Coronary artery disease (CAD) is the leading cause of death in the USA, Europe, Canada and many other industrialised nations(1, Reference Grundy, Cleeman and Merz2). According to present trends in the USA, one in two healthy 40-year-old males and one in three females will develop CAD in their lifetime(Reference Durrington3). CVD are expected to be the main cause of death globally, due to rapidly increasing rates in developing countries and the rising incidence of obesity and diabetes in the industrialised world(Reference Murray and Lopez4, 5). Epidemiological data reveal a log-linear relationship between increasing LDL-cholesterol (LDL-C) concentration and relative risk of CAD(Reference Jacobson6). Clinical trials have confirmed this log-linear relationship, showing an almost identical pattern of risk association(Reference Grundy, Cleeman and Merz2).
Dietary recommendations and exercise are the first line of therapy for individuals with elevated LDL-C; however, using these methods only very modest reductions in LDL-C can be realised even with the highest levels of compliance(Reference Talbert7). Patients who find it too difficult to make lifestyle modifications, or those who simply cannot realise enough LDL-C reduction, are offered statin therapy to reduce their risk profile(Reference Stancu and Sima8–Reference Pedersen, Kjekshus and Berg10). Unfortunately, less than half the number of patients who qualify for lipid-modifying treatment are receiving it, and only a third of treated patients are achieving their LDL-C goal due to associated cost and other limitations(Reference Grundy, Cleeman and Merz2). Thus, an effort is underway to functionalise food products and develop nutraceuticals that can help lower LDL-C and the risk of CAD.
Probiotic bacteria are defined by the WHO as ‘live micro-organisms which when administered in adequate amounts confer a health benefit on the host’ and are being examined for their efficacy in lowering total cholesterol (TC) and LDL-C in humans. A double-blind, randomised, placebo-controlled crossover study, by Schaafsma et al. (Reference Schaafsma, Meuling and van Dokkum11), reported a decrease in TC and LDL-C by 4·4 and 5·4 %, respectively, after consumption of yoghurt enriched with Lactobacillus acidophilus and fructo-oligosaccharides three times daily in thirty normolipidaemic male subjects. A double-blind, randomised, placebo-controlled crossover study, by Bertolami et al. (Reference Bertolami, Faludi and Batlouni12), reported a decrease in TC and LDL-C by 5·3 and 6·15 %, respectively, after consumption of a fermented milk product containing Enterococcus faecium in thirty-two subjects with mild to moderate hypercholesterolaemia. A double-blind, randomised, placebo-controlled study, by Agerbaek et al. (Reference Agerbaek, Gerdes and Richelsen13), reported a decrease in LDL-C by 10 % after consumption of a fermented milk product containing E. faecium and two strains of Streptococcus thermophilus, in fifty-eight non-obese, normocholesterolaemic 44-year-old Danish men. A double-blind, randomised, placebo-controlled parallel study, by Agerholm-Larsen et al. (Reference Agerholm-Larsen, Raben and Haulrik14) reported a significant reduction in LDL-C, but only after adjusting for body weight, after consumption of a yoghurt fermented with E. faecium and two strains of S. thermophilus. While these studies have reported positive findings, several placebo-controlled studies have reported little or no effect after daily consumption of various probiotic supplements and foods containing probiotic bacteria(Reference Andersson, Bosaeus and Ellegard15–Reference Lin, Ayres and Winkler19).
Lactobacillus reuteri NCIMB 30242 was selected for its cholesterol-lowering traits and overall strain safety using a rigorous process. Extensive in vitro characterisation was performed on the strain using a combination of molecular and metabolic techniques to support its safety for use in human subjects(Reference Branton, Jones and Tomaro-Duchesneau20). One phenotypic characteristic of the strain is its intrinsic capacity to deconjugate bile acids due to expression of a bile salt hydrolase (BSH) enzyme. The BSH enzyme hydrolyses the C-24 N-acyl amide bond linking the free bile acid to its amino acid conjugate glycine or taurine. It has been hypothesised that deconjugation of bile acids leads to a reduction of serum cholesterol by increasing cholesterol catabolism during the formation of new bile acids, or by reducing cholesterol absorption from dietary and bile sources in the intestinal lumen(Reference Taranto, Sesma and Holgado21–Reference De Smet, Van Hoorde and Vande23). More recently, several groups have proposed other mechanisms by which bile may act to modulate cholesterol absorption and metabolism in humans(Reference Davidson24–Reference Watanabe, Houten and Mataki27).
Extensive research on probiotic survival in the gastrointestinal (GI) tract and in various food products has revealed reduced probiotic bacterial cell viability due to exposure to organic acids, hydrogen ions, oxygen and antibacterial components(Reference Holzapfel, Haberer and Snel28, Reference Huang and Adams29). For this reason, the present study was designed to evaluate the cholesterol-lowering potential of alginate-poly-l-lysine-alginate (APA) microencapsulated L. reuteri NCIMB 30242, which allows for the delivery of highly viable and metabolically active cells to the proximal small intestine. Microencapsulation provides a physical barrier against Ig and digestive enzymes, buffers against an acidic gastric environment, concentrates the bacteria within the microcapsule and provides a microenvironment that aids in precipitation of the deconjugate(Reference Chang30–Reference Jones, Chen and Wei32). In fact, we have shown that APA microencapsulated BSH-active Lactobacillus plantarum and L. reuteri strains maintain cell viability and bile acid deconjugation activity through sequential transit in a simulated GI model(Reference Martoni, Bhathena and Jones33, Reference Martoni, Bhathena and Urbanska34). Furthermore, in BioF1B hamsters, a significant cholesterol-lowering effect was observed by APA microencapsulated Lactobacillus as compared to sham (empty) APA microcapsule-treated control(Reference Bhathena, Martoni and Kulamarva35).
Despite improved pharmacotherapy for hypercholesterolaemia, a discrepancy between target cholesterol levels and those clinically realised remains. Thus, additional treatment modalities such as probiotics should be evaluated for their cholesterol-lowering efficacy and safety profile. Accordingly, the main objective of the present study was to assess the cholesterol-lowering clinical efficacy and safety of microencapsulated L. reuteri NCIMB 30242 supplemented in a yoghurt formulation in a double-blind, randomised, placebo-controlled, multi-centre study.
Experimental methods
Subjects
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the central ethics committee (multi-centric ethics committee) and the local ethics committee in the Czech Republic. Written informed consent was obtained from all subjects. The trial was registered on clinicaltrials.gov, USA, identifier NCT01185795.
Otherwise healthy hypercholesterolaemic adult men and women were recruited from five centres in Prague, Czech Republic. Inclusion criteria for randomisation were otherwise healthy males and females between the ages of 18 and 74 years (inclusive); LDL-C levels >3·4 mmol/l with < 15 % variation between successive screening visits; TAG levels < 4·0 mmol/l; BMI of 22–32 kg/m2; the ability to understand dietary procedures; judged by the investigators as motivated. Exclusion criteria for randomisation were the use of statin or other cholesterol-lowering prescription drugs within the last 6 months; use of plant sterols, n-3 fatty acids, fish oil, soya protein, soluble oat fibre, psyllium seed husk or other cholesterol-lowering supplements within the last 3 months; history of chronic use of alcohol (>2 drinks/d); use of systemic antibodies, corticosteroids, androgens or phenytoin; myocardial infarction, coronary artery bypass or other surgical procedures within the last 6 months; lactose intolerance or allergies to dairy products; history of angina, congestive heart failure, inflammatory bowel disease, pancreatitis or diabetes; GI, renal, pulmonary, hepatic or biliary disease, or cancer (evidence of active lesions, chemotherapy or surgery in the past year); chronic use of probiotics or fibre laxatives (>2 doses/week), or stimulant laxatives; history of eating disorders; exercise greater than walking 15 miles/week, or an equivalent energy expense of 16 736 kJ/week (4000 kcal/week); pregnancy, breast feeding or intent to get pregnant.
Preparation of treatment and placebo yoghurts
Lactobacillus reuteri NCIMB 30242 (Cardioviva™) was propagated in an FV8 fermenter and concentrated in compliance with standard operating procedures and quality control procedures at Microbial Developments Limited (Malvern, UK). Microbiological analyses and bacterial culture purity were confirmed immediately after each production batch. APA microcapsules containing L. reuteri NCIMB 30242 were prepared in compliance with standard operating procedures and quality-control procedures at Brace GmbH (Karlstein, Germany) to a viability of 5 × 109 colony-forming units/g microcapsule. Placebo and treatment yoghurts were produced and prepared at Milcom (Prague, Czech Republic) with compositions as shown in Table 1. Placebo yoghurts were filled to a weight of 125 g in plastic cups. Treatment yoghurts contained 115 g of yoghurt and 10 g of microcapsules containing BSH-active L. reuteri NCIMB 30242. Placebo and treatment yoghurts were produced five times during the study with the batch numbers 1 to 5. The expiry date of placebo and treatment yoghurts was maintained at 3 weeks after yoghurt production. For details of analyses of cell viability and bile salt hydrolase activity of free and microencapsulated L. reuteri NCIMB 30242 in simulated upper GI tract conditions, see Appendix A (to be found in the online Supplementary material; http://www.journals.Cambridge.org/bjn).
Table 1 Composition of placebo and L. reuteri yoghurts
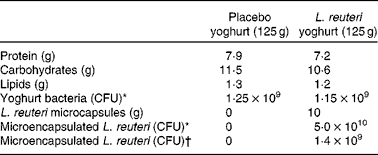
CFU, colony forming unit.
* Measured after production.
† Measured after international shipping at 4°C.
Study design
The study design was double-blind, placebo-controlled, randomised, parallel-arm and multi-centred, lasting a total of 10 weeks. This included a 2-week washout period in which general dietary recommendations (Canada's Food Guide, Health Canada) were followed, a 2-week run-in period in which general dietary recommendations were followed and subjects consumed placebo yoghurts twice daily at breakfast or dinner, and a 6-week treatment period in which general recommendations were followed and subjects consumed either placebo or treatment yoghurts twice daily at breakfast or dinner. Subjects met with the investigational team at five different time points: Visit V0 (Week − 4), V1 (Week − 2), V2 (Week 0, randomisation and treatment baseline), V3 (Week 3, treatment midpoint) and V4 (Week 6, treatment endpoint). Dietary intake, including information on total energy, percentage total fat, percentage total carbohydrates and percentage total protein, for subjects consuming placebo yoghurts and treatment yoghurts, was measured at baseline (Week 0) and endpoint (Week 6) of the treatment period.
Sample analysis
Blood for assessment of lipid profile was collected at each visit. Serum samples were analysed enzymatically for LDL-C (primary efficacy variable), TC, HDL-cholesterol (HDL-C), TAG and apoB-100. Absolute changes in lipid parameters for each subject at midpoint and endpoint were calculated by subtracting the baseline value (Week 0) from the midpoint (Week 3) or endpoint (Week 6) value, respectively. Relative change in lipid parameters for each subject at midpoint and endpoint was calculated by dividing the absolute change at midpoint (Week 3) or endpoint (Week 6) by baseline values (Week 0) and multiplying by 100 %. Blood for assessment of safety profile was collected at visits V1 (Week − 2) and V4 (Week 6, treatment endpoint). Serum biochemistry was analysed for urea, creatinine, bilirubin, aspartate aminotransferase, alanine transaminase, γ-glutamyl transpeptidase, alkaline phosphatase, glucose, Ca2+, ![]() , K+, Na+, Cl−,
, K+, Na+, Cl−, ![]() and lipase. Serum analysis was performed on a Dimension RxL biochemistry analyser using appropriate reagent kits (Dade Behring, Siemens, Munich, Germany). Whole blood (haematology) was analysed for Hb, haematocrit, erythrocytes, leucocytes and platelets using a Celltac F haematology analyser (Nihon Kohden, Tokyo, Japan).
and lipase. Serum analysis was performed on a Dimension RxL biochemistry analyser using appropriate reagent kits (Dade Behring, Siemens, Munich, Germany). Whole blood (haematology) was analysed for Hb, haematocrit, erythrocytes, leucocytes and platelets using a Celltac F haematology analyser (Nihon Kohden, Tokyo, Japan).
Faecal samples were collected in the 3 d before visits V1 (Week − 2) and V4 (treatment endpoint). Faecal deconjugated bile acid concentration was analysed on 10–15 μg of lyophilised stool samples by GLC as described by Batta et al. (Reference Batta, Salen and Batta36).
Statistical methods
The number of subjects was calculated by taking into account a critical difference in LDL-C of 0·44 (sd 0·8) mmol/l between the treatment and placebo groups with α = 5 % and a power of 80 %. Given these constraints, fifty-three evaluable subjects per group or 106 in total were required. To take into account possible premature withdrawal, a total of 120 subjects was planned to be included for randomisation.
The primary null hypothesis was that treatment was not more effective than placebo in reducing serum LDL-C concentrations. All analyses were performed according to the intention-to-treat principle. Continuous variables are presented as means with standard errors of the mean. The Shapiro–Wilk test was used to determine if variables were parametrically distributed. Differences between groups for baseline characteristics were analysed using a one-way ANOVA for continuous variables or χ2 test for categorical variables. Differences in dietary intake of macronutrients and faecal deconjugated bile acids between and within groups were analysed using mixed-model ANOVA. For lipid variables, multiple-linear regression was used to identify variables systematically contributing to any changes from baseline. To test the differences between groups, ANCOVA were performed to adjust for any systematic contribution to the changes from baseline using covariates identified by multiple-linear regression. Lipid parameters not accepting parametric description were analysed by means of Kruskal–Wallis tests. Data analyses were performed using SPSS software package version 17.0 (SPSS Inc., Chicago, IL, USA).
Forward, stepwise and backward selection models were completed using SAS software package version 9.2 (SAS Institute, Cary, NC, USA).
Results
Study parameters
A total of 120 subjects were randomised and 114 completed the study as part of the intention-to-treat population. One subject, randomised to the placebo group, dropped out for personal reasons and five subjects, two in the placebo group and three in the treatment group, were excluded as they did not meet the study criteria. Overall, 109 subjects completed the study as part of the per-protocol population. All subjects were considered hypercholesterolaemic and at borderline, high or very high risk of developing heart disease at baseline according to the National Cholesterol Education Program guidelines(1, Reference Grundy, Cleeman and Merz2).
Baseline characteristics of subjects
The baseline characteristics for the 114 subjects in the intention-to-treat population were evaluated and are presented in Table 2. The two groups produced by randomisation were homogeneous in terms of demographic and clinical characteristics. Male and female study subjects were equally distributed with 34 :66 % males–females in the placebo group and 38 :62 % males–females in the treatment group. There were no significant differences between groups at baseline in age, body weight, BMI, systolic, diastolic and mean blood pressure, pulse and temperature. Subjects were selected based on fasting serum LDL-C (>3·4 mmol/l) and TAG ( < 4·0 mmol/l). The mean serum concentrations of LDL-C at baseline were not significantly different between placebo and treatment groups (4·23 (sem 0·06) mmol/l compared with 4·37 (sem 0·08) mmol/l, respectively; P = 0·15). Additionally, there were no significant differences between placebo and treatment groups for TC, HDL-C, TAG, apoB-100, LDL-C:HDL-C and non-HDL-C. Statin and other lipid-lowering formulation intake among subjects was 0 % in the 6 months before the study start date.
Table 2 Demographic and clinical characteristics at baseline
(Mean values with their standard errors)

BP, blood pressure; bpm, beats per min; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
* One-way ANOVA for continuous variables or χ2 test for categorical variables.
Dietary assessment
A dietary assessment of total energy, percentage total lipids, percentage total carbohydrates and percentage total proteins was performed at baseline (Week 0) and at the treatment endpoint (Week 6). There were no significant differences between placebo and treatment groups at baseline or endpoint, and no difference within groups over the treatment period (Table 3).
Table 3 Dietary total energy and macronutrient intake
(Mean values with their standard errors)
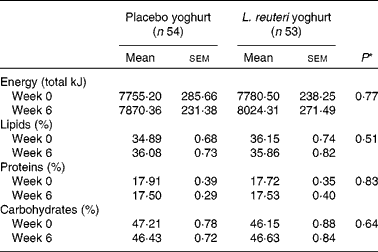
* Mixed-model ANOVA.
Serum lipid profile
The mean relative changes of LDL-C, TC, HDL-C, TAG, apoB-100, LDL-C:HDL-C and non-HDL-C from baseline to Week 3 and Week 6 are summarised in Table 4. The LDL-C-lowering effect observed at the 6-week endpoint of the intervention period was − 0·37 (sem 0·11) mmol/l with a significant mean change over placebo of 8·92 % (P = 0·016). Over the 6-week treatment period, other lipid-lowering effects were observed for TC of − 0·77 (sem 0·13) mmol/l, apoB-100 of − 0·19 (sem 0·03) mmol/l and non-HDL-C of − 0·68 (sem 1·11) mmol/l, and significant mean changes over placebo for TC of 4·81 % (P = 0·031) and non-HDL-C of 6·01 % (P = 0·029). Serum concentrations of TAG and HDL-C were unchanged over the course of the study. Three multivariate regression models were used to show that treatment was the primary predictor of LDL-C reduction: a stepwise selection approach showed that treatment was associated with a − 0·44 mmol/l change in LDL-C (P = 0·0008); a forward selection approach showed that treatment was associated with a − 0·39 mmol/l change in LDL-C (P = 0·0027); and a backward selection approach showed that treatment was associated with a − 0·40 mmol/l change in LDL-C (P = 0·0019). Finally, a side-by-side comparison of individual endpoint LDL-C changes from baseline indicates a clear LDL-C-reducing effect across the spectrum of responses (Fig. 1).
Table 4 Relative changes in lipid parameters from baseline at midpoint (Week 3) and endpoint (Week 6)
(Mean values with their standard errors)

LDL-C, LDL-cholesterol; TC, total cholesterol; HDL-C, HDL-cholesterol.
* ANCOVA adjusted by baseline values.
† Kruskal–Wallis test.

Fig. 1 LDL-cholesterol (LDL-C) response showing per subject percentage change from baseline to endpoint of treatment period for groups consuming placebo yoghurt (n 58, ![]() ) and Lactobacillus reuteri NCIMB 30242 yoghurt (n 56,
) and Lactobacillus reuteri NCIMB 30242 yoghurt (n 56, ![]() ) in the intention-to-treat population.
) in the intention-to-treat population.
Faecal assessment
Faecal samples collected before treatment (Week − 2) and at endpoint (Week 6) were analysed for faecal deconjugated bile acid concentration. A total of forty samples collected was deemed adequate for analysis; twenty-one in the treatment group and nineteen in the placebo group. The mean faecal deconjugated bile acid concentration was not found to be significantly different between groups, before treatment and at endpoint, or within groups over the treatment period (Table 5).
Table 5 Faecal deconjugated bile acids
(Mean values with their standard errors)
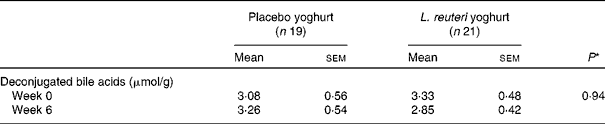
* Mixed-model ANOVA.
Safety parameters
Biochemical markers of safety were measured at baseline and endpoint and analysed for significant changes. Haematologic markers were assessed by complete blood cell count, platelets, haematocrit and Hb; kidney function was determined by urea and creatinine; liver function was determined by alanine transaminase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase and bilirubin; pancreatic function was determined by lipase; endocrine function was determined by glucose, Ca2+ and ![]() ; and electrolyte balance was determined by K+, Na+, Cl− and
; and electrolyte balance was determined by K+, Na+, Cl− and ![]() . Results show that the placebo and treatment groups were comparable for biomarkers of safety at the study endpoint, and the number of subjects with clinically significant values outside the normal range was determined to be six subjects in the placebo group and one subject in the treatment group. No changes in biochemical markers of safety were considered to be a result of treatment (data not shown).
. Results show that the placebo and treatment groups were comparable for biomarkers of safety at the study endpoint, and the number of subjects with clinically significant values outside the normal range was determined to be six subjects in the placebo group and one subject in the treatment group. No changes in biochemical markers of safety were considered to be a result of treatment (data not shown).
Discussion
This double-blind, randomised, placebo-controlled, parallel-arm, multi-centre study demonstrates the cholesterol-lowering effect of a novel microencapsulated BSH-active probiotic over 6 weeks. Subjects consuming yoghurts containing microencapsulated L. reuteri NCIMB 30242 attained significant reductions over placebo in LDL-C of 8·92 %, TC of 4·81 % and non-HDL-C of 6·01 %, and a significant absolute change in apoB-100 of − 0·19 (sem 0·03) mmol/l over the intervention period. Serum concentrations of TAG and HDL-C were unchanged over the course of the study. As well, three multivariate regression models were used to show that treatment was the primary predictor of LDL-C reduction. Finally, a side-by-side comparison of individual LDL-C responses at the study endpoint indicates that despite a proportion of subjects who experienced elevated LDL-C values, an LDL-C-lowering effect was observed over the spectrum of LDL-C responses (Fig. 1).
When examining the cholesterol-lowering trend over the course of the study, it is apparent that the time to maximal therapeutic effect may be longer than other cholesterol-lowering therapies(Reference Stancu and Sima8, Reference Andrews, Ballantyne and Hsia37–Reference Gagne, Gaudet and Bruckert41). Although only baseline, 3- and 6-week data were collected, a cholesterol-lowering trend was observed over the course of the study, indicating that maximal therapeutic effect may not have been reached by the study endpoint. Thus, future studies should evaluate the cholesterol-lowering efficacy of L. reuteri NCIMB 30242 at later time points. Also, subjects on statin monotherapy were excluded from the study to accurately determine lipid-lowering efficacy of the microencapsulated probiotic alone; however, evidence exists for improved effectiveness of dietary cholesterol-reducing agents in subjects having high cholesterol absorption and low biosynthesis(Reference Ostlund42). Thus, the potential for greater cholesterol reductions in subjects with reduced cholesterol biosynthesis should be explored.
One possible mechanism of action for cholesterol-lowering with BSH-active probiotics is that increased intra-luminal BSH activity may lead to increased excretion of deconjugated bile acids and subsequent removal of serum cholesterol by the liver replacing bile acids lost from the enterohepatic recirculation (de novo synthesis of bile acids by 7α-hydroylase catabolism of cholesterol)(Reference De Smet, Van Hoorde and De Saeyer22, Reference De Smet, De Boever and Verstraete43). The conversion of cholesterol to bile acids in the liver and their subsequent secretion and faecal excretion provides the major route for elimination of excess cholesterol. Previously, we have shown that APA microencapsulated BSH-active L. plantarum and L. reuteri strains maintain cell viability and bile acid deconjugating activity in simulated upper GI conditions(Reference Martoni, Bhathena and Jones33, Reference Martoni, Bhathena and Urbanska34). In the present study, despite significant reductions in serum LDL-C, no significant change in faecal deconjugated bile acid excretion was seen in the samples collected. A recent randomised, placebo-controlled clinical trial by Ooi et al. (Reference Ooi, Ahmad and Yuen44) showed a significant reduction in plasma TC and LDL-C as a result of synbiotic capsule feeding containing BSH-active L. acidophilus CHO-220 and inulin. However, no significant differences in the levels of plasma deconjugated primary or plasma deconjugated secondary bile acids were observed over the 12-week treatment period. The authors postulated that the BSH activity of L. acidophilus CHO-220, shown in vitro, either was not exhibited in human subjects or was too minimal to produce an observed effect.
Previous studies(Reference De Smet, De Boever and Verstraete43, Reference Jeun, Kim and Cho45, Reference Kumar, Grover and Batish46) in animals have shown hypocholesterolaemic effects of BSH-active probiotics along with increased bile acid excretion. In a porcine model, the strain B. animalis DN-173010, chosen from thirty-eight strains for its bile acid deconjugation capacity, was shown to increase serum deconjugated bile acids after 1 and 2 weeks of treatment(Reference Lepercq, Relano and Cayuela47). It has been suggested that increasing bile acid deconjugation activity in the small intestine could render the deconjugated primary bile acids more susceptible to 7α-dehydroxylation activity by the resident microflora, potentially leading to increased secondary bile acids. However, an increase in the formation of secondary bile acids was not observed in the portal vein of pigs(Reference Lepercq, Relano and Cayuela47) or in the faeces of healthy human subjects(Reference Marteau, Cuillerier and Meance48) upon B. animalis DN-173010 intervention. Evidence for the expression of 7α-dehydroxylase in lactic acid bacteria has not been reported in the literature, and is a genotype that appears to be limited to Enterobacter and Clostridium species within the gut microflora(Reference Begley, Hill and Gahan49). Furthermore, it has also been reported that active and passive absorption of bile acids complement one another and bring about nearly complete absorption of bile acid, whether conjugated or deconjugated, from the small-intestinal contents of rodents(Reference Schiff, Small and Dietschy50). Therefore, one hypothesis states that deconjugation of bile acids proximal to the terminal ileum does not disrupt the enterohepatic circulation of bile acids but rather alters the bile acid pool in circulation. Future studies should therefore assess the complete bile acid profile in circulation as well as in faeces.
A study by Jeun et al. (Reference Jeun, Kim and Cho45) demonstrated increased gene expression changes, together with increased bile acid excretion, on account of L. plantarum feeding in mice. Several mechanisms were postulated to explain the hypocholesterolaemic effect in vivo, including inhibition of hepatic cholesterol synthesis, induction of cellular LDL-C uptake, decreased dietary cholesterol uptake and elevation of bile acid excretion. Given the result in the present study, other mechanisms of action were considered, including down-regulation of farnesoid × receptor (FXR) and consequent effect of liver × receptor (LXR) down-regulation and deconjugated bile acids on adenosine triphosphate-binding cassette G5/G8 heterodimer cholesterol efflux in hepatocytes and enterocytes (Fig. 2). Such a mechanism of action would result in a significant increase in faecal total neutral sterol excretion; thus, future studies should also evaluate neutral sterol excretion in faeces.

Fig. 2 Bile salt hydrolase (BSH)-active microencapsulated Lactobacillus reuteri NCIMB 30242, by reducing the concentration of bile acids (BA) returning to the liver or by changing the BA pool profile, may down-regulate farnesoid × receptor (FXR) leading to increased catabolism of cholesterol and synthesis of BA by 7α-hydoxylase. Down-regulation of FXR may lead to up-regulation of liver × receptor (LXR) which has been shown to enhance reverse cholesterol transport, improve glycaemic control(Reference De Smet, Van Hoorde and De Saeyer22) and increase the export of free cholesterol from cells through up-regulation of the adenosine triphosphate-binding cassette (ABC) transports(Reference De Smet, Van Hoorde and De Saeyer22). Particularly, ABCG5 and ABCG8 function as heterodimers (ABCG5/G8) at the apical membrane of enterocytes and hepatocytes and limit the accumulation of cholesterol by transporting it into the gastrointestinal (GI) lumen and bile canaliculi. BA, together with cholesterol, promote an active conformation of ABCG5/G8 and increase the efflux of cholesterol(Reference De Smet, Van Hoorde and Vande23). Thus, there may be a net efflux of cholesterol by enterocytes and hepatocytes into the GI lumen and bile canaliculi(Reference De Smet, Van Hoorde and Vande23) resulting in a decrease in serum cholesterol and an increase in cholesterol excretion in faeces. LDL-C, LDL-cholesterol; LDL-R, LDL receptor; SHP, small heterodimer partner; CBA, conjugated BA.
Analysis of safety parameters did not show deleterious effects of consuming yoghurts containing microencapsulated L. reuteri NCIMB 30242. There were more subjects with safety parameters that fell outside the clinically normal range in the placebo group, as compared to the treatment group, at the study endpoint. While there were no significant changes in leucocyte count, a crude measure of inflammation, future studies should substantiate this result by looking at acute-phase reactants such as C-reactive protein which have important cardiovascular implications(Reference Ridker, Glynn and Hennekens51–Reference Roberts55).
Future studies should continue to look at improving delivery technologies for BSH-active probiotics. Microencapsulation technologies, including APA microencapsulation, should continue to focus on minimising microcapsule diameter, for improved functionality and palatability, while maximising cell loading, ultimately resulting in minimising the dose size required for clinical efficacy.
In summary, several probiotic clinical studies have shown some cholesterol-lowering efficacy(Reference Schaafsma, Meuling and van Dokkum11–Reference Agerholm-Larsen, Raben and Haulrik14), while others have shown negative results(Reference Andersson, Bosaeus and Ellegard15–Reference Lin, Ayres and Winkler19), which may have been due to poor strain selection, method of delivery or clinical design. In most cases, BSH activity is not mentioned as a characteristic of the strain administered. Previously, a BSH-active strain was shown to significantly decrease TC and LDL-C in humans, when taken as a synbiotic(Reference Ooi, Ahmad and Yuen44). For the present study, L. reuteri NCIMB 30242 was selected using a rigorous cholesterol-lowering and strain safety screening process, including BSH activity, and was delivered using a gastroprotective microencapsulation technology. The present results support efficacy and safety of the formulation in lowering LDL-C, TC, apoB-100 and non-HDL-C in hypercholesterolaemic adults over 6 weeks. The hypocholesterolaemic effect of microencapsulated L. reuteri NCIMB 30242 in yoghurt compares favourably with other cholesterol-lowering food ingredients(Reference Demonty, Ras and van der Knaap38).
Acknowledgements
The authors thank J. Lahovský, A. Vajikova, N. Barannikova, K. Beber, L. Coupal and S. Grover for their contribution to the study. The authors also thank all volunteers who participated in the study. M. L. J., S. P. and C. J. M. designed the study and prepared the manuscript; J. Lahovský, A. Vajikova and N. Barannikova conducted the research; K. Beber, M. P., L. Coupal and S. Grover performed the statistical analysis. All authors have read and approved the final manuscript. This work was supported by Micropharma Limited. M. L. J., C. J. M., M. P. and S. P. are with Micropharma Limited and report a conflict of interest. All other contributors have no conflicts of interest to report.









