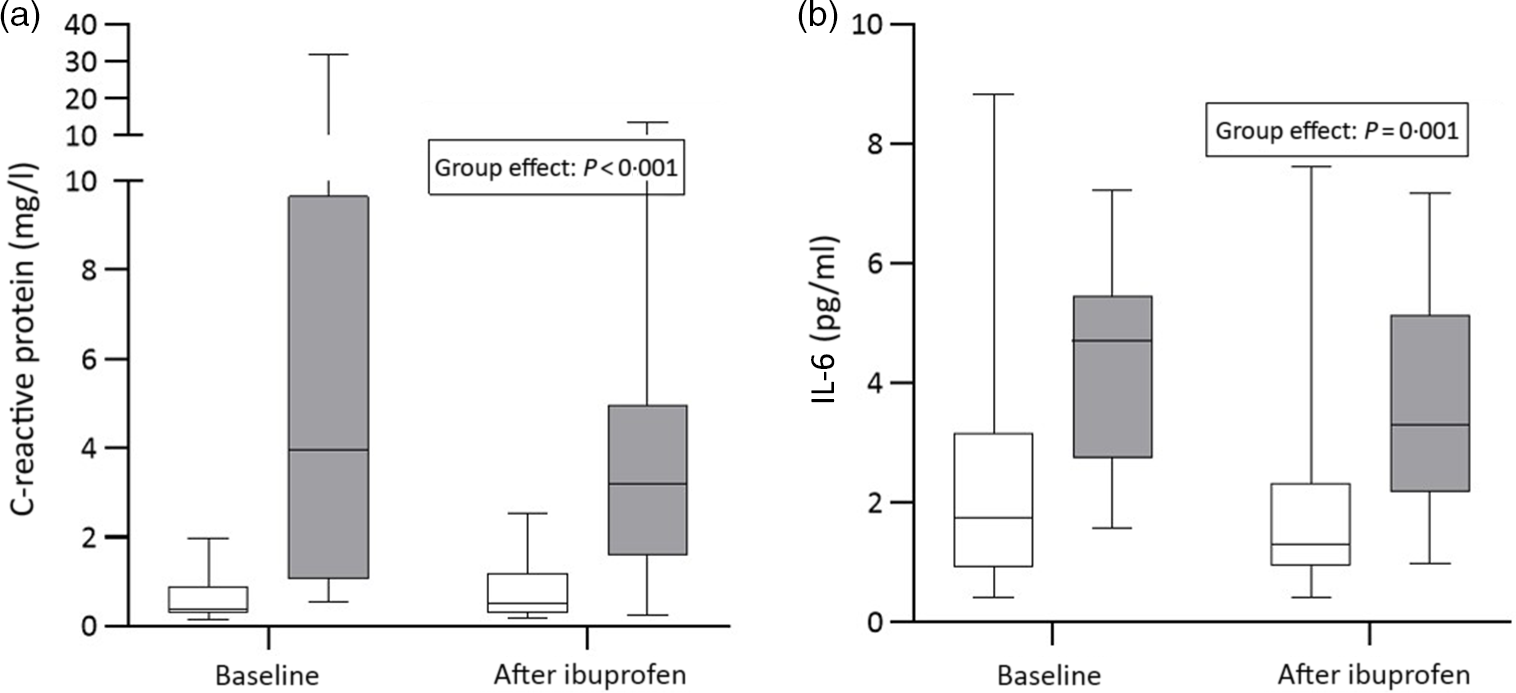
Fig. 3. C-reactive protein and IL-6 response to ibuprofen treatment (3 × 600 mg/d) for 14 d. (a) C-reactive protein and (b) IL-6 concentrations before and after ibuprofen intake for 14 d in normal-weight (n 17) and overweight/obese (n 15) women. Boxes indicate medians and interquartile ranges and whiskers describe the range of the data (minimum to maximum). ![]() , Normal-weight;
, Normal-weight; ![]() , overweight/obese.
, overweight/obese.
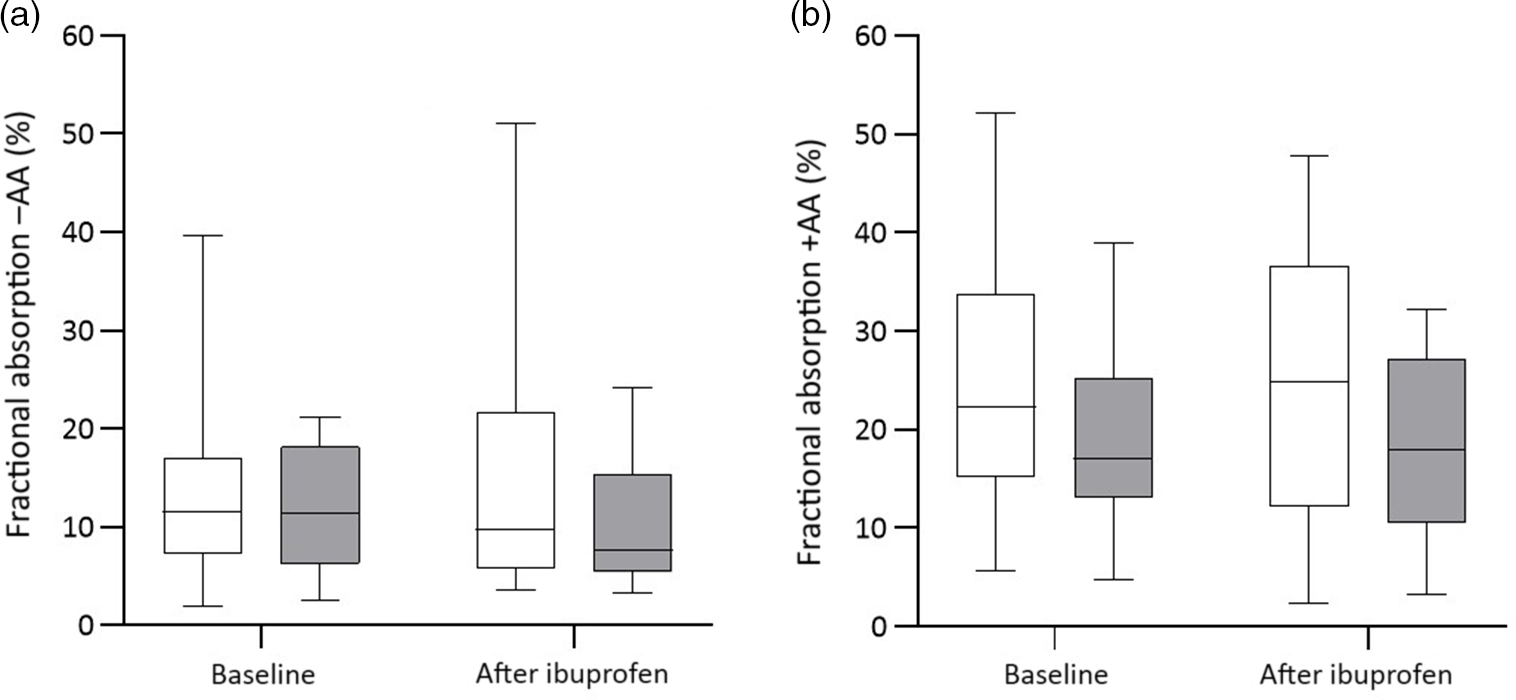
Fig. 4. Fractional iron absorption without ascorbic acid (−AA) and with ascorbic acid (+AA) at baseline and after ibuprofen treatment (3 × 600 mg/d) for 14 d. (a) Fractional iron absorption −AA and (b) fractional iron absorption +AA before and after ibuprofen intake for 14 d in normal-weight (n 17) and overweight/obese (n 15) women. Boxes indicate medians and interquartile ranges and whiskers describe the range of the data (minimum to maximum). ![]() , Normal-weight;
, Normal-weight; ![]() , overweight/obese.
, overweight/obese.
Table 2. Fractional iron absorption without ascorbic acid (FIA −AA) and with ascorbic acid (FIA +AA) at baseline and after 14 d treatment with ibuprofen in normal-weight women without inflammation and overweight/obese women with low-grade inflammation*
(Medians and interquartile ranges (IQR))

* Comparisons of the treatment effect between groups (normal weight and overweight/obese) on FIA were done using linear mixed model analysis with post hoc Bonferroni correction. Group, treatment and ascorbic acid were defined as fixed effects.
As shown in Table 3, we performed separate hierarchical regression analyses with SHep, IL-6, serum Fe, TSAT and FIA with AA or without AA as dependent variables. For SHep at baseline and after ibuprofen treatment, including BIS and IL-6 as covariates, the model explained 58·2 and 61·0 % of the variance in SHep; BIS was a significant positive predictor of SHep (P < 0·001), while IL-6 was not. For IL-6 at baseline and after ibuprofen treatment, including BMI and age as covariates, the model explained 46·6 and 30·9 % of the variance in IL-6; BMI was a significant positive predictor of IL-6 (P < 0·05) at baseline and after ibuprofen treatment, while age was only a significant positive predictor at baseline (P < 0·01). For serum Fe at baseline and after ibuprofen treatment, including BIS and SHep as covariates, the model explained 54·0 and 22·5 % of the variance in serum Fe; BIS was a significant positive predictor of serum Fe (P < 0·05) at baseline, while SHep was not.
Table 3. Multiple linear regression analyses on serum hepcidin, IL-6, serum iron, transferrin saturation and fractional iron absorption (FIA) without and with ascorbic acid (AA) at baseline and after ibuprofen treatment for 14 d
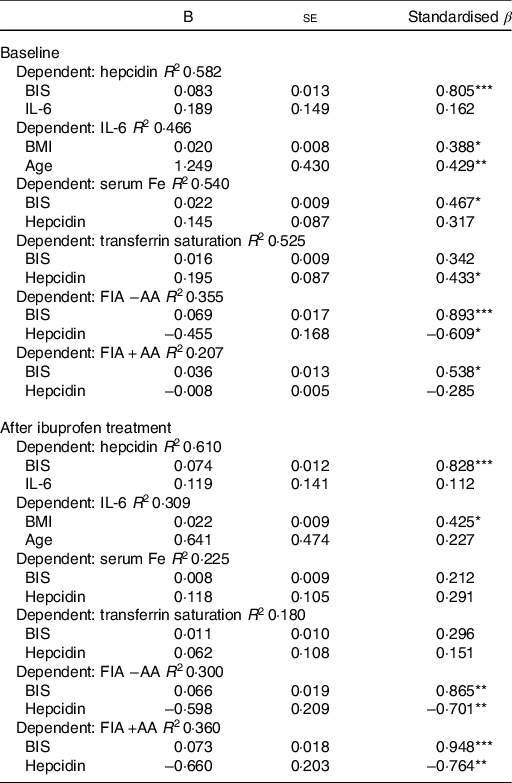
BIS, body Fe stores.
* P < 0·05, ** P < 0·01, *** P < 0·001.
Discussion
Our main findings are, comparing the effects of ibuprofen treatment in OW/OB women with inflammation and NW women without inflammation: (1) there were significant group effects on SF, IL-6, CRP, AGP, TIBC and SHep (for all, P < 0·05) and a borderline group effect on BIS (P = 0·055); (2) there were significant treatment effects on SHep, serum transferrin receptor and BIS (for all, P < 0·05) and a borderline effect on IL-6 (P = 0·120); (3) although AA significantly increased FIA (<0·001), there was no group × AA interaction, and no significant group or treatment effects on FIA and (4) BIS was a significant positive predictor of SHep before and after the ibuprofen treatment (P < 0·001), but IL-6 was not. These findings suggest that, in OW/OB Fe-depleted women, the main predictor of FIA is likely BIS rather than the low-grade inflammation.
In OW/OB, adipocytes produce multiple pro-inflammatory cytokines including IL-6(Reference Mohamed-Ali, Goodrick and Rawesh22,Reference Wellen and Hotamisligil23) . Higher BMI and greater central adiposity predict higher concentrations of CRP and IL-6(Reference Cepeda-Lopez, Melse-Boonstra and Zimmermann7,Reference Stoffel, El-Mallah and Herter-Aeberli24,Reference Weiss, Dziura and Burgert25) . In our study, baseline IL-6 (P < 0·01), CRP and AGP (P < 0·001 for both) were sharply higher in the OW/OB group compared with the NW group; thus, the choice of OW/OB as a model of chronic low-grade inflammation was appropriate. Ibuprofen is an non-steroidal anti-inflammatory drug commonly used for relief of inflammation(Reference Rainsford26); it inhibits COX-1- and COX-2-derived pro-inflammatory prostanoids, which lowers circulating IL-6(Reference Rainsford11). In our study, ibuprofen treatment resulted in modest decreases in IL-6 (−30 %) and CRP (−20 %); this decrease in IL-6 was associated with a halving of SHep in the OW/OB group, suggesting that there was a decrease in inflammation-stimulated hepcidin synthesis. During inflammation, IL-6 activates hepcidin production through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signalling pathway in hepatocytes(Reference Nemeth, Rivera and Gabayan10,Reference Wrighting and Andrews27–Reference Pietrangelo, Dierssen and Valli29) . IL-6 is the necessary and sufficient cytokine for the induction of hepcidin expression during inflammation, and the IL-6-hepcidin axis is responsible for hypoferraemia of inflammation(Reference Nemeth, Rivera and Gabayan10). In vitro, aspirin, another non-steroidal anti-inflammatory drug, suppressed IL-6 and decreased hepcidin mRNA, without affecting Fe-regulatory protein-1 in BV-2 microglia cells(Reference Li, Li and Zhou30). This was associated with an increase in ferroportin expression and a reduction in cellular ferritin light chains(Reference Huang, Ruan and Chen31). We hypothesised a similar effect would occur in vivo, that is, a reduction in IL-6 with ibuprofen treatment would decrease SHep and this would reverse hypoferraemia and increase FIA. However, we did not find these effects despite a halving of SHep in the OW/OB group. There was no significant treatment effect on serum Fe, transferrin saturation or SF, suggesting no decrease in hepcidin-mediated Fe sequestration. Moreover, there was no significant change in FIA, with or without AA, suggesting no beneficial effect on Fe release from enterocytes despite the fall in SHep.
FIA and SHep were comparable in the OW/OB and NW groups at baseline before treatment, despite the sharply higher concentrations of CRP and IL-6 in the OW/OB group. The likely explanation for this is that the poorer baseline Fe status in the OW/OB was suppressing SHep and at least partially counteracting the inducing effect of inflammation on SHep. Thus, SHep in Fe-sufficient NW women without inflammation did not differ from that in Fe-depleted OW/OB women with low-grade inflammation. We have previously shown that SHep is higher in Fe-sufficient OW/OB women compared with Fe-deficient OW/OB, despite comparable levels of systemic inflammation(Reference Cepeda-Lopez, Allende-Labastida and Melse-Boonstra9). Two recent studies in OW/OB pregnant women showed non-significant associations between IL-6 and SHep, suggesting the level of subclinical inflammation in OW/OB may be insufficient to override the suppression of SHep by low BIS(Reference Koenig, Klikuszowian and O’Brien32,Reference Cao, Pressman and Cooper33) . In the present study, BIS were significant predictors of SHep before and after ibuprofen treatment, while inflammation (IL-6 and CRP) was not.
Previous stable Fe isotope studies have reported that FIA from labelled test meals is reduced in women with OW/OB(Reference Cepeda-Lopez, Melse-Boonstra and Zimmermann7) and that absorption is negatively correlated with SHep(Reference Zimmermann, Troesch and Biebinger34). Weight loss in OW/OB women reduces SHep and improves FIA(Reference Cepeda-Lopez, Allende-Labastida and Melse-Boonstra9). Enhancement of FIA through AA, which acts through luminal reduction of dietary ferric Fe (Fe3+) to more soluble ferrous Fe (Fe2+) on the luminal side of the enterocyte, is diminished in OW/OB, where hepcidin reduces Fe efflux at the basolateral membrane of the enterocyte(Reference Cepeda-Lopez, Melse-Boonstra and Zimmermann7). In the present study, although there was no effect of the reduction in SHep on FIA in the OW/OB group, the enhancing effect of AA was more pronounced after ibuprofen treatment in both groups. At baseline and after ibuprofen treatment, although there was no significant group by AA interaction, FIA increased by approximately 100 % in the NW but only by approximately 50 % OW/OB and by approximately 150 % in the NW but only by approximately 120 % in the OW/OB, respectively. Compared with our previous study(Reference Cepeda-Lopez, Melse-Boonstra and Zimmermann7), possible factors contributing to a less blunted effect of AA on FIA in the OW/OB in the present study could be their poorer Fe status(Reference Sangkhae and Nemeth1) and/or less visceral adipose tissue(Reference Stoffel, El-Mallah and Herter-Aeberli24), resulting in a lower SHep and a lower reduction in basolateral Fe transfer into circulation. However, comparing SHep measures between studies is difficult if different assays have been used(Reference Stoffel, Zeder and Fort35).
Strengths of our study include: (1) our subjects were young women, a risk group for ID; (2) we used OW/OB as a model for chronic low-grade inflammation; (3) we used a prospective design and included NW women as a comparison group and (4) we assessed erythrocyte incorporation of stable Fe isotopes to precisely define FIA. Limitations of our study include: (1) all participants were of White or Hispanic ethnicity; (2) we did not measure leptin, which has been shown to upregulate hepatic hepcidin expression(Reference Chung, Matak and McKie36); (3) we obtained only a modest reduction in SHep after ibuprofen treatment despite giving a high dose (1800 mg/d) for 14 d and (4) estimation of blood volume in obese subjects is challenging; although we used an algorithm, we developed in OW/OB subjects using the CO2-rebreathing method(Reference Cepeda-Lopez, Zimmermann and Wussler37); we are uncertain if an over-estimation of blood volume, particularly in the very obese, may have biased our estimates of FIA using stable isotopes.
In conclusion, in this prospective experimental study, a modest decrease in inflammation and SHep in Fe-depleted women with chronic low-grade inflammation did not increase systemic Fe availability and dietary Fe absorption. In this setting, the main predictor of Fe absorption was BIS. Future studies on the interplay between inflammation and Fe status in determining SHep and Fe absorption in young women would be valuable and could inform better treatment strategies for Fe-deficiency anaemia.
Acknowledgements
We thank the study participants for their sustained participation.
The study funded by the Human Nutrition Laboratory, ETH Zurich, Switzerland.
N. U. S., A. C. C. L., I. H. A. and M. B. Z. conceived the study. N. U. S., A. C. C. L., K. C. G., D. L. C. and E. D. G. conducted the study. N. U. S., I. H. A. and M. B. Z. analysed the data and wrote the first draft of the manuscript. All authors contributed to the editing and the finalisation of the manuscript.
The authors declare no competing interests.










