As Taiwan has the greatest incidence of end-stage renal disease (ESRD) worldwide, it is imperative to assess long-term cumulative survival in patients undergoing haemodialysis, which is the mainstay of ESRD therapy in Taiwan(1). Haemodialysis patients with chronic kidney disease often have insufficient red blood cell production, which leads to anaemia(Reference Van Wyck2–Reference Kleiner, Van Wyck, Kaupke and Kirlin4). Fe must be administered by a parenteral route to restore Fe to levels sufficient to maintain erythropoiesis. Although the improvement of anaemia among haemodialysis patients with recombinant human erythropoietin (rhEPO) therapy has been reported(Reference Silverberg, Blum, Peer, Kaplan and Aiana5–9), few studies have been conducted to investigate the effect of rhEPO use on long-term survival. In addition, evidence has been lacking regarding long-term survival after combined administration of iron sucrose and rhEPO, as suggested in recent studies(Reference Charytan, Levin, Al-Saloum, Hafeez, Gagnon and Van Wyck7, Reference Van Wyck, Cavallo, Spinowitz B, Adhikarla, Gagnon, Charytan and Levin10). Because factors affecting long-term cumulative survival are very heterogeneous, the best way to assess the effectiveness of rhEPO therapy is to use a prospective randomised controlled trial. However, ethical concerns and the logistics of follow-up preclude our doing this. To be efficient, the alternative is to examine a retrospective cohort of existing patients who are undergoing dialysis with or without rhEPO therapy, taking into account other confounding factors. The aim of the present study was to assess the effect of rhEPO, vitamin D3 and/or Fe therapy on long-term survival among ESRD patients receiving haemodialysis.
Methods
Study design and subjects
This was a retrospective cohort study to investigate the effect of supplement therapy on long-term survival, based on historic medical records. We enrolled a historical cohort of 702 ESRD patients receiving haemodialysis who were admitted to Li Shin Hospital, one of the largest regional hospitals renowned for haemodialysis in northern Taiwan, between 1993 and 2002. The primary outcome of interest in the study was all-cause mortality, assessed by following the patients until 30 June 2004. A total of 344 deaths were ascertained. The average follow-up time was 3·84 (sd 3·08) years. The independent variable of interest was the administration of supplement therapy including rhEPO, vitamin D3 and Fe, which were reported to be associated with anaemia in patients receiving dialysis(Reference Van Wyck2–Reference Kleiner, Van Wyck, Kaupke and Kirlin4).
Treatment protocol and supplement therapy
All patients underwent haemodialysis at regular intervals three or occasionally two times weekly after admission to hospital. Haemodialysis was performed with a Fresenius 4008B dialyser, with F7, F8, F70S, F80S and F100S dialysate with bicarbonate, blood flow of 200–350 ml/min and an interval of 3·5–4·5 h between sessions. Patients underwent supplement therapy with rhEPO, vitamin D3 and Fe, according to haematocrit, transferrin saturation, ferritin, intact parathyroid hormone, calcium and phosphate concentrations. Table 1 shows the protocol for supplement therapy with rhEPO, vitamin D3 and Fe. Note that patients may receive combinations of these three agents.
Table 1 Protocol for supplement therapy with rhEPO, vitamin D3 and iron among patients with ESRD receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002

rhEPO, recombinant human erythropoietin; ESRD, end-stage renal disease; Hct, haematocrit; iPTH, intact parathyroid hormone; TSST, transferrin saturation.
*Calcium × phosphorus product.
†Rocaltrol® (calcitriol) is a synthetic vitamin D analogue. All dose forms contain butylated hydroxyanisole and butylated hydroxytoluene as antioxidants. Calcitriol, chemically, is 9,10-seco(5Z,7E)–5,7,10(19)-cholestatriene-1α,3β-25-triol. Atofen® is ferric chloride hexahydrate. Niferex-150® is a polysaccharide–iron complex.
Data collection
Data on demographic features (age, gender and education level) were recorded at the inception of dialysis. Details on treatment regarding haemodialysis (supplement, vascular access and vascular access blood flow) were retrospectively extracted from historical dialysis records. Aetiology of ESRD was retrieved from medical charts. The aetiology of ESRD was classified into four groups: (i) chronic glomerulonephritis (CGN); (ii) hypertension or type 2 diabetes mellitus (DM); (iii) renal failure of unknown aetiology; and (iv) other causes, including autosomal dominant polycystic kidney disease, obstructive uropathy, autoimmune disease, congenital renal diseases, herbal-induced nephropathy, gouty nephropathy and analgesic-induced nephropathy.
Statistical analysis
The Kaplan–Meier method was used to calculate the overall cumulative survival curves and the specific curves to determine whether to use supplement therapy. The proportional hazards regression model was further adapted to estimate adjusted hazard ratios (HR) regarding the effect of supplement therapy on mortality risk after controlling for age, education and aetiology.
Results
Table 2 shows the distribution of demographic features, aetiology of ESRD and the administration of rhEPO, vitamin D3 and Fe. Patients receiving haemodialysis were equally divided according to gender but were predominantly aged ≥45 years and had relatively low levels of education. Regarding aetiology, renal failure of unknown aetiology accounted for 43 % of patients, followed by hypertension or DM, CGN and other causes. Approximately 61 % of patients were treated with rhEPO, 7 % with vitamin D3 and 20 % with Fe. The overall rate of using any of these three agents was 62 %. Administration of rhEPO alone accounted for 38 %. Combined therapy using two or three agents accounted for 23 %, including 3·56 % with rhEPO + vitamin D3, 16·67 % with rhEPO + Fe and 3·13 % with all three.
Table 2 Characteristics of patients with ESRD receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002

ESRD, end-stage renal disease; rhEPO, recombinant human erythropoietin; CGN, chronic glomerulonephritis; DM, type 2 diabetes mellitus.
*Includes congenital renal disease, obstructive uropathy, autoimmune disease, toxin- or drug-induced.
Case-fatality rates are also reported in Table 2. Case-fatality rate by age group showed a U-shaped pattern, with a 50 % fatality rate for those aged ≤20 years, declining to 20 % for those aged 20–44 years, and then increasing to 44 % for 45–64 years, 55 % for 64–75 years and 72 % for ≥75 years. Gender did not have a significant effect on the case-fatality rate. The lower the education level, the higher the case-fatality rate. Patients treated with rhEPO, vitamin D3 or Fe had a lower case-fatality rate than those not treated. Case-fatality rates in those treated with combination therapy were not significantly different from those with rhEPO only.
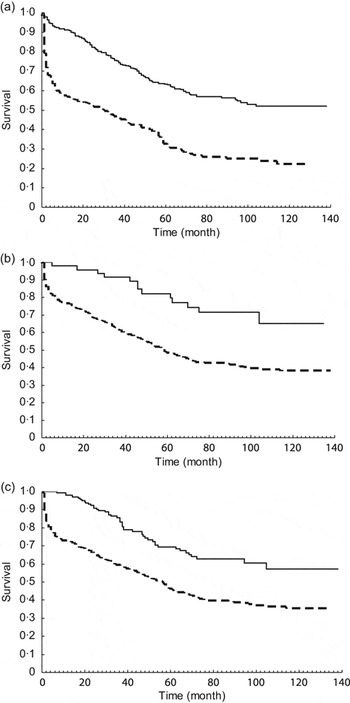
The cumulative survival rate among these patients at 1, 3, 5 and 10 years was 77.78 %, 64.27 %, 51.16 % and 40.34 %, respectively. The survival curves stratified by supplementation of rhEPO, vitamin D3 and Fe are shown in Fig. 1(a) to 1(c). The survival rate among patients with rhEPO supplementation was significantly better than among those without rhEPO (log-rank χ 2 = 87·74, P < 0·0001). Similar findings of better survival were also noted for the patients administered vitamin D3 (log-rank χ 2 = 13·84, P = 0·0002) and Fe (log-rank χ 2 = 27·42, P < 0·0001). When taking combination with Fe or vitamin D3 into account (Fig. 2), those with rhEPO supplementation combined with vitamin D3 or Fe had the best survival rate, followed by those with rhEPO only and then those with no rhEPO (log-rank χ 2 = 94·56, P < 0·0001).

Fig. 1 Kaplan–Meier estimates of 10-year survival according to supplement use: (a) recombinant human erythropoietin (![]() , yes, 160/429;
, yes, 160/429; ![]() , no, 184/273); (b) vitamin D3 (
, no, 184/273); (b) vitamin D3 (![]() , yes, 13/47;
, yes, 13/47; ![]() , no, 331/655) or (c) iron (
, no, 331/655) or (c) iron (![]() , yes, 298/559;
, yes, 298/559; ![]() , no, 46/143), among patients (n 702) with end-stage renal disease receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002
, no, 46/143), among patients (n 702) with end-stage renal disease receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002

Fig. 2 Kaplan–Meier estimates of 10-year survival according to combinations of supplements (![]() , recombinant human erythropoietin (rhEPO) + vitamin D3 or iron, 50/164;
, recombinant human erythropoietin (rhEPO) + vitamin D3 or iron, 50/164; ![]() , rhEPO, 110/265;
, rhEPO, 110/265; ![]() , none, 181/269) among patients (n 702) with end-stage renal disease receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002
, none, 181/269) among patients (n 702) with end-stage renal disease receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002
Table 3 shows the result of univariate analysis on the effect of relevant prognostic factors on survival. It was clear that supplementation of rhEPO, vitamin D3 or Fe reduced the risk of death by ∼62 % compared with no supplementation. Patients aged ≥45 years also had lower survival rates (HR = 3·64, 95 % CI 2·45, 5·40). Those with low education level had a 1·78-fold (95 % CI 1·21, 2·63) greater risk of death compared with those with a high education level. Regarding the aetiology of ESRD, those cases caused by hypertension or DM and those caused by renal failure or other unspecified causes had a 1·67-fold (95 % CI 1·06, 2·62) and 1·72-fold (95 % CI 1·11, 2·66) increased risk of death, respectively, compared with patients with ESRD caused by CGN. Survival rate in patients with rhEPO supplementation was statistically more favourable than in patients without rhEPO (HR = 0·38, 95 % CI 0·30, 0·47, P < 0·0001). Similar findings were noted for patients receiving vitamin D3 (HR = 0·36, 95 % CI 0·21, 0·64, P = 0.0004) and Fe (HR = 0·45, 95 % CI 0·33, 0·61, P < 0·0001).
Table 3 Univariate analysis: proportional hazards regression model of effect of relevant prognostic factors on survival among patients (n 702) with ESRD receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002

ESRD, end-stage renal disease; HR, hazard ratio; CGN, chronic glomerulonephritis; DM, type 2 diabetes mellitus; rhEPO, recombinant human erythropoietin.
*Includes congenital renal disease, obstructive uropathy, autoimmune disease, toxin- or drug-induced.
Supplementation with rhEPO was statistically significant, independent of the cause of ESRD, with (HR = 0·30, 95 % CI 0·22, 0·42) or without (HR = 0·48, 95 % CI 0·38, 0.61) combination with Fe or vitamin D3 in the multivariate model (Table 4). The corresponding figures for supplementation with rhEPO without Fe or vitamin D3 were 0·27 (95 % CI 0·18, 0·40) and 0·47 (95 % CI 0·35, 0.62) for those without DM, and 0·44 (95 % CI 0·25, 0.79) and 0·57 (95 % CI 0·35, 0·94) for those with DM. The significant results remained in patients with or without DM (Table 5) though the effect was larger in patients without DM.
Table 4 Multivariate analysis: proportional odds model of effect of relevant prognostic factors on survival among patients (n 702) with ESRD receiving haemodialysis, Li Shin Hospital, northern Taiwan, 1993–2002

ESRD, end-stage renal disease; HR, hazard ratio; CGN, chronic glomerulonephritis; DM, type 2 diabetes mellitus; rhEPO, recombinant human erythropoietin.
*Includes congenital renal disease, obstructive uropathy, autoimmune disease, toxin- or drug-induced.
Table 5 Multivariate analysis: proportional odds model of effect of supplementation on survival among ESRD haemodialysis patients (n 702), with or without DM, Li Shin Hospital, northern Taiwan, 1993–2002

ESRD, end-stage renal disease; DM, type 2 diabetes mellitus; HR, hazard ratio; rhEPO, recombinant human erythropoietin.
*Adjusted for age and aetiology.
†Adjusted for age.
Discussion
The present study demonstrated that supplement therapy with rhEPO, vitamin D3 or Fe significantly reduced mortality by 60 %, after adjustment for age, education and aetiology, in patients undergoing haemodialysis. After stratification of different combinations of supplement therapy, we found that the largest benefit was for a combination of all three supplements, with a 79 % reduction in mortality. The combination of rhEPO with vitamin D3 or Fe gave a 70 % mortality reduction. The sole use of rhEPO halved mortality.
Kalantar-Zadeh et al. demonstrated that low baseline serum Fe led to increased mortality and hospitalisation in a prospective cohort study of 1283 haemodialysis patients(Reference Kalantar-Zadeh, McAllister, Lehn, Liu and Kopple11). Recent studies have suggested that a combination of Fe and rhEPO improves anaemia(Reference Silverberg, Blum, Peer, Kaplan and Aiana5, Reference Macdougall, Chandler, Elston and Harchowal12–Reference Besarab, Frinak and Yee14). However, these studies lacked evidence to show any long-term benefit in terms of reduced mortality. Our 10-year follow-up of 702 patients corroborated a long-term benefit in the reduction of mortality attributed to the use of rhEPO, alone or combined with vitamin D3 or Fe. Combined therapy may confer an extra 30 % reduction in mortality in comparison with the use of rhEPO alone. The improvement in long-term survival may be attributed to the improvement in anaemia, which yields numerous additional benefits, including a significant decrease in left ventricular mass index and septal wall thickness and normalisation of increased cardiac output.
Concern has been raised as to whether Fe replenishment may have serious or even life-threatening drug-related adverse effects. However, recent studies have shown that Fe is safe in cases of Fe deficiency or when the maintenance of Fe stores is required(Reference Aronoff, Bennett and Blumenthal15–Reference Yee and Besarab17). The better survival rate demonstrated in our study suggests the relative safety of supplementation for patients treated with rhEPO alone or combined with vitamin D3 or Fe, in comparison to patients without these supplements. However, no empirical data on adverse effects, such as anaphylactic reaction or hypersensitivity, have been reported.
It may be argued that the number of cases treated with rhEPO + vitamin D3 and rhEPO + vitamin D3 + Fe is too small to draw conclusions. Indeed, the results from such a small number of patients should be interpreted with caution, and further studies are warranted. However, our results have presented a significant survival advantage for those treated with rhEPO + vitamin D3 and rhEPO + vitamin D3 + Fe compared with those receiving no supplement. Tables 3 and 4 also show a significant improvement with 95 % confidence intervals not including 1. These results would lessen the concern regarding insufficient statistical power.
Another limitation of the present study is that it was not a randomised controlled trial. Instead, a retrospective cohort design was adopted. The use of such a design, widely used in occupational epidemiology, is not only efficient in collecting data, but also dispenses with unnecessary long-term follow-up. The weakness is that a significant and beneficial result from the study may be blurred by other unmeasured clinical correlates. However, we believe that, although other confounding factors may attenuate the results, it is unlikely that such factors would render them non-significant.
In conclusion, we demonstrated a long-term benefit in reducing mortality related to supplement therapy with rhEPO, vitamin D3 and Fe. The findings provide a justification for the administration of combined supplement therapy in patients undergoing haemodialysis.
Acknowledgements
There is no granting support to be declared and no conflict of interest involved in the present study. H.-C.C. was responsible for the study design and practical performance. C.-L.C. contributed in practical performance and preparation of the manuscript. T.-L.C., S.-I.C. and A.M.-F.Y. were responsible for data analysis and interpretation of the results. T.H.-H.C. was responsible for study design, interpretation of the results, and manuscript preparation. All authors have read and approved the manuscript.