Introduction
Copepods are the most abundant metazoan animals in the ocean, with over 14,500 accepted species of parasitic copepods recorded to date (Walter & Boxshall, Reference Walter and Boxshall2022). Despite their abundance, the distribution of these species is patchily recorded across ocean basins, with their occurrence in remote locations poorly understood. Adding to patchily known species distributions can be useful in identifying stepping-stones of transport and colonisation, which facilitate widespread animal distributions. The order Siphonostomatoida contains 2233 species, of which an estimated 69% feed on the epidermal tissue, mucus or blood of marine fishes, ranging from small bait fish to large elasmobranchs (Austin et al., Reference Austin, Tan, Lee, Croft, Meekan, Pierce and Gan2014; Ohtsuka et al., Reference Ohtsuka, Nawata, Nishida, Nitta, Hirano, Adachi, Kondo, Venmathi Maran and Suárez-Morales2020). This order contains ~550 genera and nearly 40 families (Austin et al., Reference Austin, Tan, Lee, Croft, Meekan, Pierce and Gan2014), including the genus Alebion which is contained within the family Caligidae. Kabata (Reference Kabata1979) placed Alebion in the family Euryphoridae; however, Boxshall & Halsey (Reference Boxshall and Halsey2004) recognise Alebion and other former euryphorid genera as members of the Caligidae. The caligids are a diverse group of copepods that predominantly parasitise marine fishes (Dojiri & Ho, Reference Dojiri and Ho2013). The adults exhibit highly modified body forms. The adult female caligid body is dorso-ventrally flattened to allow the cephalothorax to serve as a suction cup to attach to the host (Dojiri & Ho, Reference Dojiri and Ho2013 and sources therein). Since the first description of the genus Alebion by Krøyer in 1863, nine species have been described (formerly 14, later redescribed as 9; Cressey, Reference Cressey1972; Dippenaar, Reference Dippenaar2018).
Alebion carchariae has been recorded on at least 16 shark and one fish species (Table 1), with three records in the South Atlantic Ocean spanning from the eastern USA (listed hosts include smooth hammerhead, Sphyrna zygaena; scalloped hammerhead, Sphyrna lewini, see Rokicki & Bychawska, Reference Rokicki and Bychawska1991), São Paulo (unspecified host, reviewed in Luque & Tavares, Reference Luque and Tavares2007) and off the coast of Senegal, West Africa (Vaissière, Reference Vaissière1959). Here we describe the occurrence of this species in the nearshore waters of Ascension Island, a remote island located in the South Atlantic Ocean. Thereafter we provide the first DNA barcode sequence for this species.
Table 1. The diversity of recorded Alebion carchariae hosts

Data on the host species ecology (water type, depth range, habitat) were extracted from https://fishbase.se.
Materials and methods
Six specimens of Alebion carchariae were collected off the north-west coast of Ascension Island in the South Atlantic Ocean (7.9467°S 14.3559°W). On 27 January 2021, one specimen was removed from the snout region of a juvenile (~90 cm TL) Galapagos shark (Carcharhinus galapagensis) at Comfortless Cove (7.9104°S 14.4030°W). On 17 April 2021, an additional five specimens were removed from the face of a juvenile (<100 cm TL) Galapagos shark (Carcharhinus galapagensis) at English Bay (7.8933°S 14.3849°W). Hosts were caught unintentionally using light or medium-action fishing rods in both incidences and released promptly after manually removing the copepod/s from the host. Copepods were preserved in 70% ethanol and stored temporarily in the Ascension Island Government Conservation Centre on Ascension Island.
The copepod specimens were sent to the College of Fisheries and Ocean Sciences at the University of Alaska, Fairbanks (USA) and examined with a Leica M205C stereomicroscope, and photographed using a Leica DMC4500 camera. Two specimens were dissected to examine the legs and genital complex in further detail. The specimens were identified as Alebion carchariae based on the taxonomic descriptors of Cressey (Reference Cressey1972), Boxshall & Halsey (Reference Boxshall and Halsey2004), Dojiri & Ho (Reference Dojiri and Ho2013) and Dippenaar (Reference Dippenaar2018). After dissection, specimens were stored in ethanol at −20°C. Subsequent DNA extraction and amplicon sequencing was conducted on two individual specimens.
DNA extraction, PCR amplification and sequencing
Prior to DNA extraction, individually selected and identified female A. carchariae were rinsed in sterile Milli-Q® water to remove all traces of ethanol to enable successful DNA extraction. Genomic DNA was extracted using a Qiagen DNeasy Blood and Tissue Kit with a final elution volume of 200 μl elution buffer. A 658 base-pair (bp) region of the mitochondrial cytochrome oxidase I (COI) gene (i.e. the barcode region) was targeted for species identification (Bucklin et al., Reference Bucklin, Steinke and Blanco-Bercial2011). Polymerase Chain Reaction (PCR) was carried out using 12.5 μl Q5® Hot Start High-Fidelity 2X Master Mix (New England Biolabs Inc.), 1.25 μl of each forward and reverse primer (10 μM), 3–5 μl DNA template, and PCR grade water for a final reaction volume of 25 μl. COI was amplified with the universal primer pair LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-′3) (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). PCR thermal cycle protocol consisted of 35 cycles of 98°C for 10 s, 62°C for 20 s, 72°C for 20 s and a final extension at 72°C for 2 min. PCR products were run on a 1.5% agarose TBE buffer gel stained with GelRed. Amplicons from successful PCR reactions were purified using ExoSAP-ITTM Express PCR cleanup, combined with either the forward or reverse primer for bi-directional sequencing, and sequenced by Azenta Life Sciences (http://www.azenta.com).
Bi-directional COI sequences were assembled to create contiguous reads and checked for ambiguous base calling using Geneious Prime (Ver. R10). COI sequences for copepods in the family Caligidae with species-level identifications were retrieved from the NCBI GenBank public repository (www.ncbi.nlm.nih.gov/genbank) and analysed with COI data for A. carchariae obtained in this study. COI reads were aligned using the MUSCLE algorithm (Edgar Reference Edgar2004) and trimmed to uniform lengths. Neighbour-joining (NJ) gene trees were analysed after 10,000 coalescence simulations using a bootstrap test of 1000 replicates. The COI sequence for Demoleus heptapus (MH242722) represents the outgroup. Pairwise proportional nucleotide distances (p-distance) within and between Caligidae species were calculated in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018).
Results
Morphological identification
The body was dorso-ventrally flattened (Figure 1) with genital complex bearing elongated posterior processes extending slightly beyond the caudal rami. Lateral processes of the first abdominal somite reached the distal margin of the second abdominal somite. The genital complex had a slight bulge in the centre and margin armed with spinules, with legs 1–3 biramous, and leg 1 possessing 2 segmented rami (Figure 2A). Importantly, the specimen's exopodal spines of legs 1–3 were modified into large paddle-like structures (Figure 2B), characteristic of the genus Alebion. Additionally, legs 2 and 3 possessed 3-segmented rami (Figure 2C, D, E), while leg 4 was reduced and uniramous (Figure 2F). Leg 5 was represented by the elongated posterior processes extending from genital segment. Both female specimens were 8.6 mm in total length. The cephalothorax lengths of the individuals were 5.1 and 5.3 mm.
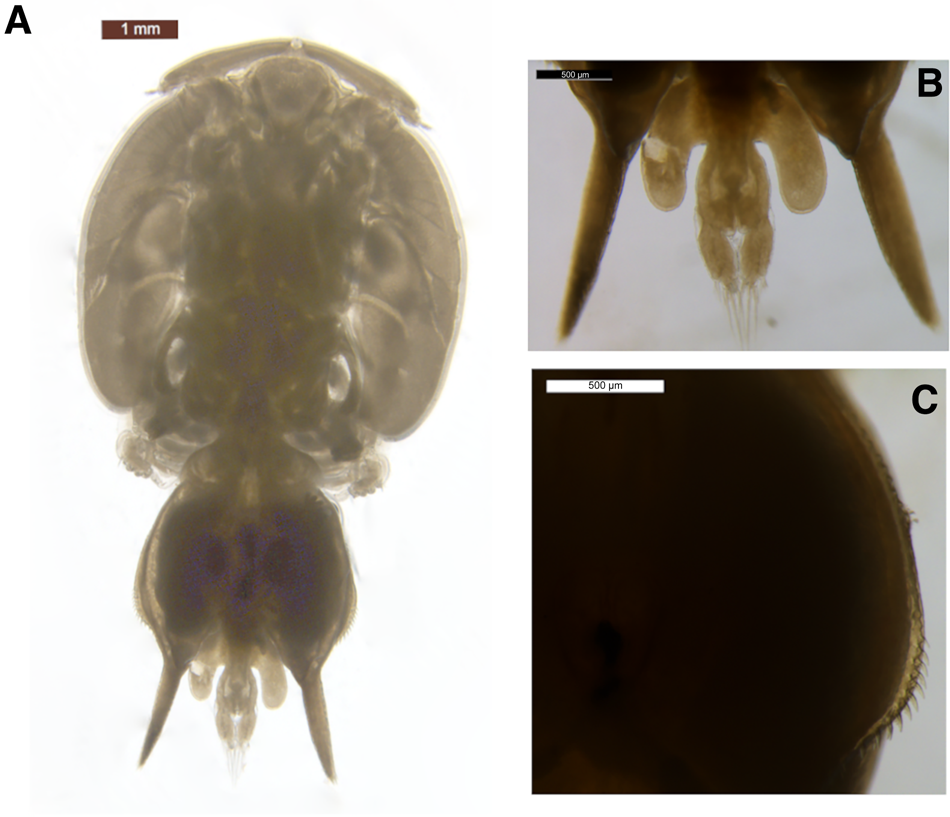
Fig. 1. Alebion carchariae female. (A) Body, dorsal view; (B) abdomen and caudal rami; posterior processes of genital segment, dorsal view; (C) spinules on margin of genital complex.
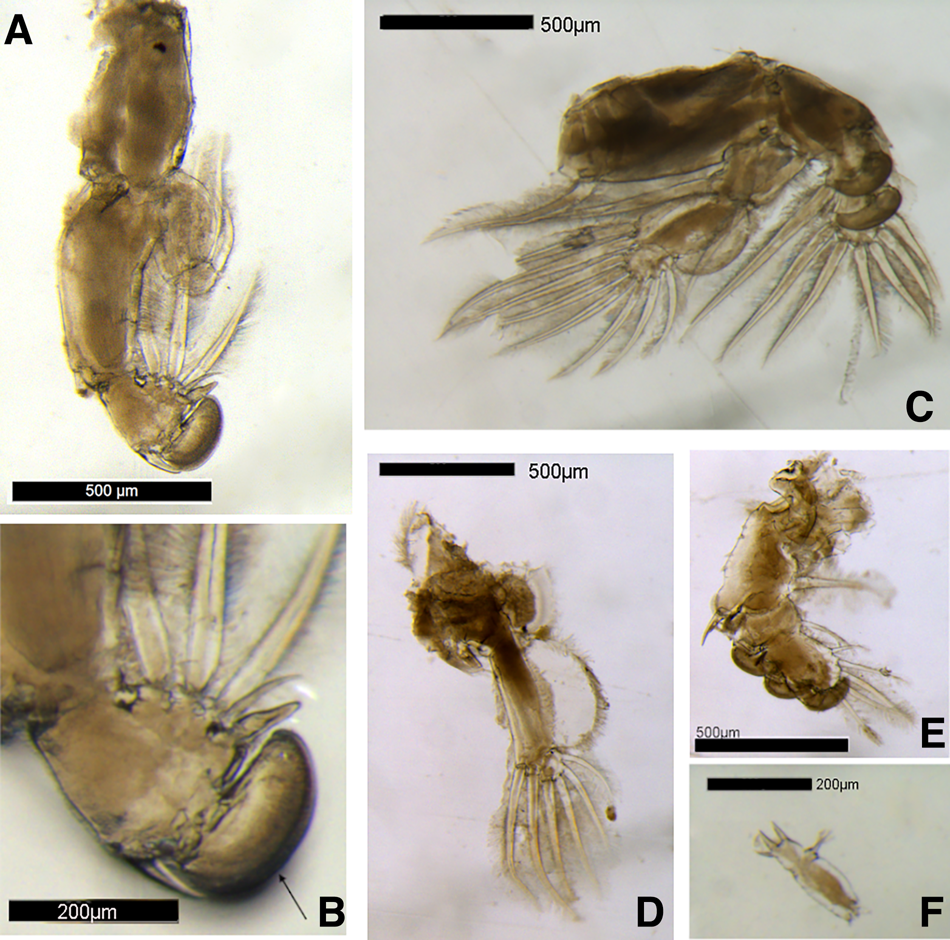
Fig. 2. Alebion carchariae female. (A) Leg 1; (B) Distal portion of exopod of leg 1; arrow indicates modified paddle-like exopodal spine; (C) Leg 2; (D) Leg 3 endopod; (E) Leg 3 exopod; (F) Leg 4.
Analysis of COI sequences
Two COI sequences of 557 base-pairs (bp) in length were sequenced for A. carchariae (GenBank accession nos ON807358 and ON807359). These data represent the first publicly available COI barcodes for A. carchariae and the first species-level COI data for the genus Alebion. Low pairwise distances (p-distances) were observed within individual A. carchariae (0.36%; N = 2) with high between-species distances observed when compared with Caligus spp. (18.0–28.1%; N = 9; Table 2). The high interspecific genetic variability for the COI barcode region is consistent with metazoan species (10–25%; Hebert et al., Reference Hebert, Ratnasingham and deWaard2003). However, phylogenetic trees based on COI variation for species in the family Caligidae (Figure 3) revealed a bifurcating pattern with A. carchariae clustering separately from the other Caligidae species (100% bootstrap support). These data suggest A. carchariae and Caligus spp. may not belong in the same family, but additional COI data for species across multiple families in the order Siphonostomatoida are needed to resolve phylogenetic lineages.

Fig. 3. COI gene tree for copepods in the family Caligidae based on COI reads for Alebion carchariae generated in this study and species-level data available in GenBank (www.ncbi.nlm.nih.gov/genbank). Demoleus heptapus (MH242772) represents the outgroup. Neighbour-joining tree with Tamura & Nei (Reference Tamura and Nei1993) substitution model; 1000× bootstrap values are indicated at nodes; values >50 are shown; scale bar indicates genetic distance.
Table 2. Pairwise genetic distances (p-distance) within and between eight species in the family Caligidae based on COI sequence variation. GenBank accession numbers for Caligus spp. are in parentheses

Discussion
Alebion carchariae (Krøyer, Reference Krøyer1863)
New record. Ascension Island (7.9467°S 14.3559°W)
The recorded diversity of A. carchariae hosts suggests this parasite has plasticity in terms of the hosts it infects and the conditions it can tolerate, given the variety of habitats and depths utilised by these hosts. Documented hosts include sharks and fishes commonly distributed across reef habitats, benthic and pelagic to oceanic realms, with preferred depths ranging between 0–500 m. Current knowledge regarding the life cycle of caligids is based on studies of sea lice genera, including Caligus and Lepeophtheirus, which have been relatively well studied due to their economic impact on aquaculture (Boxaspen, Reference Boxaspen2006; Hemmingsen et al., Reference Hemmingsen, MacKenzie, Sagerup, Remen, Bloch-Hansen and Dagbjartarson Imsland2020). The generalised caligid life cycle consists of two naupliar stages and one infective copepodid stage (Hamre et al., Reference Hamre, Eichner, Caipang, Dalvin, Bron, Nilsen, Boxshall, Skern-Mauritzen and Krkosek2013). However, the number of stages between the copepodid and adult stage reported to vary between four chalimus stages (Pseudocaligus and Caligus species; Kim, Reference Kim1993; Piasecki, Reference Piasecki1996; Ohtsuka et al., Reference Ohtsuka, Takami, Venmathi Maran, Ogawa, Shimono, Fujita, Asakawa and Boxshall2009) to four chalimus plus two preadult stages (Lepeophtheirus species; Lewis, Reference Lewis1963; Voth, Reference Voth1972; Boxshall, Reference Boxshall1974; Johnson & Albright, Reference Johnson and Albright1991). The copepodid stage of caligids is infective, and the subsequent chalimus stages bear a frontal filament used to attach to the host. Wilson (Reference Wilson1907) reported that Alebion glaber does not possess a frontal filament, but instead attaches to the host with prehensile antennae. It should also be noted that there are no published studies regarding the life cycle of caligid genera formerly included in the Euryphoridae, including Alebion. Experimental studies show the infective stage of a salmon louse (Lepeophtheirus salmonis) will aggregate in the upper water column to increase the probability of encountering potential hosts (Heuch et al., Reference Heuch, Parsons and Boxaspen1995). The lifespan of the infective stage of sea lice has been reported as lasting between 1–9 days (Johnson & Albright, Reference Johnson and Albright1991), and only upon attachment to a host can the lice fully develop and sexually reproduce. Life cycle estimates have ranged from less than two weeks (in controlled trials at 20 and 30°C; Khoa et al., Reference Khoa, Mazelan, Muda and Shaharom-Harrison2019) to <210 days under laboratory conditions (Mustafa et al., Reference Mustafa, Conboy and Burka2001). The potential longevity of these species may enable them to hitchhike onto migratory species and facilitate their distribution (Boxaspen, Reference Boxaspen2006). For example, previous records of Alebion carchariae closest to Ascension Island include South America and West Africa, and longevity could facilitate its widespread distribution via hitchhiking on hosts traversing ocean basins or transport via ocean currents (enabling subsequent host infection). The described position of attachment of A. carchariae includes shark pectoral fins (C. obscurus, Vaissière, Reference Vaissière1959) and the underside of the head (Sphyrna lewini, Vaissière, Reference Vaissière1959), and fish gills (Coryphaena equisetis, Vaissière, Reference Vaissière1959). Alebion has also been reported to wander the body surface of hosts (Cressey, Reference Cressey1972). Dorso-ventral flattening likely facilitates its successful adhesion by reducing drag when situated on the face (as observed in Nerocila, Nagler & Haug, Reference Nagler and Haug2016), and the versatility of its attachment location may increase its chance of success.
Further sampling of the parasitic assemblage around Ascension Island through opportunistic sampling, e.g. during fisheries surveys to collect mature individuals, and water sampling for pre-adult stages, could provide insight into the ecology and life cycle of this poorly studied genus and identify susceptible hosts. For example, data are required to determine the mechanisms that support its widespread distribution and its potential tolerance to a range of conditions (i.e. depths) that may facilitate its association with diverse hosts. Additionally, sampling this parasite may offer a minimally invasive technique to explore parasite-host interactions and/or the genetic structure of host populations such as C. galapagensis sharks around Ascension Island (following Meekan et al., Reference Meekan, Austin, Tan, Wei, Miller, Pierce, Rowat, Stevens, Davies, Ponzo and Gan2017). While only seven A. carchariae were collected from two sharks it was noted that one individual hosted numerous other A. carchariae that were not collected. The implications of the presence of this parasitic copepod on host health and fitness remain unknown and warrants further research.
Acknowledgements
We thank the Ascension Island Government and the Ascension Island community for supporting this work, with dedicated thanks to Shaun Scipio, Alex Knipe and Adriene Levknecht for their assistance with sample collection. We extend our sincere thanks to the anonymous reviewer of this manuscript for their contributions and recommendations.
Author contributions
DLO collected the specimens. The specimens were photographed by CS and sequenced by JQ. TS provided logistical support on Ascension Island. DLO wrote the manuscript with support from NEH, JQ, CS, TS. All authors discussed the results and contributed to the final manuscript.
Financial support
DLO is supported by the Ontario Trillium Scholarship and NEH by the NSERC Discovery Grant Program.
Conflict of interest
The authors declare none.







