Pharmacogenetics is the study of genetic differences in the metabolism and activity of exogenous agents such as drugs within human populations. The cornerstone of pharmacogenetic research is the identification of genetic variations (polymorphisms) that alter drug concentrations or responses. This genetic variation in drug response is a substantial clinical problem, and can result in treatment failure or adverse drug events. One UK study (Reference GriffinGriffin, 1997) suggested that about 1 in 15 hospital admissions are due to adverse drug events. Lazarou et al (Reference Lazarou, Pomeranz and Corey1998) estimated that adverse reactions to prescribed medication injure 2.2 million patients per year in the USA. Users of psychiatric services report high levels of dissatisfaction with medication, especially side-effects (Reference Fakhoury, Wright and WallaceFakhoury et al, 2001; Reference HellewellHellewell, 2002).
The pharmacotherapy of psychiatric illness has improved outcomes for millions of patients worldwide. However, despite effective treatments for all the major psychiatric illnesses, psychopharmacology is still practised empirically. Optimisation of therapy for individuals is generally based upon trial and error. Developments in pharmacogenetics herald the ability to tailor treatments to individuals, thereby maximising therapeutic response and minimising adverse effects. Savings in care, time in hospital and an improvement in the individual's long-term prognosis should recoup testing costs. Potential pharmacogenetic strategies to realise these aims include identifying genes that predict clinical responsiveness to drug treatment and those influencing drug metabolism.
Pharmacogenetic technologies are already in use in other medical specialties. The preferred treatment of childhood acute lymphoblastic leukaemia is with purine antimetabolites such as azathioprine and thioguanine. Thiopurine methyltransferase (TPMT) degrades these drugs and is polymorphic. Screening for TPMT activity in red blood cells showed significant variation in metabolic ability related to these polymorphisms. Children with high levels of the enzyme are routinely treated with 4 times the normal dosage, and those with low levels receive a starting dosage that is approximately 15 times lower. This tailored approach has improved 5-year survival rates from 20% to 80% and reduced the number of serious adverse drug events (Reference Mcleod, Krynetski and RellingMcLeod et al, 2000). Although this may be seen as an example of individualised therapy, it should be noted that a recent analysis indicated that 78% of thiopurine-related adverse drug events were not associated with TPMT gene polymorphisms (Reference van Aken, Schmedders and Feuersteinvan Aken et al, 2003).
Arranz et al (Reference Arranz, Collier and Sodhi1995) were the first to report a link between a polymorphism (102T→C) in the 5-HT2A receptor gene and the response to clozapine. This finding has not been replicated in other studies, but a meta-analysis has shown a trend of association between response and this polymorphism (Reference Arranz, Munro and ShamArranz et al, 1998). Other genes have been implicated in clozapine response, and additive predictive models have been developed. Nevertheless, testing for response for a single antipsychotic is not that clinically useful, given the range of antipsychotic drugs available. Although the clinician and patient may have some information on response, they will not be able to balance this against genetic vulnerability to side-effects or genetic variations in pharmacokinetics.
The majority of psychotropic drugs are metabolised by cytochrome P450 CYP2D6 (Reference Cozza and ArmstrongCozza & Armstrong, 2001). The CYP2D6 gene is located on chromosome 22, and up to 14% of white people have at least two defective, autosomal recessive alleles. Individuals with this genotype are referred to as ‘poor metabolisers’. Other genotypes result in ‘intermediate’ metabolism and ‘extensive’ metabolism. Patients who are poor metabolisers might be expected to be more likely to suffer adverse drug events, whereas patients who are extensive metabolisers are theoretically less likely to achieve therapeutic plasma drug levels. These observations resulted in an expert group recommending routine CYP2D6 screening in psychiatric patients (Reference Meyer, Amrein and BalantMeyer et al, 1996). More recently, Wolf et al (Reference Wolf, Smith and Smith2000) have echoed this view. Although prior knowledge of CYP2D6 status theoretically allows the adjustment of dosages to minimise side-effects and maximise efficacy, this has not led to the routine incorporation of screening into clinical practice.
Chou et al (Reference Chou, Yan and de Leon2000) examined the influence of CYP2D6 variability in psychiatric in-patients by evaluating adverse drug events, bed usage and total costs in a 1-year period. They reported a trend to more adverse events in patients who were poor metabolisers. Patients who were poor extended metabolisers cost between US$4000 and $6000 more to treat per year compared with patients who were intermediate metabolisers. Patients who were poor metabolisers also had the longest hospital stays. These authors’ cost-effectiveness modelling suggested that large patient numbers would be needed to establish the economic benefits of offering routine screening. However, the cost of such testing was estimated to be $100 per genotype (Reference Chou, Yan and de LeonChou et al, 2000), it is now less than $1. Accordingly, even accounting for personnel and overhead costs, the cost of genetic screening is now substantially lower, making it a more economically viable option.
Method
As part of a service development programme, the availability of cheaper testing allowed us to offer patients attending a general adult psychiatry clinic the opportunity to have their CYP2D6 status assessed. Patients were offered the test if they were currently taking psychotropic medication metabolised by CYP2D6, were complaining of side-effects and/or had a poor treatment response. Patients were genotyped for CYP2D6 status using standard polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) techniques to identify the CYP2D6*4 and *5 alleles. Case-note review was used to obtain basic demographic data and to categorise patients for reported side-effects, medication dosage, illness length and number of psychotropic drugs currently prescribed. The notes were reviewed by R.E.H., who was blind to CYP2D6 status. Data were analysed using the Statistical Package for the Social Sciences, version 10.5.
Results
A sample of 56 patients were offered CYP2D6 genetic screening; one patient declined the test. Two patients (4%) were poor metabolisers, 21 (38%) were intermediate and 32 (58%) were extensive metabolisers. The distribution of the CYP2D6 polymorphism in our clinic population was the same as in the general population (Reference Elexperu-Camiruaga, Buxton and KandulaElexperu-Camiruaga et al, 1995). There was no significant difference between metabolic status and patient gender (χ2=7.25, d.f.=1, P=0.394), diagnosis (χ2=0.980, d.f.=2, P=0.613) or length of illness (χ2=2.394, d.f.=2, P=0.302) (Table 1). The two patients who were poor metabolisers (one female and one male) were excluded from all categorical testing.
Table 1. Patient demographics categorised according to CYP2D6 genotype
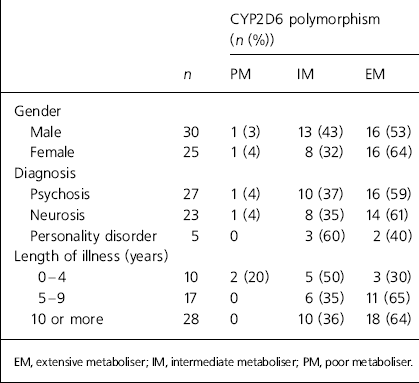
| CYP2D6 polymorphism (n (%)) | ||||
|---|---|---|---|---|
| n | PM | IM | EM | |
| Gender | ||||
| Male | 30 | 1 (3) | 13 (43) | 16 (53) |
| Female | 25 | 1 (4) | 8 (32) | 16 (64) |
| Diagnosis | ||||
| Psychosis | 27 | 1 (4) | 10 (37) | 16 (59) |
| Neurosis | 23 | 1 (4) | 8 (35) | 14 (61) |
| Personality disorder | 5 | 0 | 3 (60) | 2 (40) |
| Length of illness (years) | ||||
| 0–4 | 10 | 2 (20) | 5 (50) | 3 (30) |
| 5–9 | 17 | 0 | 6 (35) | 11 (65) |
| 10 or more | 28 | 0 | 10 (36) | 18 (64) |
The relationship between medication dosage and CYP2D6 status was statistically significant (χ2=18.04, d.f.=2, P=0.001). Four-fifths of the 22 patients on a high medication dosage were extensive metabolisers, and both the patients who were poor metabolisers were on low dosages (Table 2). Fourteen patients were taking clozapine. Although clozapine is not solely metabolised by CYP2D6 (CYP3A4 and CYP1A2 are also important), CYP2D6 is the only one of these three with definite clinically-relevant polymorphisms (Reference Cozza and ArmstrongCozza & Armstrong, 2001). Mean clozapine dosages are shown in Table 2. The patients’ CYP2D6 status was related to mean dosage, although numbers are too small for reliable statistical analysis.
Table 2. Association between CYP2D6 genotype and treatment response
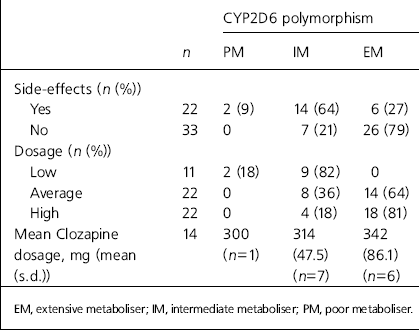
| CYP2D6 polymorphism | ||||
|---|---|---|---|---|
| n | PM | IM | EM | |
| Side-effects (n (%)) | ||||
| Yes | 22 | 2 (9) | 14 (64) | 6 (27) |
| No | 33 | 0 | 7 (21) | 26 (79) |
| Dosage (n (%)) | ||||
| Low | 11 | 2 (18) | 9 (82) | 0 |
| Average | 22 | 0 | 8 (36) | 14 (64) |
| High | 22 | 0 | 4 (18) | 18 (81) |
| Mean Clozapine | 14 | 300 | 314 | 342 |
| dosage, mg (mean (s.d.)) | (n=1) | (47.5) (n=7) | (86.1) (n=6) | |
Side-effects were also significantly related to CYP2D6 status: χ 2=12.39, d.f.=1, P=0.01 (Table 2). Only 19% of patients in the extensive metaboliser group reported side-effects, in contrast to two-thirds of the intermediate metabolisers and both of the patients who were poor metabolisers. The number of concurrently prescribed psychotropic drugs was not statistically significantly associated with CYP2D6 genotype (data not shown; Fisher's exact test, P=0.242).
Discussion
The lack of difference between the general population and the clinic population was perhaps unexpected, given the original rationale for offering CYP2D6 testing to patients who were complaining of side-effects or lack of efficacy. However, the sample was small, and was heterogeneous diagnostically as well as for illness duration and severity. Genotyping was not offered in a rigorous and systematic manner, and some patients requested the test after hearing about it from other patients. This investigation must therefore be regarded only as a pilot study. There are other methodological confounders: the sample was drawn from a secondary care population, and would be different from one obtained from a primary care setting; clinical ratings for side-effects were based on case-note review, not on standardised rating instruments. Although there is also some relationship between clozapine dosage, clozapine serum levels and clinical response (Reference PerryPerry, 2000), the dosage and serum level relationship for other antipsychotic and antidepressant drugs is less clear. Of course, categorising of doses does allow some comparability between drugs. Some patients were on more than one drug, which can lead to interactions; for example, fluoxetine and paroxetine strongly inhibit CYP2D6, leading to higher serum levels of co-administered antipsychotics (Reference RichelsonRichelson, 1997).
Our study took place in a follow-up clinic. However, to maximise the benefits of testing, it should be offered to all patients in advance of prescribing psychotropic medication: patients who were poor metabolisers would be started on lower dosages, titrated slowly upwards; patients who were extensive metabolisers would be started on higher dosages, titrated more rapidly. Consideration should also be given to prescribing dosages in excess of those recommended in the British National Formulary, although such prescribing needs to be carefully discussed with the patient. In both these types of patient, the use of psychotropic agents that are not significantly metabolised by CYP2D6 should be considered.
The results of this preliminary study suggest that CYP2D6 genotyping may be of clinical benefit. Genotyping should enhance patients’ involvement in - and satisfaction with - prescribing decisions. However, prospective randomised clinical trials are needed to demonstrate the clinical and economic effectiveness of CYP2D6 genotyping. Such trials are expensive, and further observational studies may be needed to inform their design. Such genotyping will need to be combined with many other assays, given the numerous genetic and environmental factors involved in determining treatment response and liability to side-effects, before truly individualised therapy can be said to have been achieved.
Acknowledgements
We wish to thank Professor Robert Kerwin for comments on an earlier draft of this paper.





eLetters
No eLetters have been published for this article.