Introduction
Considering congruent morphological and molecular data, Sturhan (Reference Sturhan2012) removed the subfamily Merliniinae Siddiqi, Reference Siddiqi1971 from Telotylenchidae sensu Siddiqi, Reference Siddiqi2000 and the genus Pratylenchoides Winslow, Reference Winslow1958 from Pratylenchidae Thorne, Reference Thorne1949, and amended the diagnosis of the family Merliniidae Siddiqi, Reference Siddiqi1971 (Ryss, Reference Ryss1993). According to Sturhan (Reference Sturhan2012), Merliniidae consists of two subfamilies: Merliniinae and Pratylenchoidinae Sturhan, Reference Sturhan2012. The first subfamily comprises the genera Geocenamus Thorne & Malek, Reference Thorne and Malek1968 (= Scutylenchus Jairajpuri, Reference Jairajpuri1971); Merlinius Siddiqi, Reference Siddiqi1970; Paramerlinius Sturhan, Reference Sturhan2012; Macrotylenchus Sturhan, Reference Sturhan2012; Amplimerlinius Siddiqi, Reference Siddiqi1976; and Nagelus Thorne & Malek, Reference Thorne and Malek1968; whereas the subfamily Pratylenchoidinae is monogeneric with Pratylenchoides only. Another interesting genus described by Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014, Telomerlinius Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014, differs from all known genera in the subfamily Merliniinae by having four incisures in the lateral fields of females and males, having spicules with notch at tip, and sharing the absence of deirids with Geocenamus.
However, the taxonomic status of the genera included in the family Merliniidae has been subjected to a long controversial discussion; particularly, the exact taxonomic position of Geocenamus, Merlinius, and Scutylenchus remains as an unresolved problem. The genus Geocenamus was proposed by Thorne & Malek (Reference Thorne and Malek1968), with G. tenuidens as its type species. Two years later, the genus Merlinius was proposed for 32 species of Tylenchorhynchus Cobb, Reference Cobb1913, which had six incisures in the lateral field, rather cylindroid spicules with prominently notched distal end, a non-protruding gubernaculum, and a moderately developed bursa (Siddiqi Reference Siddiqi1970). One year later, the genus Scutylenchus was proposed to accommodate Tylenchorhynchus mamillatus Tobar-Jiménez, Reference Tobar-Jimenéz1966, mainly based on the mamillate tail shape and in having enlarged, scutella-like phasmids (Jairajpuri Reference Jairajpuri1971).
Anderson (Reference Anderson1977), Hooper (Reference Hooper1978), and Fortuner & Luc (Reference Fortuner and Luc1987) considered Scutylenchus as a junior synonym of Merlinius, and Brzeski (Reference Brzeski1991, Reference Brzeski1998) regarded it as a junior synonym of Geocenamus. Siddiqi (Reference Siddiqi1979, Reference Siddiqi2000) revalidated Scutylenchus and listed the diagnostic characters as presence of longitudinal striae or grooves in the body cuticle and the absence of deirids. The validity of Scutylenchus was subsequently accepted by some nematologists (Decraemer & Hunt Reference Decraemer, Hunt, Perry and Moens2006, Reference Decraemer, Hunt, Perry and Moens2013; Andrássy Reference Andrássy, Csuzdi and Mahunka2007; Hunt et al. Reference Hunt, Bert, Siddiqi, Manzanilla-López and Marban-Mendoza2013), but Geraert (Reference Geraert2011) considered all three genera – Geocenamus, Merlinius, and Scutylenchus – under Geocenamus (Table 1).
Table 1. Classification of Merliniinae genera based on Siddiqi (Reference Siddiqi2000), Geraert (Reference Geraert2011), and Sturhan (Reference Sturhan2012)

Sturhan (Reference Sturhan2012) considered Merlinius as a separate genus but synonymised Scutylenchus with Geocenamus. In that work, certain species of Merlinius with a heavily sclerotised cephalic framework and a distinct refractive inner cuticle layer at tail terminus were transferred to Paramerlinius as a new genus. Also, he mentioned that Scutylenchus and Geocenamus are similar in having four lateral incisures in all juvenile stages, refractive inner cuticle layer at tail terminus, presence of cephalic radial grooves, and a moderate cephalic framework. Sturhan (Reference Sturhan2011, Reference Sturhan2012) concluded that the absence of longitudinal cuticular striae along the entire body appears to be the only essential character distinguishing Geocenamus species from Scutylenchus and further noted that the presence of longitudinal striation should not be considered sufficient to discriminate between genera. Ghaderi et al. (Reference Ghaderi, Karegar and Niknam2014) found that four species of Scutylenchus form a distinct clade within Merliniinae, thus supporting the view of Siddiqi (Reference Siddiqi1979, Reference Siddiqi2000) and Sturhan (Reference Sturhan2012) on Scutylenchus as a distinct genus from Merlinius. However, they stated that the relationships of Scutylenchus with other genera should be further studied and tested by inclusion of additional sequences of species of Merlinius and Geocenamus. The same authors also demonstrated phylogenetically the support for combination of Pratylenchoides and Merliniinae into a single family, the Merliniidae using D2–D3 of 28S rRNA gene. Recently, other authors performed phylogenies with additional sequences using several ribosomal genes and regions (Carta et al. Reference Carta, Skantar and Handoo2010; Alvani et al. Reference Alvani, Mahdikhani, Rouhani and Mohammadi2017; Munawar et al. Reference Munawar, Yevtushenko and Castillo2021) giving a view of the phylogenetic relationships among the different genera in Telotylenchidae. However, additional genera and species are still necessary in order to clarify the position and validity of some genera in this family.
The present study aims to i) describe unknown species of Scutylenchus under an integrative taxonomical approach; ii) add morphological and molecular data on several known species in the subfamily Merliniinae including members of Amplimerlinius, Geocenamus, Merlinius, Nagelus, Scutylenchus, and Telomerlinius; and iii) infer phylogenetic relationships based on partial 18S rRNA, the ITS region, and the D2–D3 region of the 28S rRNA genes within subfamily Merliniinae with inclusion of several representatives of these genera within the subfamily in order to verify the status of the subfamily Merliniinae, preferably by combining morphological and DNA sequence information, if possible.
Material and methods
Nematode sampling and morphological identification
Soil samples were collected from the rhizosphere of different plants in the Khuzestan and Zanjan provinces, in southwestern and northwestern Iran, respectively. Additional samples were collected from an almond orchard in Valenzuela (Córdoba province), southern Spain, and topotype specimens on Arroyo Frío (Jaén province), southern Spain.
Nematodes were extracted by the tray method (Whitehead & Hemming Reference Whitehead and Hemming1965) and then killed and fixed by hot FP 4:1 and processed to anhydrous glycerol (De Grisse Reference De Grisse1969). The nematodes were transferred to a drop of glycerol and a surrounding ring of paraffin wax on permanent slides and studied using a light microscope equipped with a Dino-eye microscope eyepiece camera in conjunction with its Dino Capture version 2.0 software. Drawings were made through a drawing tube attached to a light microscope and redrawn using Adobe Photoshop 7.0 ME software. Specimens were identified at species level using available identification keys (Geraert Reference Geraert2011; Ghaderi et al. Reference Ghaderi, Karegar, Miraeiz and Hashemi2017).
Scanning electron microscopy
For the scanning electron microscopy, specimens preserved in glycerine were selected for observation under SEM according to the Abolafia’s (Reference Abolafia2015) protocol. The nematodes were hydrated in distilled water, dehydrated in a graded ethanol-acetone series, critical-point dried with liquid carbon dioxide, mounted on SEM stubs, coated with gold, and observed with a Zeiss Merlin microscope (5 kV) (Zeiss, Oberkochen, Germany).
3D modelling
To visualise important morphological characters in the lip region and to facilitate further zoological education, 3D models were manually reconstructed by Autodesk® Maya® based on SEM images following the procedure of Qing et al. (Reference Qing, Sánchez Monge and Bert2015).
Nematode molecular identification
DNA extraction was performed from a single individual as described by Subbotin et al. (Reference Subbotin, Waeyenberge and Moens2000). Several sets of primers were used for PCR. A partial region of the 28S rRNA gene including the expansion domains D2 and D3 (D2–D3) was amplified by using the primers D2A (5′ –ACAAGTACCGTGAGGGAAAGTTG– 3′) and D3B (5′ –TCGGAAGGAACCAGCTACTA– 3′) (Nunn Reference Nunn1992). The portion of 18S rRNA was amplified using primers 988F (5′ –CTCAAAGATTAAGCCATGC– 3′), 1912R (5′ –TTTACGGTCAGAACTAGGG–3′), 1813F (5′ –CTGCGTGAGAGGTGAAAT– 3′), and 2646R (5′ –GCTACCTTGTTACGACTTTT– 3′) (Holterman et al. Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006). The internal transcribed spacer region (ITS) separating the 18S rRNA and 28S rRNA genes from the 5.8S rRNA gene was amplified using forward primer 18S (5′ –TTGATTACGTCCCTGCCCTTT– 3′) and reverse primer 26S (5′ –TTTCACTCGCCGTTACTAAGG– 3′) (Vrain et al. Reference Vrain, Wakarchuk, Lévesque and Hamilton1992). All PCR assays were carried out according to the conditions described by Archidona-Yuste et al. (Reference Archidona-Yuste, Navas-Cortés, Cantalapiedra-Navarrete, Palomares-Rius and Castillo2016). 5x HOT FIREpol® Blend Master Mix (Solis Biodyne, Tartu, Estonia) was used in all PCR reactions. The PCR products were purified after amplification using ExoSAP-IT (Affimetrix, USB products, Kandel, Germany) and used for direct sequencing in both directions with the corresponding primers. The resulting products were purified and run in a DNA multicapillary sequencer (Model 3130XL Genetic Analyser; Applied Biosystems, Foster City, CA, USA) using the BigDye Terminator Sequencing Kit v.3.1 (Applied Biosystems) at the Stab Vida sequencing facility (Caparica, Portugal). The sequence chromatograms of the two markers were analysed using DNASTAR LASERGENE SeqMan v. 7.1.0. The newly obtained sequences were deposited in the GenBank database under accession numbers indicated on the phylogenetic trees and in Table 2.
Table 2. List of species, localities, and GenBank accession numbers of specimens obtained in this study for phylogenetic analysis based on 28S rRNA, 18S rRNA, and ITS genes

Scutylenchus sp. (Semnan pop.)KX789703 (28S)KX789706 and KX789707 (18S)Semnan, Semnana province, Iran Apple tree.
Phylogenetic analyses
Sequenced genetic markers in the present study (after discarding primer sequences and ambiguously aligned regions) and sequences obtained from GenBank were used for phylogenetic reconstruction of family Merliniidae (Table 2). Outgroup taxa for each dataset were selected based on previous published studies (Carta et al. Reference Carta, Skantar and Handoo2010; Alvani et al. Reference Alvani, Mahdikhani, Rouhani and Mohammadi2017). Multiple sequence alignments of the newly obtained and published sequences were made using the FFT-NS-2 algorithm of MAFFT v. 7.450 (Katoh et al. Reference Katoh, Rozewicki and Yamada2019). Sequence alignments were visualized using BioEdit (Hall Reference Hall1999) and manually edited and trimmed of the poorly aligned positions using a light filtering strategy (up to 20% of alignment positions), which has little impact on tree accuracy and may save some computation time as suggested by Tan et al. (Reference Tan, Muffato, Ledergerber, Herrero, Goldman, Gil and Dessimoz2015), since methods for automated filtering of multiple sequence alignments frequently worsen single-gene phylogenetic inference (Tan et al. Reference Tan, Muffato, Ledergerber, Herrero, Goldman, Gil and Dessimoz2015).
Phylogenetic analyses of the sequence data sets were based on Bayesian inference (BI) using MrBayes 3.2.7a (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). The best-fitted model of DNA evolution was obtained using jModelTest v. 2.1.7 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012) with the Akaike information criterion (AIC). The base frequency, the proportion of invariable sites, and the gamma distribution shape parameters and substitution rates in the AIC-supported model were then used in phylogenetic analyses. BI analyses were performed under a transitional model of invariable sites and a gamma-shaped distribution (TIM3 + I + G) for D2–D3 region and partial 18S, and a transitional and a gamma-shaped distribution (TIM2 + G) model model for the ITS rRNA region. These BI analyses were run separately per dataset with four chains for 4 × 106 generations. The Markov chains were sampled at intervals of 100 generations. Two runs were conducted for each analysis. After discarding burn-in samples of 30% and evaluating convergence, the remaining samples were retained for more in-depth analyses. The topologies were used to generate a 50% majority-rule consensus tree. Posterior probabilities (PP) were given on appropriate clades. Trees from all analyses were visualized using FigTree software version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
Results
In this study, we identified fourteen known species of the subfamily Merliniinae under morphological taxonomic characters and molecular criteria including Amplimerlinius globigerus Siddiqi, Reference Siddiqi1979; A. longicauda Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina1990; A. macrurus (Goodey, Reference Goodey1932) Siddiqi, Reference Siddiqi1976; A. magnistylus Castillo, Gómez-Barcina, Vovlas & Navas, Reference Castillo, Gómez-Barcina, Vovlas and Navas1991; A. paraglobigerus Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina1990; Geocenamus tenuidens Thorne & Malek, Reference Thorne and Malek1968; Paramerlinius hexagrammus (Sturhan, Reference Sturhan1966) Sturhan, Reference Sturhan2012; Merlinius alboranensis (Tobar-Jiménez, Reference Tobar-Jiménez1970) Tarjan, Reference Tarjan1973; M. brevidens (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970; M. nanus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970; Nagelus obscurus (Allen, Reference Allen1955) Powers, Baldwin & Bell, Reference Powers, Baldwin and Bell1983; N. leptus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1979; Scutylenchus rugosus (Siddiqi, Reference Siddiqi1963) Siddiqi, Reference Siddiqi1979; Telomerlinius teleosus Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014; and one unknown species of Scutylenchus from Iran. Our Scutylenchus sp. as well as G. tenuidens, M. alboranensis, and T. teleosus were measured, described, and illustrated herein, whereas brief description and morphometric values are given for the other eleven previously described species (Ghaderi & Karegar Reference Ghaderi and Karegar2014; Ghaderi et al. Reference Ghaderi, Karegar and Niknam2014).
Subfamily Merliniinae Siddiqi, Reference Siddiqi1971
Diagnosis
Lateral fields each with six incisures (except Telomerlinius). Deirids present except in Geocenamus, Scutylenchus, and Telomerlinius. Phasmids usually prominent, on tail. Lip region annulated; cephalic disc indistinct or distinct (Geocenamus and Telomerlinius). Stylet under 50 μm (except Macrotylenchus, up to 137 μm), with distinct basal knobs. Median and basal bulbs well developed. Vulva small, pore-like, transversely oval or slit-like, usually with epiptygma. Ovaries paired. Spermathecae two- to four-lobed. Postrectal intestinal sac absent. Female tail conoid, subcylindroid, cylindroid, or subclavate, between two and six anal body widths long; terminal inner cuticle layer occasionally strongly thickened. Male tail conical, about as long as that of female. Bursa simple, moderately developed, enveloping tail. Hypoptygma (a pair of papillae on posterior lip of cloaca opening) always present. Spicules cylindroid in distal half, straight to slightly arcuate, with distal end broadly rounded, notched (as a main character) and devoid of ventro-lateral flanges or vela. Gubernaculum simple, trough-like, fixed.
Type Genus
Merlinius Siddiqi, Reference Siddiqi1970
Other Genera
Amplimerlinius Siddiqi, Reference Siddiqi1976
Geocenamus Thorne & Malek, Reference Thorne and Malek1968
Macrotylenchus Sturhan, Reference Sturhan2012
Nagelus Thorne & Malek, Reference Thorne and Malek1968
Paramerlinius Sturhan, Reference Sturhan2012
Scutylenchus Jairajpuri, Reference Jairajpuri1971
Telomerlinius Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014
Key to identification of the genera of subfamily Merliniinae

Genus Amplimerlinius Siddiqi, Reference Siddiqi 1976
Diagnosis
Body medium to large sized, arcuate to strongly curved. Cuticle with prominent annuli. Lateral field with six incisures in adults, fourth and third stage juveniles and four in second stage juveniles. Deirids located in six-incisures region of lateral field. Cephalic region continuous with body contour, annuli not broken by radial grooves or indentations, face view rounded, cephalic plate fused with first annulus. Amphidial apertures ovate, cephalic framework heavily sclerotised. Stylet robust, 20–47 μm long; conus about half of total stylet length; knobs large, rounded. Female tail cylindrical to subclavate, smooth or annulated terminus; with distinct hyaline but without refractive inner cuticle layer around its end. Spicules robust, slightly arcuate, blunt and notched at tip. Gubernaculum trough-shaped in lateral view.
Type species
Amplimerlinius amplus Siddiqi, Reference Siddiqi1976
Other species
A. globigerus Siddiqi, Reference Siddiqi1979
A. hornensis Bello, Mahajan & Zancada, Reference Bello, Mahajan and Zancada1987
A. icarus (Wallace & Greet, Reference Wallace and Greet1964) Siddiqi, Reference Siddiqi1976
A. intermedius (Bravo, Reference Bravo1976) Siddiqi, Reference Siddiqi1976
A. longicauda Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina1990
A. macrurus (Goodey, Reference Goodey1932) Siddiqi, Reference Siddiqi1976
A. magnistylus Castillo, Gómez-Barcina, Vovlas & Navas, Reference Castillo, Gómez-Barcina, Vovlas and Navas1991
A. nectolineatus Siddiqi, Reference Siddiqi1976
A. omentelus Kleynhans & Heyns, Reference Kleynhans and Heyns1983
A. paraglobigerus Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina1990
A. parbati Zarina & Maqbool, Reference Zarina and Maqbool1990
A. planitierus (Eroshenko, Reference Eroshenko1984) Eroshenko & Volkova, Reference Eroshenko and Volkova1988
A. quercinus Mahajan, Reference Mahajan1996
A. siddiqii Mancini, Cotroneo & Moretti, Reference Mancini, Cotroneo and Moretti1982
A. sikkimensis Shaw & Khan, Reference Shaw and Khan1992
A. socialis (Andrássy, Reference Andrássy1962) Siddiqi, Reference Siddiqi1976
A. truncatus (Poghossian, Reference Poghossian1979) Geraert, Reference Geraert2011
A. umbonatus Ivanova, Reference Ivanova1982
A. uramanatiensis Ghaderi & Karegar, Reference Ghaderi and Karegar2014
A. viciae (Saltukoglu, Reference Saltukoglu1973) Siddiqi, Reference Siddiqi1976
Amplimerlinius globigerus Siddiqi, Reference Siddiqi 1979
The Iranian populations from Naghadeh and Ahar in West Azerbaijan and East Azerbaijan provinces were brecovered in the rhizosphere of apricot and apple trees, respectively. Females can be characterised by having a straight to slightly ventrally curved body posture, distinct cuticular annuli, lip region continuous, rounded with flattened anterior end, with 7–8 annuli, stylet with rounded to slightly posteriorly directed knobs, tail cylindrical. Males similar to females except for sexual characters, bursa encircling the entire tail. The morphology and morphometric characters of the Iranian populations are consistent with the other populations from Iran (Ghaderi & Karegar Reference Ghaderi and Karegar2014).
Amplimerlinius longicauda Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina 1990
Morphology and morphometry of topotype specimens of this species agree with the original description (Castillo et al. Reference Castillo, Siddiqi and Gómez-Barcina1990) and can be characterised by having an elongate female tail measuring 3.2–3.5 anal body widths long, a stout stylet measuring 33–37 μm, and outer bands of lateral fields with few scattered striae in pharyngeal and tail regions.
Amplimerlinius macrurus (Goodey, Reference Goodey 1932 ) Siddiqi, Reference Siddiqi 1976
(Figures S2, S3; Table S3)
The Iranian populations of A. macrurus from the rhizosphere of alder trees in Mazandaran province and Astragalus sp. in Lorestan province were characterised by cylindrical and slightly ventrally curved body, rounded lip region continuous with flattened anterior end, robust stylet having rounded to posteriorly directed knobs, cylindrical tail, terminus with distinct annuli which are smaller than or as wide as other tail annuli, bearing 43 (38–52) tail annuli, hyaline region 11.0 (9.5–12.5) μm in Mazandaran population but tail cylindrical to subclavate with smooth terminus or with large irregular annuli at terminus in Lorestan population (Figures S2 & S3). The morphological and morphometric characters of the Iranian populations are consistent with the other populations from Iran (Ghaderi & Karegar Reference Ghaderi and Karegar2014).
Amplimerlinius magnistylus Castillo, Gómez-Barcina, Vovlas & Navas, Reference Castillo, Gómez-Barcina, Vovlas and Navas 1991
The Spanish population of A. magnistylus from the rhizosphere of almond trees in Córdoba province was characterised by cylindrical and slightly ventrally curved body, lip region continuous anteriorly flattened, stylet long and robust (43–45 μm in females), knobs rounded with anterior surfaces backwardly directed, basal bulb elongate-saccate, slightly longer than isthmus, vulva cavity with double sunken epiptygma, tail elongate-cylindroid; terminus hemispherical, with fine striae. The morphological and morphometric characters of the population from Valenzuela (Córdoba province) are consistent with the original population described at Bujalance (Córdoba province) (Castillo et al. Reference Castillo, Gómez-Barcina, Vovlas and Navas1991).
Amplimerlinius paraglobigerus Castillo, Siddiqi & Gómez-Barcina, Reference Castillo, Siddiqi and Gómez-Barcina1990
Morphology and morphometry of topotype specimens of this species agree with the original description (Castillo et al. Reference Castillo, Siddiqi and Gómez-Barcina1990) and can be characterised by a small body and stylet measuring under 1 mm and under 24 μm, respectively; lip region with 8–10 annuli and cephalic sclerotisation not appearing bead-like in optical section, and a slender isthmus about 1.5 times length of the basal bulb.
Genus Geocenamus Thorne & Malek, Reference Thorne and Malek 1968
Diagnosis
Body about 1 mm or longer. Body cuticle without longitudinal striae (except for the anterior body region in some species). Lateral field with six incisures in adults and four in all juvenile stages. Deirid absent. Cephalic region offset, with six radial grooves and conspicuous, perioral disc. Cephalic framework moderate. Stylet slender, 25–30 μm, conus longer than shaft. Female tail elongate-conoid to subcylindroid, terminus smooth or annulated, refractive inner cuticle layer at tail end present. Spicules slender, slightly arcuate and notched at tip. Gubernaculum crescent-shaped in lateral view.
Type species
Geocenamus tenuidens Thorne & Malek, Reference Thorne and Malek1968
Other species
G. arcticus (Mulvey, Reference Mulvey1969) Tarjan, Reference Tarjan1973
G. angelescresti Chitambar & Ferris, Reference Chitambar and Ferris2005
G. deserticola (Ivanova & Shagalina, Reference Ivanova and Shagalina1983) Fortuner & Luc, Reference Fortuner and Luc1987
G. khashanicus Volkova, Reference Volkova1995
G. superbus (Allen, Reference Allen1955) Fortuner & Luc, Reference Fortuner and Luc1990
G. tokobaevi (Sultanalieva, Reference Sultanalieva1983) Fortuner & Luc, Reference Fortuner and Luc1987
Geocenamus tenuidens Thorne & Malek, Reference Thorne and Malek 1968
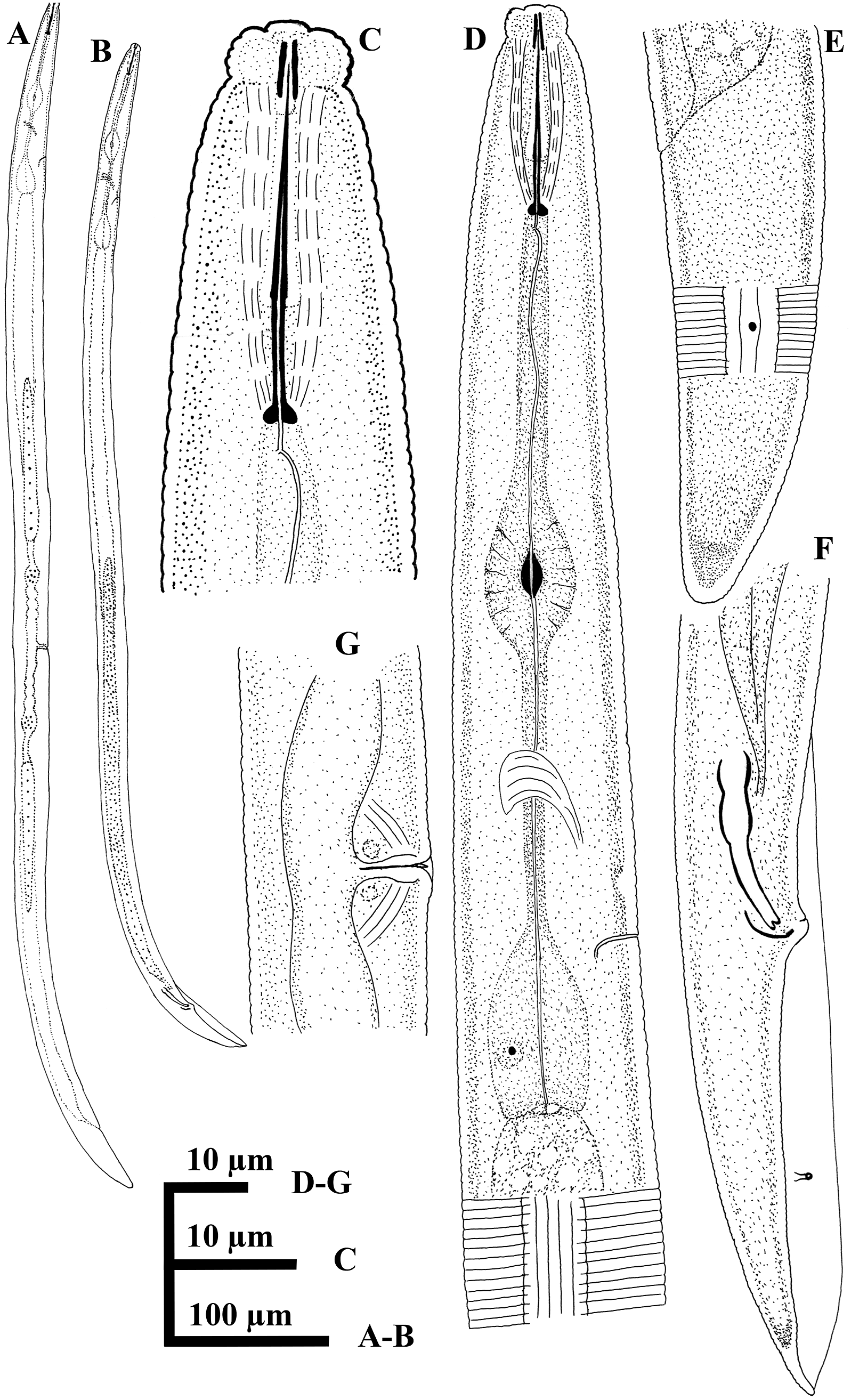
Figure 1. Line drawings of the Iranian population of Geocenamus tenuidens. A, C–E, female and B, F, male. A, B, entire body. C, anterior region. D, pharyngeal region. E, F, tail region. G, vulval region.

Figure 2. Light micrographs of the Iranian population of Geocenamus tenuidens. A–E, female and F, male. A–C, anterior region. D–F, tail region. Scale bars = 10 μm.

Figure 3. SEM micrographs of the Iranian population of Geocenamus tenuidens. A–J, female and K, L, male. A, E, F, anterior region (white arrows indicating the amphidial apertures). B, C, en face view of cephalic region. D, excretory pore in ventral view (white arrow indicating the excretory pore). G, lateral field. H, vulval region in ventral view showing small lateral flaps. I–L, tail region (white and black arrows indicating the phasmid).
Table 3. Morphometrics of Geocenamus tenuidens from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Description of female
Body slightly arcuate after heat fixation. Longitudinal striation or ridges indistinct except lateral fields. Body annuli distinct but fine, 1.2 (1.0–1.3) μm at mid-body. Lateral field originating at the level of the stylet and extending up to tail terminus, with six incisures, outer bands areolated, 7.8 (6–9) μm wide occupying 36 (30–39) % of the corresponding body diameter. Cephalic region distinctly offset by a constriction, bearing 5–6 annuli with distinct perioral disc. Cephalic framework not refractive. Stylet slender, conus 13.3 (12.5–14.0) μm or 58.5 (57.7–60.0) % of the total stylet length; basal knobs weak and posteriorly sloping, 2.7 (2.5–3.0) μm across. DGO 2.5 (2.3–2.8) μm behind stylet knobs. Median bulb oval, 10.8 (9–12) μm × 18 (14.5–21.0) μm, occupying 57 (47–64) % of body wide at that level. Basal bulb pyriform 11.6 (9.5–13.0) μm × 25 (20–30) μm. Nerve ring at 97 (92–104) μm from anterior end. Hemizonid 4 to 6 annuli anterior to excretory pore, at 117 (111–125) μm from anterior end. Deirids not seen. Reproductive system didelphic, vulva slightly sunken into the body, with small epiptygma. Vagina perpendicular, 8.3 (6.5–9.5) μm long occupying 22.5 (20–26) % of vulval body diameter, spermatheca rounded and filled with globular sperm cells. Post anal intestinal sac absent. Tail elongate, conical to subcylindrical, tail terminus coarsly striated. Phasmids located at 39 (35–45) % of tail.
Description of male
Morphologically similar to female except for sexual characters. Body slightly shorter. Body annuli 1.1 (1.0–1.2) μm at mid-body. Lateral field 6.5 (5.3–7.0) μm wide. Cephalic region offset, 6.8 (6.2–7.1) μm wide and 3.9 (3.7–4.5) μm high. Conus 58 (56–59) % of the total stylet length. DGO 2.5 (2–3) μm behind stylet knobs. Nerve ring at 96 (91–102) μm from anterior end. Hemizonid 3–5 annuli anterior to excretory pore and located at 114 (102–119) μm from anterior end. Bursa surrounded tail tip, 70 (65–81) μm in length. Spicules tylenchoid, notched at tip. Gubernaculum simple and ventrally arcuate.
Voucher specimens
Fourteen females and eight males were deposited in the nematode collection of the department of Plant Protection, College of Agriculture, University of Zanjan, Zanjan, Iran.
Host and locality
Recovered from the rhizosphere of Astragalus sp. in Mongasht Mountain in Dehdez region, Khuzestan province, southwest of Iran. GPS coordinates: 31°47’16” N, 50°25’32” E.
Diagnosis and relationships
G. tenuidens is similar to G. arcticus (Mulvey, Reference Mulvey1969) Tarjan, Reference Tarjan1973.
G. tenuidens differs from G. arcticus by a shorter stylet (22.8 (21.6–24.0) vs. 32–38 μm), shorter tail (45.6 (41–55) vs. 65–80 μm) and shorter spicules (21.0 (20.5–21.6) vs. 26–28 μm) and in tail terminus (striated vs. smooth).
Fadavi Khalajlo et al. (Reference Fadavi Khalajlo, Mahdikhani Moghaddam and Rouhani2013) described G. tenuidens from tomato plants in North Khorasan province, but the population was described without perioral disc, with strong stylet knobs, short conus, and 32–45 tail annuli, while G. tenuidens has been described originally with a distinct perioral disc, slender stylet with long conus and tail with 45–80 annuli. Morphological and morphometric characters of our population fit well with those of G. tenuidens, so we consider the present population as the first population of the species reported from Iran.
Genus Merlinius Siddiqi, Reference Siddiqi 1970
Diagnosis
Body usually 1 mm or less. Body cuticle lacking longitudinal striae or grooves. Lateral field with six incisures in adults and four in all juvenile stages. Lip region continuous or slightly offset. Perioral disc and first annulus merged, lip region hexagonal but not separated from radial incisures, the lateral sectors demarcated from defective incisures, submedian sectors wider than lateral sectores, amphidial apertures nearly rounded in first annulus. Stylet usually under 20 μm long, conus half of stylet length or shorter. Deirids distinct and in four-incisure region of lateral field. Vulva with transverse slit, epiptygma indistinct. Female tail conoid to subcylindroid, terminal cuticle normally thickened and with refractive inner cuticle layer around tail end. Spicules cylindroid, straight to slightly arcuate, tip bluntly notched. Gubernaculum crescent-shaped in lateral view.
Type species
Merlinius brevidens (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970
Other species
M. acuminatus Minagawa, Reference Minagawa1985
M. alboranensis (Tobar-Jiménez, Reference Tobar-Jiménez1970) Tarjan, Reference Tarjan1973
M. bavaricus (Sturhan, Reference Sturhan1966) Siddiqi, Reference Siddiqi1970
M. bijnorensis Khan, Reference Khan1971
M. bilqeesae Khan & Khan, Reference Khan and Khan1995
M. bogdanovikatjkovi (Kirjanova, Reference Kirjanova1941) Siddiqi, Reference Siddiqi1970
M. capitonis Ivanova, Reference Ivanova1983
M. circellus Anderson & Ebsary, Reference Anderson and Ebsary1982
M. communicus Sultan, Singh & Sakhuja, Reference Sultan, Singh and Sakhuja1989
M. gatevi Budurova, Reference Budurova1988
M. graminicola (Kirjanova, Reference Kirjanova1951) Siddiqi, Reference Siddiqi1976
M. indicus Zarina & Maqbool, Reference Zarina and Maqbool1995
M. joctus (Thorne, Reference Thorne1949) Sher, Reference Sher1974
M. khuzdarensis Handoo, Khan & Islam, Reference Handoo, Khan and Islam2007
M. lineatus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970
M. loofi Siddiqi, Reference Siddiqi1979
M. microdorus (Geraert, Reference Geraert1966) Siddiqi, Reference Siddiqi1970
M. mollicephalus Eroshenko & Volkova, Reference Eroshenko and Volkova1988
M. montanus Maqbool & Shahina, Reference Maqbool and Shahina1987
M. nanus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970
M. niazae Maqbool, Fatima & Hashmi, Reference Maqbool, Fatima and Hashmi1983
M. nothus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970
M. obesus (Gagarin, Reference Gagarin and Sonin2004) Sturhan, Reference Sturhan2012
M. pistaciei Fatema & Farooq, Reference Fatema and Farooq1992
M. plerorbus Anderson & Ebsary, Reference Anderson and Ebsary1982
M. processus Siddiqi, Reference Siddiqi1979
M. productus (Thorne, Reference Thorne1949) Sher, Reference Sher1974
M. pseudobavaricus Saltukoglu, Geraert & Coomans, Reference Saltukoglu, Geraert and Coomans1976
M. pyri Fatema & Farooq, Reference Fatema and Farooq1992
M. tetylus Anderson & Ebsary, Reference Anderson and Ebsary1982
M. tortilis Kazachenko, Reference Kazachenko1980
Species inquirendae
M. salechardicus Nesterov, Reference Nesterov1985
M. kirjanovae (Karapetjan, Reference Karapetjan1979) Eroshenko & Volkova, Reference Eroshenko and Volkova1987
Merlinius alboranensis (Tobar-Jiménez, Reference Tobar-Jiménez 1970 ) Tarjan, Reference Tarjan 1973

Figure 4. Line drawings of the Iranian population of Merlinius alboranensis. A, C, D, F–H, J, female and B, E, I, male. A, B, entire body. C, pharyngeal region. D, basal bulb of pharynx. E, F, anterior region. G, reproductive system. H–J, tail region.

Figure 5. Light micrographs of the Iranian population of Merlinius alboranensis. A, B (right), C–H, female and B (left), male. A, pharyngeal region. B, entire body. C, D, anterior region. E, lateral field and deirid. F–H, tail region. Scale bars: A, C–H = 10 μm; B= 100 μm.

Figure 6. SEM micrographs of the Iranian population of Merlinius alboranensis. A–L, female and M, N, male. A, anterior region (black arrow indicating the excretory pore). B, C, lateral view of cephalic region (black arrows indicating the amphidial apertures). D, en face view of cephalic region. E, excretory pore in ventral view (white arrow indicating the excretory pore). F, lateral field at deirid region. G, lateral field at mid-body. H, Lateral field at vulval region. I, vulval region in ventral view. J, anus in ventral view. K–N, tail region (white arrows indicating the phasmid).
Table 4. Morphometrics of Merlinius alboranensis from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Table 5. Morphometrics of Nagelus leptus from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Description of female
Body ventrally arcuate to C-shaped after heat fixation. Longitudinal striation or ridges except lateral fields, absent. Body annuli distinct, 1.1 (0.9–1.2) μm at mid-body. Lateral field originating at the level of the procorpus and extending up to tail terminus, with six incisures at mid-body, 6.3 (5.4–7.5) μm wide occupying 31 (28–34) % of body diameter. Lip region slightly offset by constriction, flattened at front, bearing 4–5 annuli. Cephalic framework not refractive. Stylet delicate, conus 5.3 (5.0–5.6) μm or 49 (48–52) % of total stylet length, basal knobs small and posteriorly directed, 2.8 (2.6–3.0) μm across. DGO 1.7 (1.5–2.0) μm behind stylet knobs. Median bulb oval, 10.4 (9.5–11.0) μm × 16 (14–19) μm, occupying 64 (56–69) % of body wide at that level. Basal bulb pyriform, 11.1 (9.5–12.5) μm × 21 (19–23) μm. Nerve ring at 72 (63–76) μm from anterior end. Hemizonid 1 to 2 annuli anterior to secretory-excretory pore, at 85 (68–95) μm from anterior end. Deirids in four-insicures region. Reproductive system didelphic, with epiptygma, vagina perpendicular, 7.9 (7.0–9.5) μm occupying 39 (33–43) % of vulval body diameter. Spermatheca slightly ovate and filled with globular sperm cells. Post anal intestinal sac absent. Tail elongate, conical to subcylindrical with 41 (32–50) annuli in ventral side, tail terminus smooth. Phasmids located at 45 (40–47) % of tail.
Description of male
Morphologically similar to female except for sexual characters. Body straight to ventrally curved. Body annuli 1.1 (0.9–1.2) μm at mid-body. Lateral field 6.5 (5.2–8.3) μm wide. Lip region offset, 7.3 (6.8–7.5) μm wide and 2.3 (2.1–2.5) μm high. Conus 48 (47–49) % of total stylet length. DGO 1.5 μm behind stylet knobs. Nerve ring at 73 (63–80) μm from anterior end. Hemizonid 1–2 annuli anterior to excretory pore and located at 82 (76–87) μm from anterior end. Bursa enveloping entire tail, 61 (58–68) μm. Spicules tylenchoid, at tip with a notch. Gubernaculum simple, ventrally arcuate.
Voucher specimens
Seven females and five males were deposited in the collection of the department of Plant Protection, College of Agriculture, University of Zanjan, Zanjan, Iran. Five females and a male were deposited in the collection of the Department of Plant Protection, School of Agriculture, Shiraz University, Shiraz, Iran.
Host and locality
Recovered from the rhizosphere of Hawthorn (Crateagus aronia L.), collected in Dezfule Region in Khuzestan province, southwest of Iran. GPS coordinates: 32°90’42” N, 48°57’06” E; altitude: 1107 m. a.s.l.
Relationships
M. alboranensis comes close to M. brevidens (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970; M. capitonis Ivanova, Reference Ivanova1983; M. loofi Siddiqi, Reference Siddiqi1979; M. microdorus (Geraert, Reference Geraert1966) Siddiqi, Reference Siddiqi1970; and M. nanus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1970 according to stylet length, tail shape, body length, tail annuli, and cephalic region shape. It differs from M. brevidens by a shorter stylet (10.8 (10.0–11.5) vs. 13.0–16.5 μm) and a weakly developed cephalic framework (vs. distinctly refractive). From M. capitonis, it differs by a shorter body (523 (487–604) vs. 680–810 μm) and a slightly offset cephalic region (vs. offset). From M. loofi, it differs by a shorter tail (40.6 (32–50) vs. 62 μm), smaller c’ ratio (2.9 (2.5–3.6) vs. 4.3–5.7), and lower number of tail annuli (42 (34–52) vs. 50–60). From M. microdorus, it differs by a shorter stylet (10.8 (10.0–11.5) vs. 12.0–15.5 μm) and a slightly but visible lower lip region. Finally, it differs from M. nanus only in tail terminus (smooth vs. annulated) and cuticular annulations (shallow vs. deep).
The Iranian population of M. alboranensis is very similar to the original description (Tobar-Jiménez, Reference Tobar-Jiménez1970) but differs only by a slightly longer body (523 (487–604) vs. 450 (430–470) μm). Ghaderi et al. (Reference Ghaderi, Kashi and Karegar2018) proposed that M. alboranensis may be synonymised with M. microdorus. This species has been reported several times from Iran (Seraji et al. Reference Seraji, Pourjam and Kheiri2000; Jahanshahi Afshar et al. Reference Jahanshahi Afshar, Pourjam and Kheiri2006; Palashi et al. Reference Palashi, Ardali, Khosrawi, Jahanbazian and Abdollahi2012; and Hatami et al. Reference Hatami, Eskandari and Asghari2014) but not described or illustrated in those works. In the present paper, it was described and illustrated for the first time from Iran.
Merlinius brevidens (Allen, Reference Allen 1955 ) Brzeski, Reference Brzeski 1991
The Iranian population of M. brevidens from the rhizosphere of wheat in Ardabil province could be characterised by having a slightly ventrally arcuate to C-shaped body, lip region slightly set off by constriction, with 5–7 annuli, and a distinct refractive basal plate of framework. Stylet delicate, knobs rounded and posteriorly directed. Tail conoid with rounded terminus, tail tip smooth. The morphological and morphometric characters of this population fit well with those of the original description (Allen, Reference Allen1955) and other Iranian populations (Ghaderi et al. Reference Ghaderi, Karegar and Niknam2014).
Merlinius nanus (Allen, Reference Allen 1955 ) Brzeski, Reference Brzeski 1991
The Iranian population of M. nanus from the rhizosphere of barley in East Azerbaijan can be characterised by body ventrally curved to C-shaped, lip region with 5–6 annuli, cephalic framework lightly sclerotised and tail subcylindrical with 45 (38–56) annuli, ending to an annulated terminus. The Iranian population of M. nanus is consistent with the original description (Allen Reference Allen1955) and other Iranian populations (Naseri et al. Reference Naseri, Pourjam and Tanha Maafi2008; Ghaderi et al. Reference Ghaderi, Karegar and Niknam2014).
Genus Nagelus Thorne & Malek, Reference Thorne and Malek 1968
Diagnosis
Body about 1 mm or longer. Lateral fields with six incisures in adults, fourth and third stage juveniles and four in second stage juveniles. Cephalic region slightly offset by expansion, annuli not broken by radial grooves of indentations, perioral disc merged with first annulus, cephalic region hexagonal but not separated from radial incisures, amphidial apertures nearly rounded, located at labial disc. Lip region continuous or slightly offset by a constriction. Cephalic framework lightly sclerotised. Deirids conspicuous in six-incisure region of lateral field. Stylet robust, conus about as long as shaft, knobs posteriorly sloping. Basal bulb saccate. Vulval slit with epiptygma. Famale tail elongate-conoid to subcylindrical, terminus with distinct hyaline region but without refractive inner cuticle layer around its end. Spicules robust. Gubernaculum simple and curved in lateral view.
Type species
Nagelus leptus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1979
Other species
N. borealis Powers, Baldwin & Bell, Reference Powers, Baldwin and Bell1983
N. exacutus Volkova, Reference Volkova1993
N. gerriae Khan & Singh, Reference Khan and Singh1999
N. jamalensis (Nesterov, Reference Nesterov1973) Siddiqi, Reference Siddiqi1979
N. macrophasmidus (Khan & Darekar, Reference Khan and Darekar1979) Siddiqi, Reference Siddiqi1986
N. obscurus (Allen, Reference Allen1955) Powers, Baldwin & Bell, Reference Powers, Baldwin and Bell1983
N. parobscurus (Mulvey, Reference Mulvey1969) Siddiqi, Reference Siddiqi1986
N. sobaekensis (Choi & Geraert, Reference Choi and Geraert1993) Siddiqi, Reference Siddiqi2000
N. varians (Thorne & Malek, Reference Thorne and Malek1968) Siddiqi, Reference Siddiqi1986
Nagelus leptus (Allen, Reference Allen 1955 ) Siddiqi, Reference Siddiqi 1979

Figure 7. Light micrographs of the Iranian population of Nagelus leptus. A–D, female. A, B, anterior region. C, D, tail region. Scale bars = 10 μm.
Description of female
Body ventrally arcuate to C-shaped after heat fixation. Body annuli distinct, 0.9–1.0 μm at mid-body. Lateral field originating at the level of the precorpus and extending up to tail terminus, with six incisures, 6.5 (6.0–7.0) μm wide that occupied 27 (28–34) % of body diameter. Cephalic region narrower than adjective of body and slightly marked by a depression, rounded, 8.5 (8.0–9.0) μm in width, bearing 8 annuli. Cephalic framework lightly sclerotised. Stylet robust, conus 12 (11–13) μm or 48 (47–50) % of total stylet length, basal knobs small and posteriorly directed, 6.0–6.5 μm across. DGO 3.0–3.5 μm behind stylet knobs. Median bulb oval, 18.5 (18–19) μm × 12.5 (12–13) μm. Basal bulb pyriform, 13–15 μm × 20–24 μm. Nerve ring at anterior half of isthmus. Hemizonid 1 to 2 annuli anterior to excretory pore and located at 124 (121–128) μm from anterior end. Deirids located in six-incisures region. Reproductive system didelphic, with small epiptygma, vagina perpendicular, 10–11 μm occupying 37 (34–41) % of vulval body diameter. Spermatheca rounded, offset, without sperm cell. Post anal intestinal sac absent. Tail tapering, with 61 (58–66) annuli in ventral side, tail terminus annulated. Hyaline 9.0 (8.5–9.5) μm. Phasmids located at 48 (46–53) % of tail.
Voucher specimens
Five females were deposited in the collection of the department of Plant Protection, College of Agriculture, University of Zanjan, Zanjan, Iran.
Host and locality
Recovered from the rhizosphere of Willow, collected in Abr forest, Semnan province, northwest of Iran. GPS coordinates: 36°04’59” N, 53°30’20” E.
Diagnosis and relationships
N. leptus is similar to N. obscurus and differs from it by a higher number of lip region annuli (8–9 annuli vs. 5–6 annuli) and a slightly wider tail tip.
Nagelus obscurus (Allen, Reference Allen 1955 ) Powers, Baldwin & Bell, Reference Powers, Baldwin and Bell 1983
The Iranian population of N. obscurus from rhizosphere of Johnson grass, apple in Ardabil province can be characterised by lip region continuous from body contour to slightly offset by a depression, cephalic framework lightly sclerotised, deirids located at six incisures region, basal bulb pyriform, spermatheca filled with sperm, tail conical, tail tip annulated, and hyaline 10–11 μm. This population corresponds well with the original description of N. camelliae (Kheiri, Reference Kheiri1972). Brzeski (Reference Brzeski1998) synonymised N. camelliae Kheiri, Reference Kheiri1972 with N. obscurus. This population morphologically and morphometrically fits well with the original description and other Iranian populations (Kheiri Reference Kheiri1972; Ghaderi et al. Reference Ghaderi, Karegar and Niknam2014).
Genus Paramerlinius Sturhan, Reference Sturhan 2012
Diagnosis
Body medium or large. Lateral field with six incisures in adults, fourth and third stage juveniles and four in second stage juveniles. Deirids situated in four-incisures region of lateral fields. Lip region continuous or slightly offset by constriction, 5–9 annuli, without longitudinal grooves, occasionally with indentations or grooves, prioral disc mostly indistinct. Framework heavily sclerotised. Stylet robust, 20–50 μm long, conus as long as shaft. Median bulb well developed. Basal bulb pyriform to saccate. Reproductive system amphidelphic. Tail broadly conoid or subcylindrical with smooth terminus, with refractive inner cuticle layer surrounding the tail tip. Phasmid mostly prominent. Second-stage juveniles with four incisures in each lateral field. Spicules robust, with blunt and notched tip. Gubernaculum simple, curved in lateral view.
Type species
Paramerlinius hexagrammus (Sturhan, Reference Sturhan1966) Sturhan, Reference Sturhan2012
Other species
P. adakensis (Bernard, Reference Bernard1984) Sturhan, Reference Sturhan2012
P. affinis (Allen, Reference Allen1955) Sturhan, Reference Sturhan2012
P. alpinus (Allen, Reference Allen1955) Sturhan, Reference Sturhan2012
P. arenosus (Ivanova & Shagalina, Reference Ivanova and Shagalina1983) Sturhan, Reference Sturhan2012
P. clavicaudatus (Choi & Geraert, Reference Choi and Geraert1975) Ghaderi, Reference Ghaderi2019
P. ekbali (Khan & Singh, Reference Khan and Singh1999) Sturhan, Reference Sturhan2012
P. elongatus (Ivanova & Shagalina, Reference Ivanova and Shagalina1983) Sturhan, Reference Sturhan2012
P. falcatus (Eroshenko, Reference Eroshenko, Eroshenko and Belogurov1981) Sturhan, Reference Sturhan2012
P. grandis (Allen, Reference Allen1955) Sturhan, Reference Sturhan2012
P. macrodens (Allen, Reference Allen1955) Sturhan, Reference Sturhan2012
P. neohexagrammus (Ivanova, Reference Ivanova1978) Sturhan, Reference Sturhan2012
Paramerlinius hexagrammus (Sturhan, Reference Sturhan 1966 ) Siddiqi, Reference Siddiqi 1979
The Iranian population of P. hexagrammus from the rhizosphere of Sloe (Prunus divaricata) in Abr forest of Semnan province can be characterised by body almost straight to slightly curved, cylindrical. Cephalic region continuous from body to slightly offset by constriction, with 5–6 annuli, tail subcylindrical, with smooth terminus, and 46 (40–54) annuli. Males similar to female except for sexual characters, bursa encircling the entire tail, spicules and gubernaculum measuring 32–37 μm and 9.0–10.0 μm, respectively. The morphological and morphometric characters of the Iranian population are consistent with the other populations from Iran (Ghaderi & Karegar Reference Ghaderi and Karegar2014).
Genus Scutylenchus Jairajpuri, Reference Jairajpuri 1971
Diagnosis
Body medium-sized, about 1 mm. Body cuticle marked by longitudinal striae or grooves. Lateral field with six incisures in adults and four in all juvenile stages. Deirid absent. Lip region continuous or slightly offset, with six radial grooves (six lobs) and conspicuous, oral disc rounded and offset from first annuli. Vulva usually in a cavity or depression, with epiptygma. Female tail conoid to subcylindrical with bluntly to finely rounded tip, terminal cuticle normally thickened and with refractive inner cuticle layer around tail end. Phasmid conspicuous. Spicules robust with notched tip. Gubernaculum crescent-shape in lateral view.
Type species
Scutylenchus mamillatus (Tobar-Jiménez, Reference Tobar-Jimenéz1966) Jairajpuri, Reference Jairajpuri1971
Other species
S. baluchiensis Maqbool, Ghazala, Fatima & Qasim, Reference Maqbool, Ghazala, Fatima and Qasim1985
S. boghiae (Choi & Geraert, Reference Choi and Geraert1993) Siddiqi, Reference Siddiqi2000
S. brevicaudatus Peng & Hunt, Reference Peng and Hunt1995
S. chengi (Munawar, Miao, Castillo and Zheng, Reference Munawar, Miao, Castillo and Zheng2020) comb. nov.
= Geocenamus chengi Munawar, Miao, Castillo and Zheng, Reference Munawar, Miao, Castillo and Zheng2020
S. conicaudatus (Ghaderi & Karegar, Reference Ghaderi and Karegar2016) comb. nov.
= Geocenamus conicaudatus Ghaderi & Karegar, Reference Ghaderi and Karegar2016
S. dongtingensis Xu, Xie, Zhao, Zhang & Su, Reference Xu, Xie, Zhao, Zhang and Su2012
S. hexincisus (Jairajpuri & Baqri, Reference Jairajpuri and Baqri1968) Siddiqi, Reference Siddiqi1979
S. koreanus (Choi & Geraert, Reference Choi and Geraert1971) Siddiqi, Reference Siddiqi1979
S. laminatus (Wu, Reference Wu1969) Anderson & Ebsary, Reference Anderson and Ebsary1982
S. lenorus (Brown, Reference Brown1956) Siddiqi, Reference Siddiqi1979
S. longus (Wu, Reference Wu1969) Skwiercz, Reference Skwiercz1984
S. myungsugae (Choi & Geraert, Reference Choi and Geraert1993) Siddiqi, Reference Siddiqi2000
S. ordinarius (Volkova, Reference Volkova1993) comb. nov.
= Geocenamus ordinarius Volkova, Reference Volkova1993
S. paniculoides (Vovlas & Esser, Reference Vovlas and Esser1990) Siddiqi, Reference Siddiqi2000
S. patternus (Eroshenko & Volkova, Reference Eroshenko and Volkova1987) Xu, Xie, Zhao, Zhang & Su, Reference Xu, Xie, Zhao, Zhang and Su2012
S. persici (Zhang, Munawar, Castillo, Han & Zheng, Reference Zhang, Munawar, Castillo, Han and Zheng2022) comb. nov.
= Geocenamus persici Zhang, Munawar, Castillo, Han & Zheng, Reference Zhang, Munawar, Castillo, Han and Zheng2022
S. quadrifer (Andrássy, Reference Andrássy1954) Siddiqi, Reference Siddiqi1979
S. quercinus Sheikhzadeh, Mobasseri, Valizadeh & Pedram, Reference Sheikhzadeh, Mobasseri, Valizadeh and Pedram2022
S. rugosus (Siddiqi, Reference Siddiqi1963) Siddiqi, Reference Siddiqi1979
S. siddiqii (Mulk, Reference Mulk1978) Skwiercz, Reference Skwiercz1984
S. seonunensis (Choi & Kim, Reference Choi and Kim2001) comb. nov.
= Geocenamus seonunensis Choi & Kim, Reference Choi and Kim2001
S. sobolevi (Mukhina, Reference Mukhina1970) Siddiqi, Reference Siddiqi1979
S. sphaerocephalus Ivanova, Reference Ivanova1982
S. squamatus (Eroshenko & Volkova, Reference Eroshenko and Volkova1988) Xu, Xie, Zhao, Zhang & Su, Reference Xu, Xie, Zhao, Zhang and Su2012
S. stegus (Thorne & Malek, Reference Thorne and Malek1968) Siddiqi, Reference Siddiqi1979
S. tartuensis (Krall, Reference Krall1959) Siddiqi, Reference Siddiqi1979
S. tessellatus (Goodey, Reference Goodey1952) Siddiqi, Reference Siddiqi1979
S. thomasi Skwiercz, Reference Skwiercz1984
S. tumensis Skwiercz, Reference Skwiercz1984
S. variabilis Ivanova & Shagalina, Reference Ivanova and Shagalina1983
S. vietnamensis (Nguyen, Linh, Nguyen, Liebanas, Nguyen & Trinh, Reference Nguyen, Le, Nguyen, Liebanas, TAD and Trinh2019) comb. nov.
= Geocenamus vietnamensis Nguyen, Linh, Nguyen, Liebanas, Nguyen & Trinh, Reference Nguyen, Le, Nguyen, Liebanas, TAD and Trinh2019
Remark
All six new combinations transferred from the genus Geocenamus to Scutylenchus due to having longitudinal incisures in entire body with lateral field.
Scutylenchus sp.
(Figures 8–11, S4–S6; Tables 6, 7)
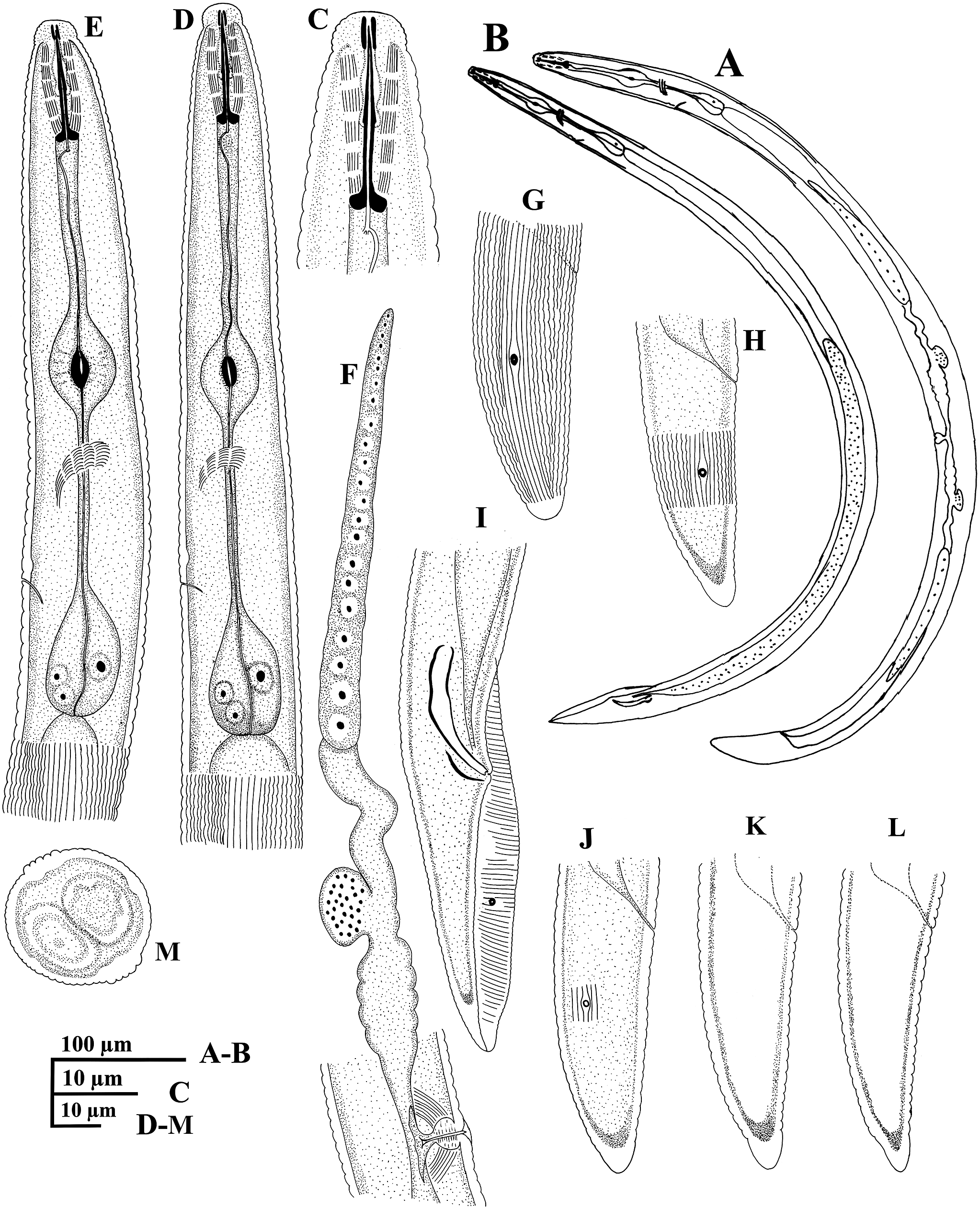
Figure 8. Line drawings of the Iranian (Sendan) population of Scutylenchus sp. A, C, D, F–H, J–M, female and B, E, I, male. A, B, entire body. C, anterior region. D, E, pharyngeal region. F, reproductive system; G–L, tail region. M, cross section at mid-body.

Figure 9. Light micrographs of the Iranian (Sendan) population of Scutylenchus sp. A–J, female. A–C, H, anterior region. D–G, tail region. I, J, cross section at mid-body. Scale bars = 10 μm.

Figure 10. Light micrographs of the Iranian (Sendan) population of Scutylenchus sp. A–C, male. A–C, tail region. Scale bars = 10 μm.

Figure 11. SEM micrographs of the Iranian population of Scutylenchus sp. A–M, female and N, O, male. A, anterior region. B, C, lateral view of cephalic region (white arrows indicating the amphidial apertures). D, en face view of cephalic region (white arrows indicating the amphidial apertures). E, excretory pore in ventral view (white arrow indicating the excretory pore). F, lateral field at pharyngeal region. G, lateral field at mid-body. H, I, vulval region. J, anus. K–O, tail region (white arrows indicating the anus and black arrows indicating the phasmid).

Figure 12. Line drawings of the Iranian population of Telomerlinius teleosus. A, C, E, G, I–M, O, female and B, D, F, H, N, male. A, B, entire body. C, pharyngeal region. D, E, anterior region. F, spicules and gubernaculum. G, reproductive system. H–J, basal bulb of pharynx. K–N, tail region. O, vulval region.

Figure 13. Light micrographs of the Iranian population of Telomerlinius teleosus. B, C, E, F–J, female and A, D, K, male. A, B, entire body. C, pharyngeal region. D, E, anterior region. F, basal bulb of pharynx. G, vulval region. H, I, K, tail region. J, lateral field at mid-body. Scale bars: A–B = 100 μm; C–K = 10 μm.

Figure 14. SEM micrographs of the Iranian population of Telomerlinius teleosus. A–L, female and M–O, male. A, anterior region (black arrow indicating the excretory pore). B–D, lateral view of cephalic region. E, F, en face view of cephalic region. G, lateral field at mid-body. H, excretory pore in ventral view (black arrow indicating the excretory pore). I, vulval region. J, anus (black arrow indicating the anus). K–N, tail region (black arrows indicating the phasmid). O, cloacal region.
Table 6. Morphometrics of Scutylenchus sp. from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Table 7. Morphometrics of Damavand mountain population of Scutylenchus sp. from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Description of female
Body ventrally arcuate to C-shaped after heat fixation. Cuticular annuli distinct, 2.2 (1.6–2.7) μm across at mid-body. Cuticle with 35–50 incisures (35–42 in Damavand population, 40–42 in Semnan population, 36–40 in Zanjan population, 40–46 in Sabalan population, and 40–50 in Sendan population) excluding lateral fields (200 cross sections from 70 specimens: 35 incisures = 11.5%; 36 incisures = 20%; 37 incisures = 9.5%; 38 incisures = 4.5%; 39 incisures = 1.5%; 40 incisures = 3%; 41 incisures = 1%; 42 incisures = 14%; 44 incisures = 5%; 45 incisures = 3.5%; 47 incisures = 7.5%; 48 incisures = 18.5%; 49 incisures = 5.5%; 50 incisures = 6.5%). Lateral field prominent, with six longitudinal incisures, 7.8 (6.5–9.6) μm wide or 30 (20–35) % of the corresponding body diameter. Lip region slightly offset by a constriction, flattened at front end, bearing 5–7 annuli, 8.3 (7.4–9.4) μm wide and 4.2 (3.8–5.1) μm high; in SEM images, with six radial grooves and a conspicuous offset, rounded oral disc. Amphidial apertures small and slightly ovate, located at lateral view of oral disc in SEM images. Cephalic framework not refractive. Stylet robust, conus 10.0 (8.5–12.5) μm or 51 (49–53) % of total stylet length, basal knobs round and slightly posteriorly directed, 4.3 (3.9–4.7) μm wide. DGO 2.1 (1.5–2.6) μm behind stylet knobs. Pharyngeal median bulb oval with prominent valve, 12.2 (10.0–13.5) μm × 17.4 (16–20) μm, occupying 59 (57–69) % of body wide at that level. Basal bulb pyriform 13.5 (11.5–15.0) μm × 25 (23–27) μm. Nerve ring at 91 (80–104) μm from anterior end. Hemizonid 1 to 2 annuli anterior to excretory pore and located at 112 (90–131) from anterior end. Deirids absent. Reproductive system amphidelphic-didelphic, vulva with small lateral flaps and epiptygma. Vagina perpendicular, 11.0 (8.0–12.0) μm occupying 37 (31–41) % of the vulval body diameter; spermatheca round and filled with globular sperm cells. Post anal intestinal sac absent. Tail elongate conical, dorsally convex, with 24 (19–28) annuli in ventral side, ending to a smooth terminus. Phasmids located at 38 (33–44) % of tail length.
Description of male
Generally similar to female except for sexual characters. Body straight to ventrally curved. Body annuli 2.1 (1.7–2.7) μm at mid-body. Lateral field 7.5 (6.5–8.5) μm wide. Cephalic region offset, 7.6 (7.2–8.3) μm wide and 4.3 (3.8–4.6) μm high. Conus 50 (49–51) % of total stylet length. DGO 2.2 (1.7–2.6) μm behind stylet knobs. Nerve ring at 90 (83–101) μm from anterior end. Hemizonid 1–2 annuli anterior to excretory pore and located at 105 (90–115) μm from anterior end. Bursa surrounded tail tip, 74 (65–87) μm in length. Spicules tylenchoid, notched at tip. Gubernaculum simple, crescent-shaped.
Host and locality
Recovered from the rhizosphere of Astragalus sp. in five localities at Alborz mountains: Sendan mountain, Zanjan province (GPS coordinates 36°23’45” N, 49°08’09”E); Sabalan mountain, Ardabil province (GPS coordinates 38°29’57” N, 47°70’03”E), Damavand mountain, Mazandaran province (GPS coordinates 35°59’20” N, 52°07’16”E) in October 2019, and Semnan population (GPS coordinates 36°02’40” N, 53°28’16”E) and Zanjan population (GPS coordinates 36°41’42” N, 48°44’13”E).
Diagnosis and relationships
Scutylenchus sp. characterised by having 35–50 longitudinal incisures at mid-body (except lateral fields); cephalic region slightly offset by a constriction with 5-7 annuli; cephalic framework not refractive; stylet 18.3–27.3 μm; tail 32–63 μm and 19–28 annuli in ventral side with smooth tail tip and spicules 24.5–31 μm.
Scutylenchus sp. in regard to morphological and morphometric characters comes close to S. siddiqii (Mulk, Reference Mulk1978) Skwiercz, Reference Skwiercz1984, S. laminatus, S. tessellatus (Goodey, Reference Goodey1952) Siddiqi, Reference Siddiqi1979, and S. mamillatus (Tobar-Jiménez, Reference Tobar-Jimenéz1966) Jairajpuri, Reference Jairajpuri1971. Our population differs from S. siddiqii by a shorter body (598–838 vs. 820–1220 μm), shorter spicules (24.5–31.0 vs. 30–36 μm), and tail characteristics (dorsally convex, smooth at tip vs. regularly tapering, annulated at tip). From S. laminatus, it differs by a shorter body (598–838 vs. 800–1200 μm), the number of longitudinal striae excluding lateral field (35–50 vs. 56–58), and tail characteristics (smooth at tip, bearing 16–33 annuli and 35–63 μm long vs. annulated at tip, bearing about 50 annuli and 62–88 μm long). The Iranian population of Scutylenchus sp. can be distinguished from mamillatus by a shorter body (598–833 vs. 890–990 μm), lower number of lip region annuli (5–6 vs. 6–7), tail characteristics (elongate conical, with rounded tip vs. subcylindrical with digitate tip), and longer spicules (24.5–31.0 vs. 21–24 μm). Finally, it differs from S. tessellatus by lip region (slightly offset by a constriction vs. offset by a deep groove), slightly stylet length (18.3–27.0 vs. 16.5–20.5 μm), tail shape (dorsally convex in posterior half vs. striaght), and tail tip (smooth vs. annulated). Our population of Scutylenchus sp. differs from S. quercinus by number of longitudinal striae without lateral fields (35–50 vs. 22–25), number of lateral fields (six vs. four), number of tail annuli (19–28 vs. 14–18), and tail shape (elongate conical, dorsally convex vs. subcylindrical).
Scutylenchus rugosus (Siddiqi, Reference Siddiqi 1963 ) Brzeski, Reference Brzeski 1991
The Iranian population of S. rugosus from the rhizosphere of Astragalus sp. in Zanjan province can be characterised by straight to slightly ventrally curved body, having 29–34 longitudinal striae excluding lateral field, lip region slightly offset by a constriction, with 5–6 annuli, and tail with 29 (23–35) annuli, with annulated rarely smooth terminus. This population corresponds well with the original description (Siddiqi Reference Siddiqi1963) and other populations from Iran (Hasanzadeh et al. Reference Hasanzadeh, Karegar and Kheiri2005; Ghaderi & Karegar Reference Ghaderi and Karegar2016) except for tail terminus striation (usually annulated vs. usually smooth).
Genus Telomerlinius Siddiqi & Sturhan, Reference Siddiqi and Sturhan 2014
Diagnosis
Body about 1 mm or less, straight or slightly ventrally curved after relaxed. Cuticle distinctly annulated, without longitudinal striae excluding lateral field. Lateral field with four incisures forming three longitudinal bands. Cephalic region distinctly offset from the body, perioral disc present and prominent, offset from cephalic region annuli, with six radial conspicuous grooves, framework slightly sclerotised. Amphidial apertures ovate, located in first annulus. Stylet delicate slender, conus being longer than shaft; basal knobs small rounded, posteriorly directed. Deirid absent. Median bulb muscular with distinct valve. Basal bulb large, sac-like, usually slightly overlapping intestine in dorsal side. Female genital system didelphic-amphidelphic. Vulva at mid-body, with transverse slit, equipped with prominent epiptygma. Spermatheca axial, rounded or oval, with small rounded sperm. Post anal sac lacking. Female tail conoid with smooth rounded terminus and without refractive inner cuticle layer around its end. Male similar to female. Spicules thick, slightly curved ventrally, with notched tip. Gubernaculum small, saucer-shaped. Cloaca opening with distinct pair of hypoptygma on the posterior lip.
Type species
Telomerlinius mellumensis Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014
Other species
Telomerlinius teleosus Siddiqi & Sturhan, Reference Siddiqi and Sturhan2014
Telomerlinius teleosus Siddiqi & Sturhan, Reference Siddiqi and Sturhan 2014
(Figures 12–14, Table 8)
Table 8. Morphometrics of Telomerlinius teleosus from Iran. All measurements are in μm and in the form: mean ± s.d. (range)

Description of female
Body elongate, straight to slightly ventrally curved when killed by heat. Cuticle annuli fine, 2.3 (2.2–2.4) μm at pharyngeal region and 1.6 (1.5–1.8) μm at mid-body. Lateral field with four distinct incisures, 8.4 (8.0–9.4) μm wide occupying 41 (39–45) % of body diameter. Cephalic region rounded, offset from body by a sharp constriction, with usually 5–6 annuli, showing six longitudinal grooves and perioral disc. Cephalic framework lightly sclerotised. Stylet delicate and slender, 3.0 (2.9–3.2) times head wide long, conus needle-like, 13.8 (13.8–14.0) μm or 62.2 (60.9–65.2) % of total stylet length. Basal knobs rounded, posteriorly sloping 3.0 (2.5–3.3) μm across. DGO 1.2 (1.0–1.5) μm behind stylet knobs. Median bulb well developed and oval, 18.8 (18.0–19.0) μm long and 11.7 (11.0–12.0) μm wide that occupied 61 (55–63) % of body wide at adjacent level, with distinct valvular aperture. Basal bulb elongate-cylindrical, with 9.5 (5.0–13.0) μm overlapping intestine. Cardia distinct and rounded. Nerve ring located at 86 (78–90) μm from anterior end. Hemizonid 1–2 annuli anterior to excretory pore and located at 98 (95–103) μm from anterior end. Deirid absent. Reproductive system didelphic. Vulva a transverse slit with anterior flap. Vagina not sclerotised, perpendicular to body axis. Spermatheca spherical, not offset, 8–11 μm long and filled with globular sperm. Rectum slightly curved. Anus distinct. Tail elongate-conoid with smooth rounded terminus, with 23 (21–25) large annuli at ventral side. Phasmid not so large, just anterior to middle of tail, 48 (42–51) % of tail.
Description of male
Similar to female in general morphology. Body annuli finer than female, 1.4 (1.3–1.6) μm at mid-body. Lateral field with four incisures that occupied 40 (37–46) % of body wide in diameter. Lip region with 7.2 (6.6–7.8) μm wide and 4.7 (4.0–5.3) μm high. Basal bulb 7 (5.0–10) μm overlapping intestine. Testis single, outstretched, occupied about half or 49 (43–55) % of whole body length. Spicules tylenchid-shape with notch at tip. Gubernaculum very curved and saucer-shape. Hypoptygma present and slightly prominent. Bursa enveloped tail, 100 (90–110) μm long.
Voucher specimens
Five females and six males were deposited in the collection of the Department of Plant Protection, College of Agriculture, University of Zanjan, Zanjan, Iran.
Host and locality
Recovered from the rhizosphere of Khejri tree (Prosopis cineraria (L.) Druce), collected in Dezful region of Khuzestan province, southwest of Iran, in September 2019. GPS coordinates: 32°06’50” N, 48°27’16” E.
Diagnosis and relationships
Telomerlinius teleosus differs from the other species of the genus, T. mellumensis Siddiqi and Sturhan, Reference Siddiqi and Sturhan2014, by a shorter stylet (21–23 vs. 25–30 μm), shorter spicules (18.5–21.0 vs. 22–25.5 μm) and cuticular annuli width (1.2–1.8 vs. 2.4–3.4 μm). Our population is in an intermediate position but more comes close to T. teleosus; however, it differs from the original description by a wider lateral field (41 (39–45) vs. about 26% of the corresponding body diameter), longer body (803 (743–856) vs. 530–740 μm), longer tail (63 (57–69) vs. 45–55 μm), slightly longer spicules (22 (20–24) vs. 18.5–21.0 μm), and longer gubernaculum (8.5 (7.5–9.5) vs. 6.0–7.5 μm).
3D modelling of lip region in the genera of Merliniinae
3D modelling in the genera of Merliniidae (except Macrotylenchus) showed four types of lip regions (Figure 15). However, the SEM images of the genus Macrotylenchus not available.

Figure 15. 3D modelling of the genera in Merliniinae. Type 1: Cephalic radial grooves absent; the lip region is not divided into sectors, and all lips are completely fused and merged with perioral disc, lip region continuous from body. Type 2: Lip region with two separated lateral lips, reduced in size; two sectors of each subdorsal and subventral lips are fused. Perioral disc not distinct and merged with the first annulus of lip region, lip region with slightly offset from body. Type 3: Lip region distinctly separated into six sectors by radial grooves; lateral lips are slightly reduced in size. Perioral disc distinct and separated but not elevated from the lip region, lip region offset from body. Type 4: Similar to Type 3, but perioral disc is distinctly elevated from the lip region, lip region distinctly offset from body.
Amplimerlinius
Labial radial grooves absent; the lip region is not divided into sectors, and all lips are completely fused and merged with perioral disc (Type-1).
Nagelus
Similar to Amplimerlinius, but lip region is broadly oval and compressed dorso-ventrally (vs. spherical in Amplimerlinius) (Type-1).
Merlinius
Lip region with two separated lateral lips, reduced in size; two sectors of each subdorsal and subventral lips are fused. Perioral disc not distinct and merged with the first annulus of lip region (Type-2).
Scutylenchus and Geocenamus
Lip region distinctly separated into six sectors by radial grooves; lateral lips are slightly reduced in size. Perioral disc distinct and separated but not elevated from the lip region (Type-3).
Paramerlinius
More similar to Scutylenchus and Geocenamus than Amplimerlinius, as having six obscure sectors divided by radial grooves; perioral disc is hexagonal (Type-3).
Telomerlinius
Similar to Scutylenchus and Geocenamus, but perioral disc is distinctly elevated from the lip region (Type-4).
Molecular characterisation and phylogenetic relationships between representatives of family Merliniidae
The sequences obtained for the described species in this paper are listed in Table 2. The majority are from D2–D3 region of 28S rRNA and partial 18S rRNA; in some cases, the ITS region was also provided.
The D2–D3 domains of the 28S rRNA gene alignment after manual edition (754 bp long) included 109 sequences from the family Merliniidae and two outgroup species [Psilenchus sp. (DQ328716) and Psilenchus hilarulus (EU915489)]. Forty new sequences with their morphological characterisation were included in this analysis. The Bayesian 50% majority rule consensus tree inferred from the D2–D3 alignment is given in Figure 16. Smaller clades are coincident with other recent studies with related phylogenetic families (Alvani et al. Reference Alvani, Mahdikhani, Rouhani and Mohammadi2017; Munawar et al. Reference Munawar, Yevtushenko and Castillo2021). This tree showed that many species from GenBank are difficult to distinguish using only this marker as a separated clade in the phylogenetic tree (i.e., Pratylenchoides crenicauda-P. erzurumensis-P. variabilis, Scutylenchus tartuensis-S. rugosus, and others). However, it seems fine for separating the majority of the genera in this family, but with low clade support in some genera clades (i.e., Amplimerlinius) or, in some cases, consisting of several clades distributed in the phylogenetic tree, but not clearly supported and phylogenically relationship not well resolved between them and other genera (i.e., Scutylenchus).
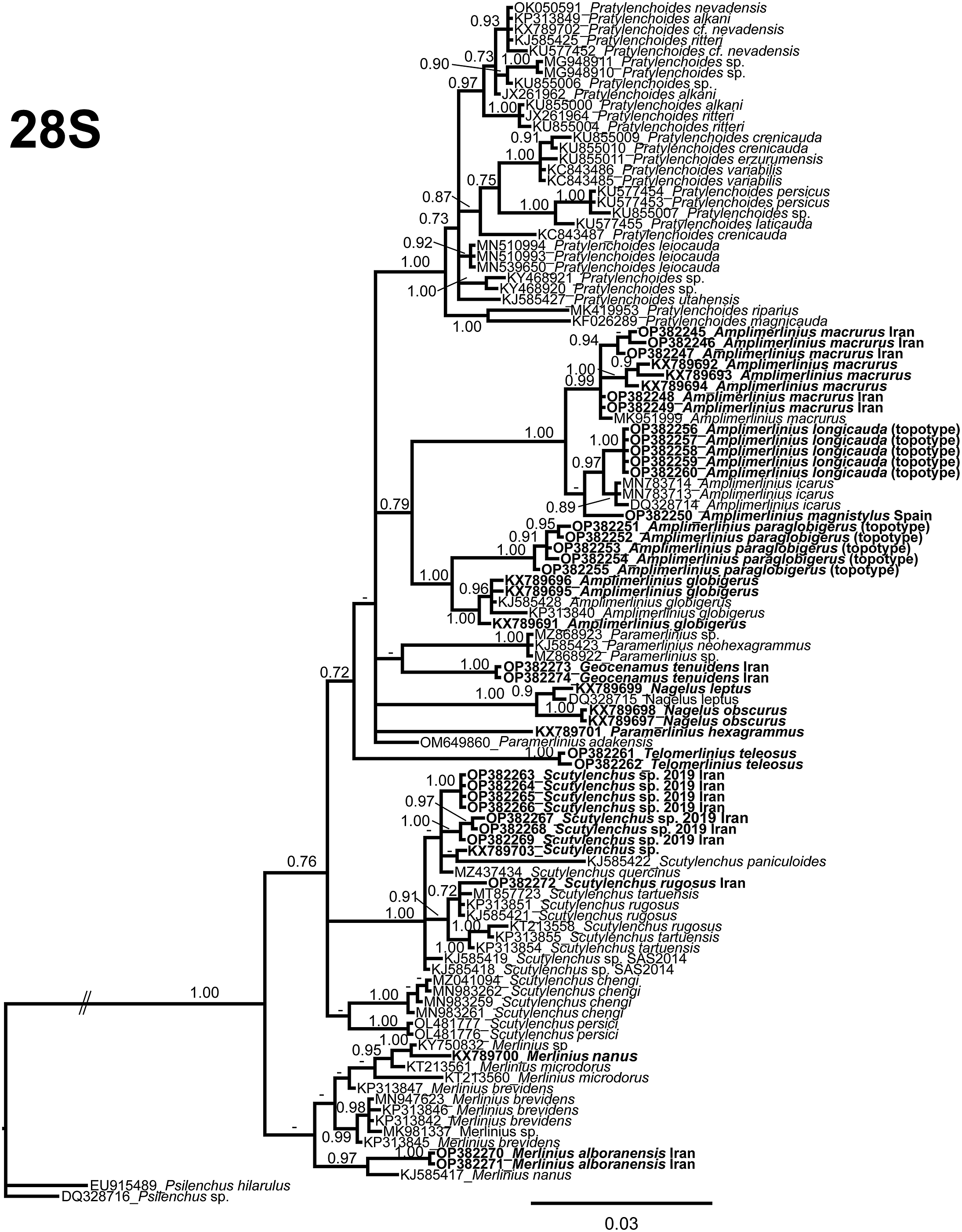
Figure 16. Phylogenetic relationships among Merliniidae species. Bayesian 50% majority rule consensus tree as inferred from D2–D3 expansion segments of 28S rRNA sequence alignment under a transitional model of invariable sites and a gamma-shaped distribution (TIM3 + I + G). Posterior probabilities greater than 0.70 are given for appropriate clades. Newly obtained sequences in this study are shown in bold. (Scale bar = expected changes per site).
The partial 18S rRNA alignment after manual edition (1693 bp long) included 59 sequences from the family Merliniidae and two outgroup species [Psilenchus hilarulus de Man, Reference De Man1921 (KX789728) and Psilenchus vinciguerrae Brzeski, Reference Brzeski1991 (KX789733)]. Twenty-six new sequences with their morphological characterisations were included in this analysis. The Bayesian 50% majority rule consensus tree inferred from the 18S rRNA sequence alignment is given in Figure 17. The tree contained two highly supported major clades (one with 0.96 PP support and the another with 1.00 PP support). These clades were partially coincident with another study (Carta et al. Reference Carta, Skantar and Handoo2010), but in our case with a higher number of sequences and additional genera. Similarly, to the D2–D3 domains of the 28S rRNA the use of this marker for species separation is complicated due to the low variability associated with this marker. At the genus level, only some genera are clearly defined in the phylogenetic tree (i.e., Pratylenchoides and Nagelus).

Figure 17. Phylogenetic relationships among Merliniidae species. Bayesian 50% majority rule consensus tree as inferred from partial 18S rRNA gene sequence alignment under a transitional model of invariable sites and a gamma-shaped distribution (TIM3 + I + G). Posterior probabilities greater than 0.70 are given for appropriate clades. Newly obtained sequences in this study are shown in bold. (Scale bar = expected changes per site).
The ITS rRNA gene alignment (778 bp long) included 41 sequences from the family Merliniidae and two outgroup species [Coslenchus rhombus Andrássy, Reference Andrássy1982 (MK874506) and Filenchus sp. (MH842880)]. Eighteen new sequences with their morphological characterisations were included in this analysis. The Bayesian 50% majority rule consensus tree inferred from the ITS alignment is given in Figure 18. The tree contained two low-supported major clades. In this case, this marker has an important amount of differences between species, clustering the majority of them in individual clades with the exception of A. paraglobigeru and P. leiocauda-P. ritteri. Genera are well separated with the exception of Geocenamus and Scutylenchus with different clades distributed in the phylogenetic tree. However, the amount of available sequences is minimal in comparison to the other ribosomal markers used in this study.
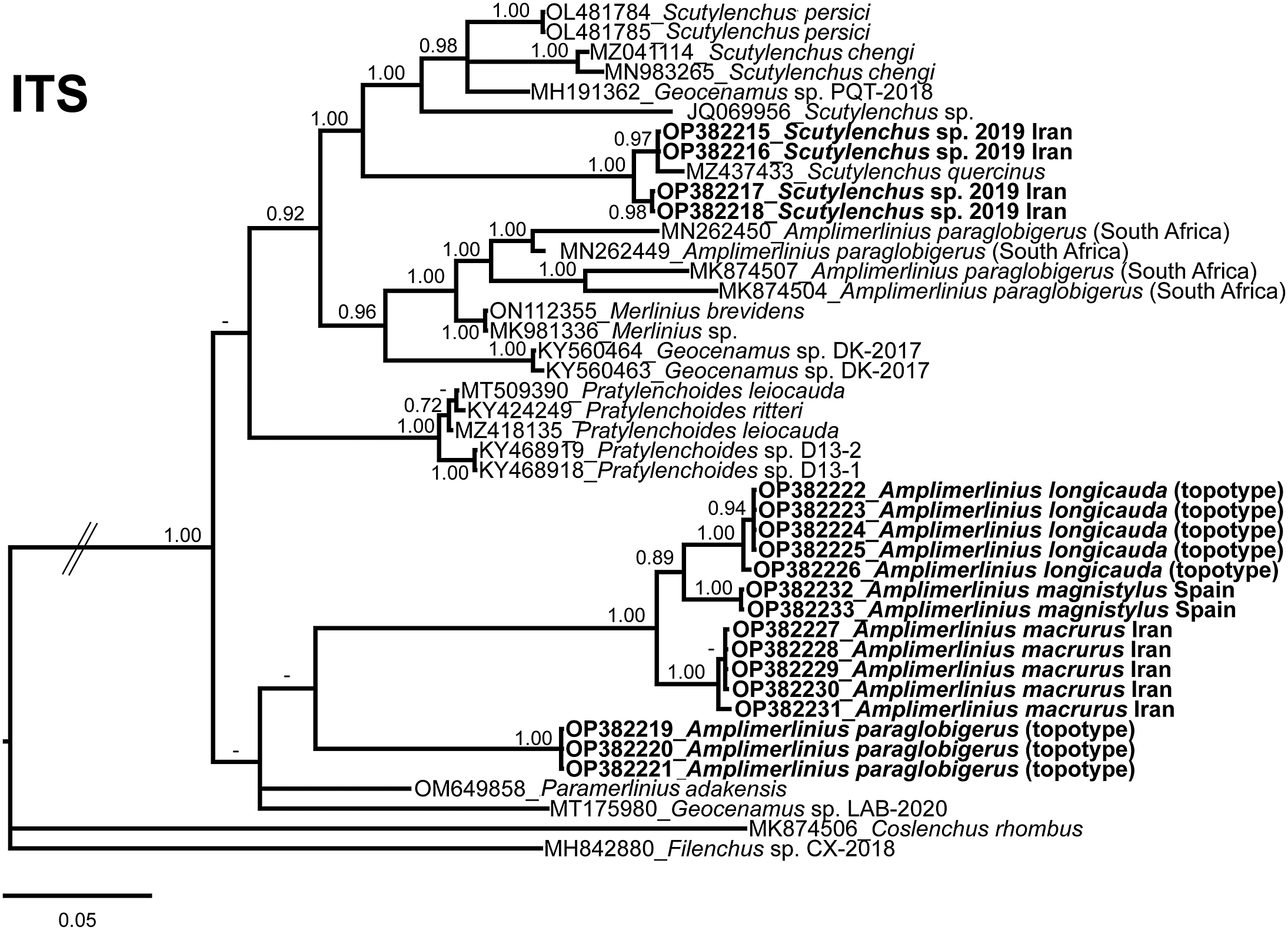
Figure 18. Phylogenetic relationships among Merliniidae species. Bayesian 50% majority rule consensus tree as inferred from ITS rRNA sequence alignment under transitional and a gamma-shaped distribution (TIM2 + G) model. Posterior probabilities greater than 0.70 are given for appropriate clades. Newly obtained sequences in this study are shown in bold. (Scale bar = expected changes per site).
Scutylenchus sp. is clustered in a low-supported clade in the D2–D3 domains of the 28S rRNA phylogeny with S. quercinus (MZ437434), an unidentified Scutylenchus species (KX789703) that could be considered as our Scutylenchus sp. by the authors, and S. paniculoides (KJ585422). For partial 18S rRNA, this species occupies a clade with other Scutylenchus spp. (including S. quercinus, MZ437435 and S. quadrifer, AY284599, AY993977) and other genera as Geocenamus spp. and Merlinius spp. Some of these sequences formerly described as an unidentified Scutylenchus species (KX789706 and KX789707) could be considered as Scutylenchus sp. Scutylenchus sp. (OP382215-OP382218) in the ITS region is grouped in a highly supported clade with S. quercinus (MZ437433). The three populations of Scutylenchus sp. sequenced in this study show 0, 5–6 nucleotides differences (99.1–99.3% similarity) and 13 nucleotides differences (3 were indels and 98.2% similarity) for the partial 18S rRNA, D2–D3 expansion segments of the 28S rRNA and ITS region, respectively. Intra-population variability (5 populations) was only 1 nucleotide difference for the Alborz mountains population in the D2–D3 expansion segments of the 28S rRNA, and no intra-population was found for the ITS region. Our studied species (Scutylenchus sp.) was identical for partial 18S rRNA with S. quercinus (MZ437435, 974 bp long) and was 99.8% similar to several species including an unidentified Scutylenchus species in the GenBank found in Iran (KX89706 and KX89707) and in this study assumed to be Scutylenchus sp. by the authors, S. rugosus (KX89704 and KX89705), Merlinius brevidens (KX89708), and M. nanus (KX89709) for partial 18S rRNA. Also, high similarities were found for the D2–D3 region marker. In this case, the marker for this species was 99.4% similar (4 nucleotides in difference) to S. quercinus (MZ437434) and by an unidentified Scutylenchus species in the GenBank found in Iran (KX89703) and in this study assumed to be Scutylenchus sp. by the authors, followed by S. tartuensis with 98.6–98.6% similarity (KP313853 and MT857723), and another unidentified Scutylenchus species with 98.9% similarity (KJ585419). The ITS region showed the closest similarity to S. quercinus with similarity of 96.8% (MZ437433, shorter than our sequence, with 414 bp, 12 nucleotide difference including 3 indels), followed by Merlinius sp. with 87.8% similarity (MK981336) and Geocenamus sp. PQT-2018 with 86.7% similarity (MH191362). The closest Scutylenchus species is an undescribed species with 85.1% similarity (JQ069956).
Amplimerlinius globigerus and A. paraglobigerus (topotype specimens) could be separated molecularly using D2–D3 domains of the 28S rRNA (KX789691, KX789695, KX789696, KJ585428, KP313840, and OP382251–OP38512255, respectively) with 97.4% (18 nucleotide differences) and the ITS region (MN262449, MN262450, MK874507, MK874504, annotated in GenBank as A. paraglobigerus, and OP382219–OP382221, respectively) with 82.8% (125 nucleotide differences) similarity. Geocenamus tenuidens showed a no-resolved phylogenetic relationship with Merlinius and other related genera for the partial 18S rRNA. For the D2–D3 domains of the 28S rRNA, Geocenamus tenuidens is related to Paramerlinius neohexagrammus (KJ585423) in a low-supported clade. Sequences of M. alboranensis (OP382270 and OP382271, D2–D3 region), M. brevidens (KX789708, partial 18S rRNA), and M. nanus (KX789700 and KX789709, 28S rRNA and partial 18S rRNA, respectively) grouped with other species of Geocenamus in the partial 18S rRNA, and they formed a low-supported clade with other species of Merlinius in the case of D2–D3 domains of the 28S rRNA. In the case of D2–D3 domains of the 28S rRNA, M. nanus is closely related phylogenetically with M. alboranensis (KJ585417) in a well-supported clade (PP = 0.97) and is not clearly related with other sequences of M. nanus (KX789700). The sequences provided in this study for the genus Nagelus (N. leptus [KX789699 and KX789718 for 18S rRNA and 28S rRNA, respectively] and N. obscurus [KX789697-KX789698 and KX789716- KX789717 for 18S rRNA and 28S rRNA, respectively]) clustered together in a unique clade for partial 18S rRNA and D2–D3 domains of the 28S rRNA in high-supported clades in both molecular markers (PP = 1.00).
In this study, we provided for the first time molecular data for the genus Telomerlinius with only one species (T. teleosus). This genus is located phylogenetically in the same clade with the genera Scutylenchus and Geocenamus but is clearly separated molecularly from them for the partial 18S rRNA in a moderatelly-supported clade (PP = 0.98). For the D2–D3 domains of the 28S rRNA phylogeny, the position of this genus is clearly separated from the other genera of the family Merliniidae, but the relationship with other genera is not clearly defined inside a major clade with a low support (PP = 0.72).
Discussion
In the present work, we provided an outline on the taxonomic position of the genera and species in the subfamily Merliniinae. We included representatives from all known genera in Merliniinae (except Macrotylenchus) and provided morphological and molecular data for recovered mambers. Genera in the subfamily as well as species in each genus could be distinguished based on previously published works (Geraert Reference Geraert2011; Sturhan Reference Sturhan2012; Ghaderi & Karegar Reference Ghaderi and Karegar2014; 2016; Ghaderi et al. Reference Ghaderi, Karegar, Miraeiz and Hashemi2017).
In this study, integrative taxonomy of fourteen known species including Amplimerlinius globigerus, A. longicauda, A. macrurus, A. magnistylus, A. paraglobigerus, Geocenamus tenuidens, Merlinius alboranensis, M. brevidens, M. nanus, Nagelus leptus, N. obscurus, Paramerlinius hexagrammus, Scutylenchus rugosus, Telomerlinius teleosus, and one species described herein as Scutylenchus sp. are performed. However, only two of these species described were collected from the type localities (A. longicauda and A. paraglobigerus), and future molecular analyses of type material of the studied species might clarify the species identification or prevent possible cases of cryptic speciation in this complicated family of nematodes. Partial 18S rRNA does not have enough resolution for species separation as shown with the mixture of species in different clades, and in some cases, even genera (i.e., Merlinius, Scutylenchus vs. Geocenamus) (Figure 17), but only a few genera could be separated phylogenetically using this molecular marker (Paramerlinius, Pratylenchoides, Nagelus, and Telomerlinius). However, the D2–D3 domains of the 28S rRNA have a better resolution for all genera studied (Amplimerlinius, Pratylenchoides, Merlinius, Nagelus, Scutylenchus, and Telomerlinius), but Geocenamus and Paramerlinius occupied polyphyletic positions in the majority of the cases with low-medium support. This confirms the hypothesis about the validity of the genera even with the low differences found in both of these markers. In this sense, Ghaderi et al. (Reference Ghaderi, Karegar and Niknam2014) supported the grouping of Pratylenchoides and Merliniinae into a single family – Merliniidae as proposed by Sturhan (Reference Sturhan2012) – but these authors did not accept the monophyly of the genus Amplimerlinius using the Shimodaira–Hasegawa (SH) test using D2–D3 domains of the 28S rRNA. The ITS region has a good differentiation among genera, but our analysis is very restricted and needs additional effort to get more species sequenced. However, this marker could have a potential problem with additional taxa. The sequences could have less similarity, increasing the alignment problems that occurred with other groups of nematodes (i.e., Longidoridae) being the only accepted phylogenies for closely related species (Palomares-Rius et al. Reference Palomares-Rius, Cantalapiedra-Navarrete, Archidona-Yuste, Subbotin and Castillo2017). Munawar et al. (Reference Munawar, Yevtushenko and Castillo2021) explored this marker within selected genera and a restricted selection of sequences from the subfamilies Telotylenchinae and Merliniinae.
Nematode identification using molecular markers for this family with the sequencing data deposited in GenBank could be difficult for partial 18S rRNA and D2–D3 domains of the 28S rRNA, as many of the species are mixed between the different genera clades and interspecies differentiation is low (see branches length in trees). Additional markers as COI or ITS regions are needed in the future for a clear molecular separation between closely related species. Additionally, some of the species deposited in the GenBank are not published, or they need an integrative taxonomical study including morphological-morphometrical data with molecular markers.
Depending on the absence or presence of deirids, the subfamily Merliniinae is divided into two groups: the genera Amplimerlinius, Nagelus, Merlinius, and Paramerlinius with deirid and Geocenamus, Telomerlinius, and Scutylenchus without deirid. In our phylogenetic trees, the genera with deirid are not clearly separated from those without deirid. According to the lip region, we can divide the family Merliniidae into three groups; group 1: lip region with offset perioral disc (may be elevated or not elevated) and head annuli with six sectors (four in Merlinius), including genera Geocenamus, Scutylenchus, Merlinius, and Telomerlinius; group 2: perioral disc simple and fused with the first annulus of lip region without radial longitudinal grooves, including genera Amplimerlinius, Nagelus, and Paramerlinius; and group 3: lip region fused with the first annulus and without radial longitudinal grooves but the first annulus with six sectors, including Pratylenchoides. Our partial D2–D3 of 28S rRNA and 18S rRNA partially supported this grouping. The 18S rRNA tree supported Geraert’s (Reference Geraert2011) decision on the synonymisation of Scutylenchus and Merlinius with Geocenamus, but this marker can not separate the valid species. Our 18S rRNA phylogeny tree supported the validity of the genus Paramerlinius proposed by Sturhan (Reference Sturhan2012) and separated it from Nagelus spp.
In three markers used in our phylogeny, the genus Amplimerlinius formed two separated clades, one clade including A. globigerus and A. paraglobigerus with an average of less than 25 μm for stylet length and the latter including A. macrurus, A. icarus, A. longicauda, and A. magnistylus with an average of more than 25 μm for stylet length. Phylogenetic position in relation to stylet length was also confirmed for Paramerlinius hexagrammus and P. neohexagrammus in the 28S rRNA tree. In the genus Pratylenchoides, the Iranian population of P. riparius (Andrássy, Reference Andrássy1985) Luc, Reference Luc1986 (Hosseinvand et al. Reference Hosseinvand, Eskandari and Ghaderi2019) and P. magnicauda (Thorne, Reference Thorne1935) Baldwin, Luc & Bell, Reference Baldwin, Luc and Bell1983, with an average of stylet 25 μm or more, formed a separated clade from other species of Pratylenchoides spp. bearing a stylet less than 25 μm.
The Lorestan population of A. macrurus [OP382245–OP382247 (D2–D3 region), OP382230 and OP382231 (ITS region)] slightly differs from our other populations of A. macrurus [KX789693, KX789694, OP382248 and OP382249, OP382227–OP382229 (D2–D3 region)] by its tail (Figures 2, 3), which is subclavate and smooth with wide annuli at the tip vs. cylindrical with fine annuli at the tip. However, this population is in other populations of A. macrurus phylogenetically and cannot be considered as a separate species.
Brzeski (Reference Brzeski1997) synonymised N. camelliae with N. obscurus; in this paper, we follow his action, but according to the 18S rRNA tree, the Iranian population of N. obscurus (identified as N. camelliae) (KX789716 and KX789717) occupied different phylogenetic positions from two other isolates/populations of N. obscurus deposited in GenBank (KJ636353 and AY593904). More sequences are needed to clarify inter-species variability in this genus.
This study provides for the first time molecular markers for Telomerlinius, and our phylogenetic analysis in both the markers studied (18S and 28S rRNA) supports the genus validity. The genus Telomerlinius with four lateral fields and the absence of deirids is similar to the family Telotylenchidae, but according to spicules shape, it comes close to Merliniinae. Our phylogenetic trees supported placing this genus in the family Merliniidae as proposed by Siddiqi & Sturhan (Reference Siddiqi and Sturhan2014). The lip region of Telomerlinius is similar to Geocenamus and Scutylenchus but with an elevated perioral disc.
The taxonomic position of certain species in Merliniinae is yet unclear and under question. Eight species including G. angelescresti Chitambar & Ferris, Reference Chitambar and Ferris2005; N. conicus (Allen, Reference Allen1955) Siddiqi, Reference Siddiqi1979; N. djungaricus (Razzhivin, Reference Razzhivin1974) Kapoor, Reference Kapoor1983; G. dobroticus Budurova, Baicheva & Milkova, Reference Budurova, Baicheva and Milkova1996; G. kirjanovae (Sagitov, Reference Sagitov1973) Fortuner & Luc, Reference Fortuner and Luc1987; M. salechardicus Nesterov, Reference Nesterov1985; G. superbus (Allen, Reference Allen1955) Fortuner & Luc, Reference Fortuner and Luc1990; and G. tokobaevi (Sultanalieva, Reference Sultanalieva1983) Fortuner & Luc, Reference Fortuner and Luc1987 were listed by Sturhan (Reference Sturhan2012) under species of uncertain generic position. Unfortunately, there is neither morphological nor molecular evidence sufficient to assign them to one of the known genera. However, we believe that deirids in G. angelescresti in the original description (Chitambar & Ferris Reference Chitambar and Ferris2005) are misidentified, as under light or scanning microscopes, a real deirid can be seen as a smaller dot than represented in Fig. 3D and 4J of the original description. Moreover, other morphological characteristics (particularly typical stylet bearing long conus) as well as its cephalic region pattern (similar to type 3 in our study) confirm its position under the genus Geocenamus.
In summary, this study provides for the first time an integrative taxonomy for several species and in some cases for certain genera of the family Merliniidae. The complexity of the family, the possible cryptict nature of some species, and the possible misidentification of species in the sequences deposited in GenBank necessitate the use of an integrative taxonomy. The low diversity in partial 18S rRNA and D2–D3 domains of 28S rRNA genes urges the use of a multilocus approach involving additional markers such as the ITS region and/or COI.
Supplementary material
To view supplementary material for this article, please visit http://doi.org/10.1017/S0022149X23000640.
Acknowledgements
None.
Financial support
This project was supported by the Iranian National Science Foundation (INSF) for financial support (Grant no. 99025922) and by the University of Jaén, Spain, through the Research Support Plan “PAIUJA 2019/2020: EI_RNM02_2019”.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.




























